The Effect of Starting Blood Glucose Levels on Serum Electrolyte Concentrations during and after Exercise in Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Baseline Testing and Measurement of VO2peak
2.2. Experimental Design
2.3. Exercise Testing Sessions
2.4. Blood and Urine Sample Collection and Analysis
2.5. CGM Data Assessment
2.6. Statistical Analysis
3. Results
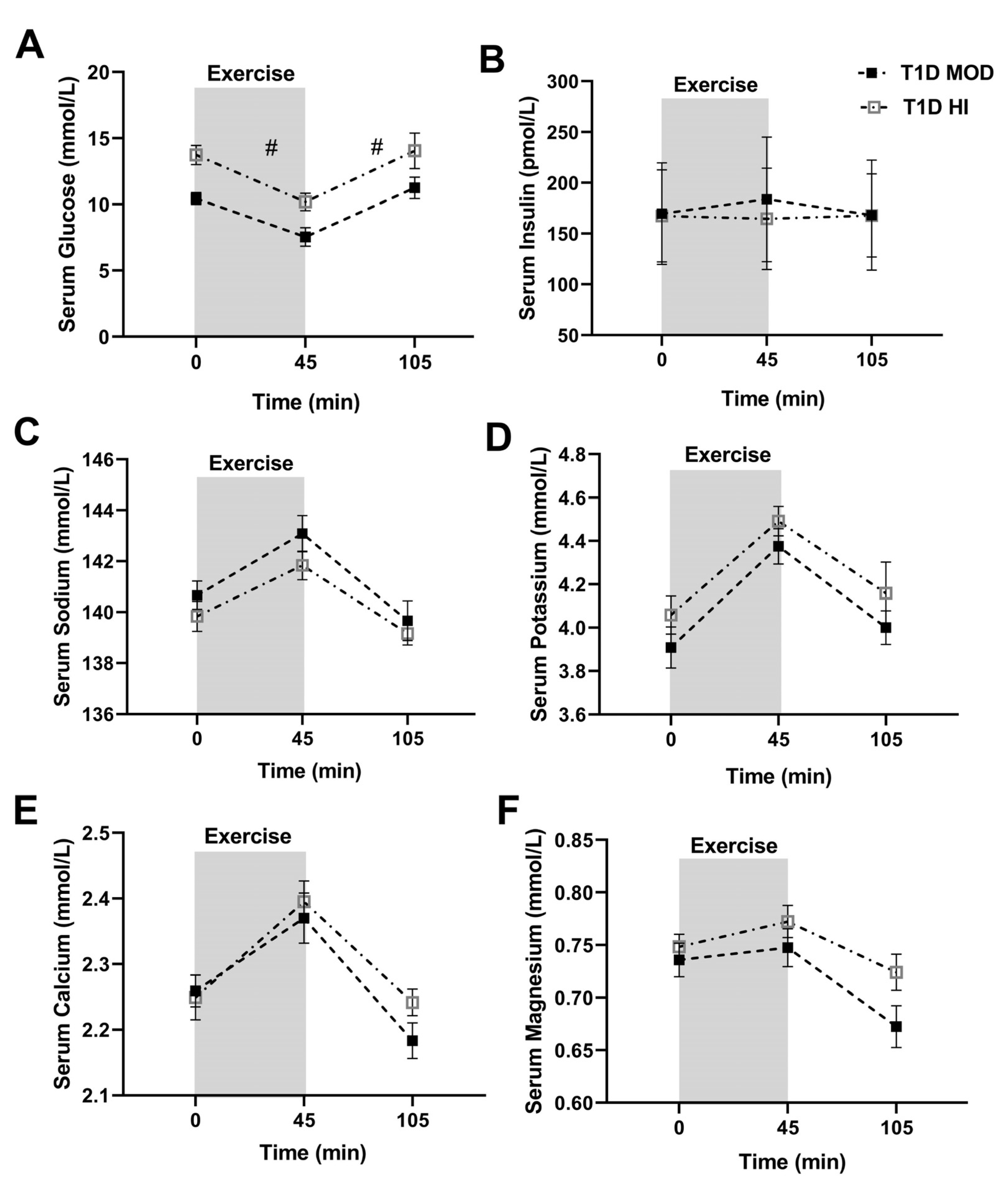
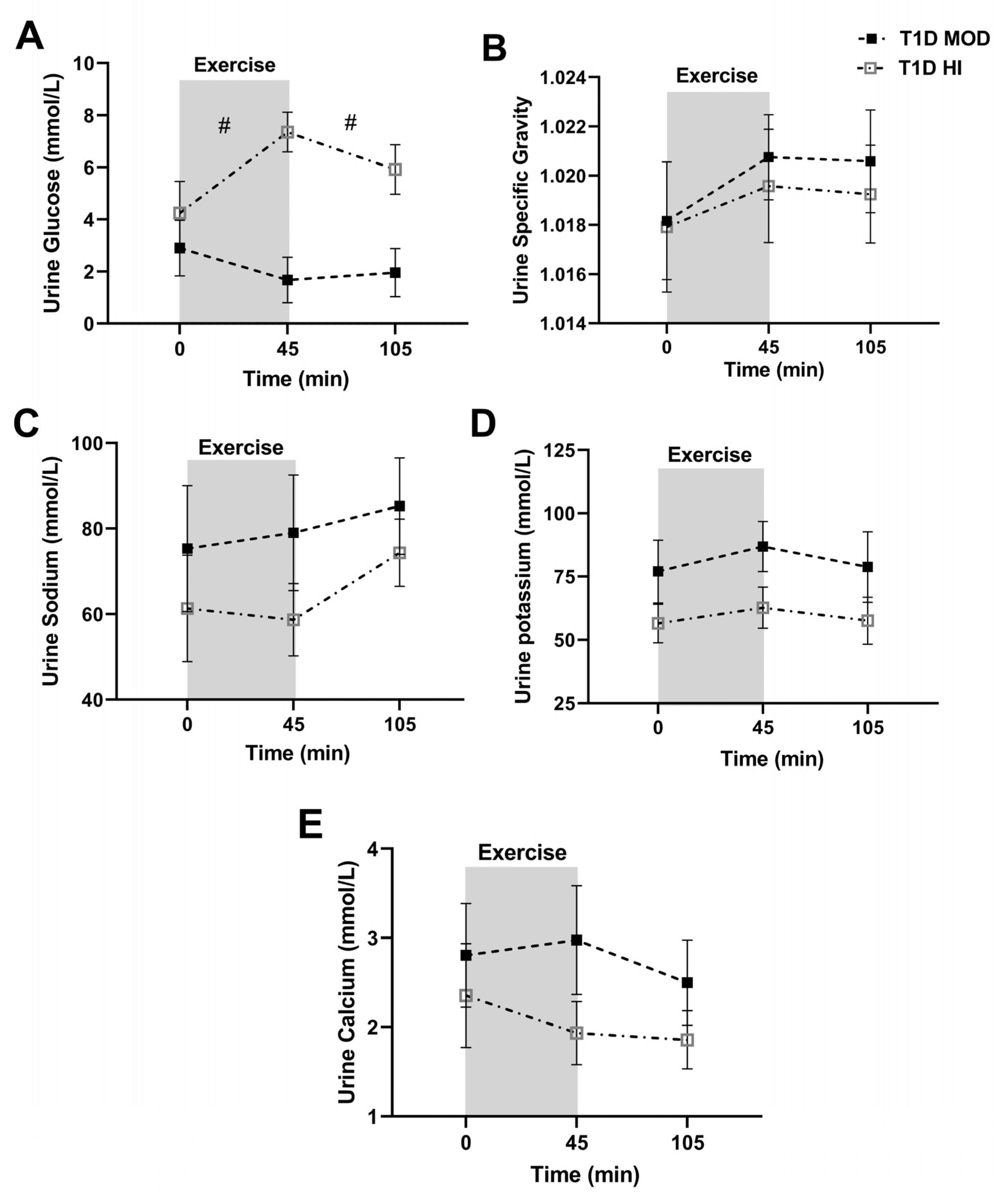
