Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species
Abstract
1. Introduction
2. Materials and Methods
2.1. First-Aid Protocol Experiments
2.1.1. Jellyfish Origin
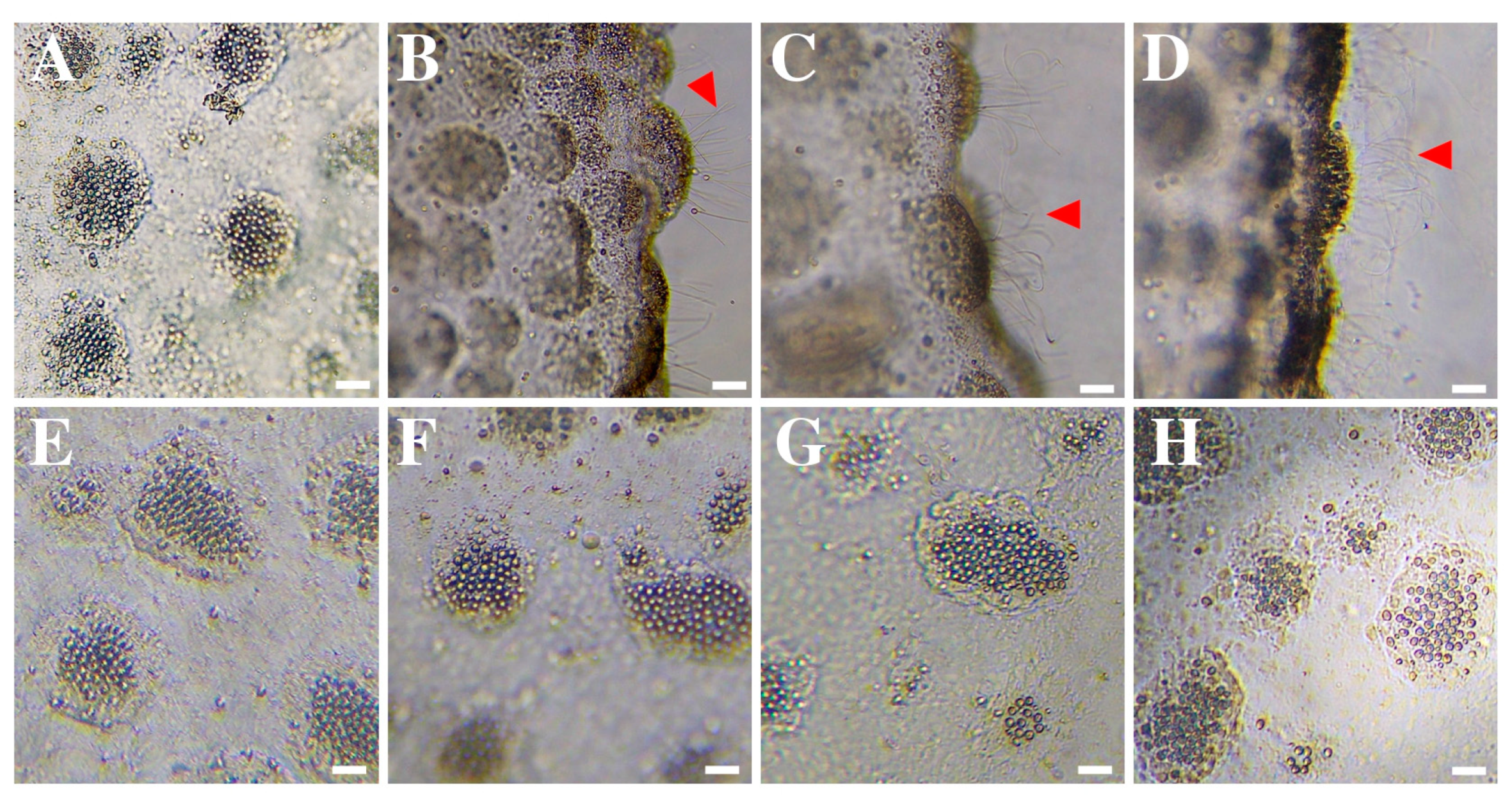
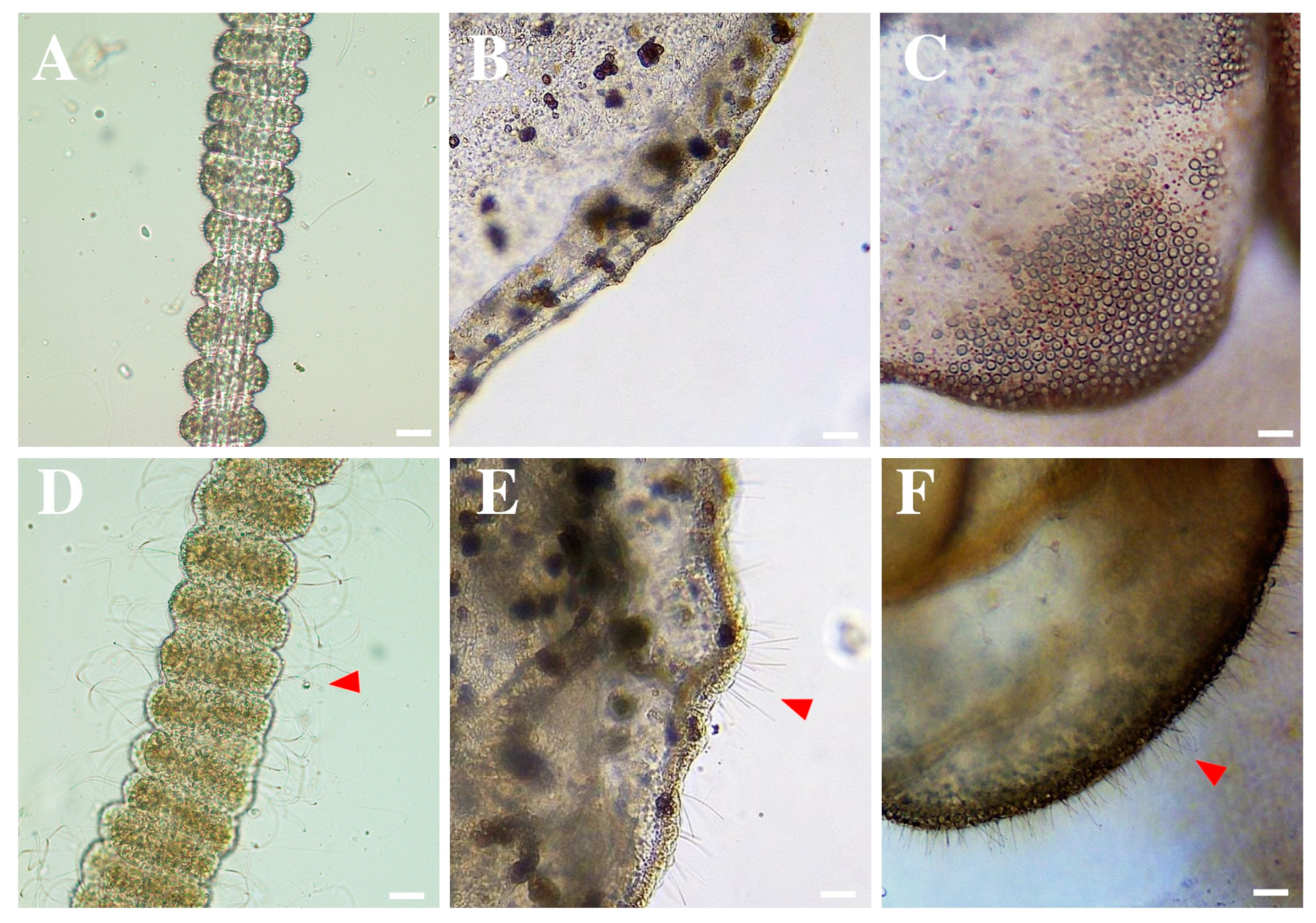
2.1.2. Tentacle Solution Assay (TSA)—Nematocyst Discharge
- 0: no discharge observed;
- +: low discharge of nematocysts;
- ++: medium discharge of nematocysts;
- +++: maximum discharge of nematocysts.
- Activator effect solution: nematocysts were activated after the application of the solution;
- Neutral effect solution: nematocysts were not activated after the application of the solution.
3. Results
3.1. Response of Rhizostoma pulmo Nematocysts to Rinse Solutions
3.2. Response of Aurelia sp., Cassiopea sp., and Rhizostoma luteum Nematocysts to Vinegar
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boulware, D.R. A Randomized, Controlled Field Trial for the Prevention of Jellyfish Stings With a Topical Sting Inhibitor. J. Travel Med. 2006, 13, 166–171. [Google Scholar] [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F.; et al. Impact of Stinging Jellyfish Proliferations along South Italian Coasts: Human Health Hazards, Treatment and Social Costs. Int. J. Environ. Res. Public Health 2014, 11, 2488–2503. [Google Scholar] [CrossRef]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed]
- Östman, C. A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Sci. Mar. 2000, 64, 31–46. [Google Scholar] [CrossRef]
- Fautin, D.G. Structural diversity, systematics, and evolution of cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef]
- Al-Rubiay, K.; Al-Musaoi, H.; Alrubaiy, L.; Al-Freje, M. Skin and Systemic Manifestations of Jellyfish Stings in Iraqi Fishermen. Libyan J. Med. 2009, 4, 75–77. [Google Scholar] [CrossRef]
- Mghili, B.; Analla, M.; Aksissou, M. Epidemiology of the cnidarian Pelagia noctiluca stings on Moroccan Mediterranean beaches. Trop. Dr. 2020, 50, 322–325. [Google Scholar] [CrossRef]
- Ballesteros, A.; Salazar, J.; Marambio, M.; Tena, J.; García-March, J.R.; López, D.; Tellez, C.; Trullas, C.; Jourdan, E.; Granger, C.; et al. Trial Assay for Safe First-Aid Protocol for the Stinging Sea Anemone Anemonia viridis (Cnidaria: Anthozoa) and a Severe Toxic Reaction. Toxins 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Dinis-Oliveira, R.J. Raising Awareness on the Clinical and Forensic Aspects of Jellyfish Stings: A Worldwide Increasing Threat. Int. J. Environ. Res. Public Health 2022, 19, 8430. [Google Scholar] [CrossRef]
- Remigante, A.; Costa, R.; Morabito, R.; La Spada, G.; Marino, A.; Dossena, S. Impact of Scyphozoan Venoms on Human Health and Current First Aid Options for Stings. Toxins 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, L.; Seys, J.; Mees, J. To Pee, or Not to Pee: A Review on Envenomation and Treatment in European Jellyfish Species. Mar. Drugs 2016, 14, 127. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Becken, S.; Bordehore, C.; Fuentes, V.L.; Pitt, K.A.; Yangihara, A.A. Empowering Stakeholders to Manage Stinging Jellyfish: A Perspective. Coast. Manag. 2018, 46, 1–18. [Google Scholar] [CrossRef]
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish Stings and Their Management: A Review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F. Epidemiology of jellyfish stings reported to summer health centres in the Salento peninsula (Italy). Contact Dermat. 2009, 60, 330–335. [Google Scholar] [CrossRef]
- Bordehore, C.; Alonso, C.; Sánchez-Fernández, L.; Canepa, A.; Acevedo, M.; Nogué, S.; Fuentes, V.L. Lifeguard assistance at Spanish Mediterranean beaches: Jellyfish prevail and proposals for improving risk management. Ocean Coast. Manag. 2016, 131, 45–52. [Google Scholar] [CrossRef]
- Canepa, A.; Fuentes, V.; Sabatés, A.; Piraino, S.; Boero, F.; Gili, J.-M. Pelagia noctiluca in the Mediterranean Sea. In Jellyfish Blooms; Pitt, K., Lucas, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 237–266. [Google Scholar] [CrossRef]
- Simmons, B.J.; Griffith, R.D.; Falto-Aizpurua, L.A.; Nouri, K. Moon Jellyfish Stings. JAMA Dermatol. 2015, 151, 453–454. [Google Scholar] [CrossRef]
- Friedel, N.; Scolnik, D.; Adir, D.; Glatstein, M. Severe anaphylactic reaction to mediterranean jellyfish (Ropilhema nomadica) envenomation: Case report. Toxicol. Rep. 2016, 3, 427–429. [Google Scholar] [CrossRef]
- Killi, N.; Mariottini, G.L. Cnidarian Jellyfish: Ecological Aspects, Nematocyst Isolation, and Treatment Methods of Sting. In Marine Organisms as Model Systems in Biology and Medicine. Results and Problems in Cell Differentiation; Kloc, M., Kubiak, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 477–513. [Google Scholar] [CrossRef]
- Bs, K.G.E.; Claire, K.S.; Daveluy, S.; Claire, M.K.S. Acetic acid and the skin: A review of vinegar in dermatology. Int. J. Dermatol. 2021, 61, 804–811. [Google Scholar] [CrossRef]
- Doyle, T.K.; Headlam, J.L.; Wilcox, C.L.; MacLoughlin, E.; Yanagihara, A.A. Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models. Toxins 2017, 9, 215. [Google Scholar] [CrossRef]
- Pyo, M.-J.; Lee, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Yoon, W.D.; Kang, C.; Kim, E. Modulation of jellyfish nematocyst discharges and management of human skin stings in Nemopilema nomurai and Carybdea mora. Toxicon 2016, 109, 26–32. [Google Scholar] [CrossRef]
- Ballesteros, A.; Marambio, M.; Fuentes, V.; Narda, M.; Santín, A.; Gili, J.-M. Differing Effects of Vinegar on Pelagia noctiluca (Cnidaria: Scyphozoa) and Carybdea marsupialis (Cnidaria: Cubozoa) Stings—Implications for First Aid Protocols. Toxins 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Fitzpatrick, P.F. Experiments with the nematocysts of Cyanea capillata. Med. J. Aust. 1986, 145, 174. [Google Scholar] [CrossRef] [PubMed]
- Birsa, L.M.; Verity, P.G.; Lee, R.F. Evaluation of the effects of various chemicals on discharge of and pain caused by jellyfish nematocysts. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 426–430. [Google Scholar] [CrossRef]
- Marambio, M.; Canepa, A.; Lòpez, L.; Gauci, A.; Gueroun, S.; Zampardi, S.; Boero, F.; Yahia, O.; Yahia, M.; Fuentes, V.; et al. Unfolding Jellyfish Bloom Dynamics along the Mediterranean Basin by Transnational Citizen Science Initiatives. Diversity 2021, 13, 274. [Google Scholar] [CrossRef]
- Leoni, V.; Bonnet, D.; Ramírez-Romero, E.; Molinero, J.C. Biogeography and phenology of the jellyfish Rhizostoma pulmo (Cnidaria: Scyphozoa) in Southern European seas. Glob. Ecol. Biogeogr. 2021, 30, 622–639. [Google Scholar] [CrossRef]
- Fuentes, V.; Straehler-Pohl, I.; Atienza, D.; Franco, I.; Tilves, U.; Gentile, M.; Acevedo, M.; Olariaga, A.; Gili, J.-M. Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the Mar Menor (Spain, NW Mediterranean). Mar. Biol. 2011, 158, 2247–2266. [Google Scholar] [CrossRef]
- Marambio, M. (ICM-CSIC-Institute of Marine Sciences, Barcelona, Spain). Personal communication, 2022.
- Avian, M.; DEL Negro, P.; Sandrini, L.R. A comparative analysis of nematocysts in Pelagia noctiluca and Rhizostoma pulmo from the North Adriatic Sea. Hydrobiologia 1991, 216–217, 615–621. [Google Scholar] [CrossRef]
- Kokelj, F.; Plozzer, C. Irritant contact dermatitis from the jellyfish Rhizostoma pulmo. Contact Dermat. 2002, 46, 179–180. [Google Scholar] [CrossRef]
- Marambio, M.; Ballesteros, A.; López-Castillo, L.; Fuentes, V.; Gili, J.M. Guía de Identificación de Medusas y Otros Organismos Gelatinosos; CSIC-Instituto de Ciencias del Mar (ICM): Barcelona, Spain, 2021. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.; King, R.; Hurwitz, K.; Castelfranco, A.M. Experimental Assays to Assess the Efficacy of Vinegar and Other Topical First-Aid Approaches on Cubozoan (Alatina alata) Tentacle Firing and Venom Toxicity. Toxins 2016, 8, 19. [Google Scholar] [CrossRef]
- Colin, S.; Costello, J.H. Functional characteristics of nematocysts found on the scyphomedusa Cyanea capillata. J. Exp. Mar. Biol. Ecol. 2007, 351, 114–120. [Google Scholar] [CrossRef]
- Ballesteros, A.; Trullas, C.; Jourdan, E.; Gili, J.-M. Inhibition of Nematocyst Discharge from Pelagia noctiluca (Cnidaria: Scyphozoa)—Prevention Measures against Jellyfish Stings. Mar. Drugs 2022, 20, 571. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, A.A.; Wilcox, C.L. Cubozoan Sting-Site Seawater Rinse, Scraping, and Ice Can Increase Venom Load: Upending Current First Aid Recommendations. Toxins 2017, 9, 105. [Google Scholar] [CrossRef]
- Ballesteros, A.; Marambio, M.; Fuentes, V.; Gili, J.-M. The use of a citizen science tool (IMEDJELLY App) to deliver first aid protocols for Mediterranean jellyfish stings. In Proceedings of the VII International Symposium on Marine Sciences, Barcelona, Spain, 1–3 July 2020. [Google Scholar]
- Nogué, S.; Gili, J.M. Toxicidad por picadura de medusas. Jano 2006, 1816, 45–46. [Google Scholar]
- Wilcox, C.L.; Headlam, J.L.; Doyle, T.K.; Yanagihara, A.A. Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins 2017, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.; Ruhnau, C.; Schütt, C.; Prange, A. Comparative study on the cell toxicity and enzymatic activity of two northern scyphozoan species Cyanea capillata (L.) and Cyanea lamarckii (Péron & Léslieur). Toxicon 2007, 50, 53–64. [Google Scholar] [CrossRef]
- Marino, A.; Crupi, R.; Musci, G.; La Spada, G. Morphological integrity and toxicological properties of Pelagia noctiluca (Scyphozoa) nematocysts. Chem. Ecol. 2006, 22, S127–S131. [Google Scholar] [CrossRef]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
| Rinse Solution | Discharge 1 | Effect 2 |
|---|---|---|
| Seawater (control) | 0 | Neutral |
| Vinegar | ++ | Activator |
| 5% acetic acid in freshwater | ++ | Activator |
| Scented ammonia | +++ | Activator |
| 10% baking soda mixed in seawater | 0 | Neutral |
| Freshwater | 0 | Neutral |
| Urine | 0 | Neutral |
| Hydrogen peroxide | 0 | Neutral |
| Aurelia sp. | ||
| Rinse Solution | Discharge 1 | Effect 2 |
| Seawater (control) | 0 | Neutral |
| Vinegar | ++ | Activator |
| Cassiopea sp. | ||
| Rinse solution | Discharge 1 | Effect 2 |
| Seawater (control) | 0 | Neutral |
| Vinegar | ++ | Activator |
| Rhizostoma luteum | ||
| Rinse solution | Discharge 1 | Effect 2 |
| Seawater (control) | 0 | Neutral |
| Vinegar | ++ | Activator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros, A.; Marambio, M.; Trullas, C.; Jourdan, E.; Tena-Medialdea, J.; Gili, J.-M. Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species. Int. J. Environ. Res. Public Health 2023, 20, 2344. https://doi.org/10.3390/ijerph20032344
Ballesteros A, Marambio M, Trullas C, Jourdan E, Tena-Medialdea J, Gili J-M. Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species. International Journal of Environmental Research and Public Health. 2023; 20(3):2344. https://doi.org/10.3390/ijerph20032344
Chicago/Turabian StyleBallesteros, Ainara, Macarena Marambio, Carles Trullas, Eric Jourdan, Jose Tena-Medialdea, and Josep-Maria Gili. 2023. "Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species" International Journal of Environmental Research and Public Health 20, no. 3: 2344. https://doi.org/10.3390/ijerph20032344
APA StyleBallesteros, A., Marambio, M., Trullas, C., Jourdan, E., Tena-Medialdea, J., & Gili, J.-M. (2023). Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species. International Journal of Environmental Research and Public Health, 20(3), 2344. https://doi.org/10.3390/ijerph20032344