3.1. MOD vs. HI Comparisons for the Main Outcomes
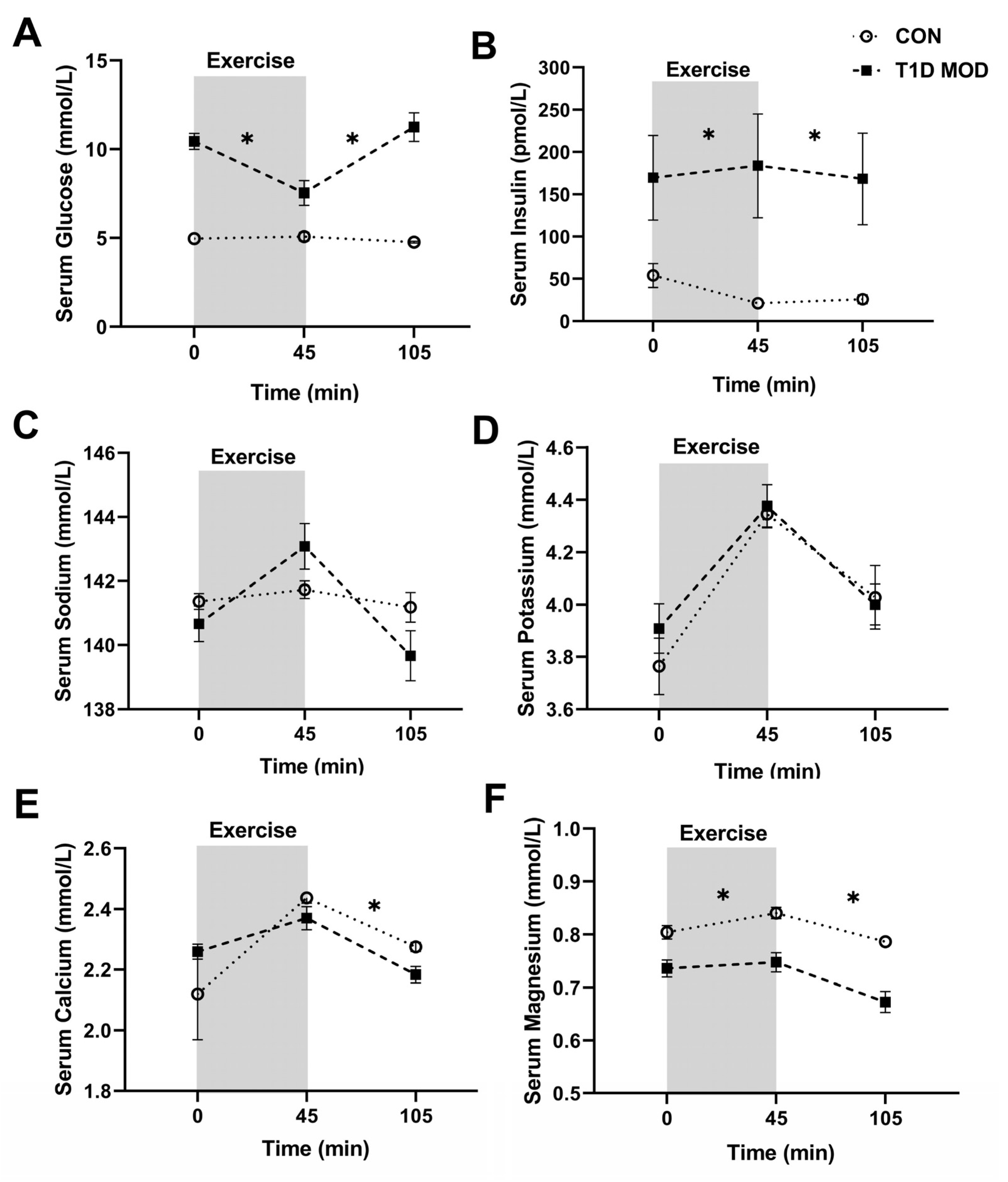
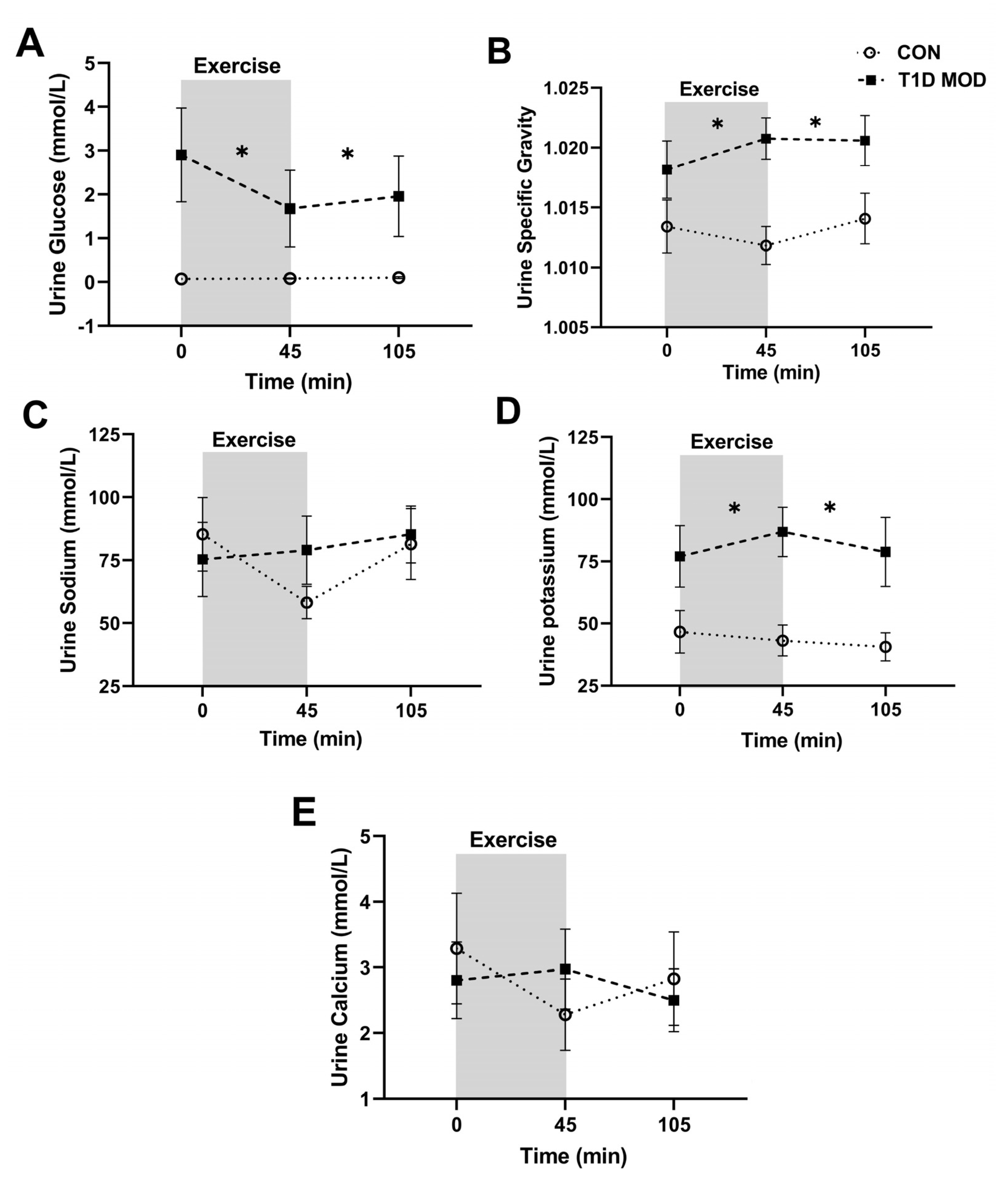
3.2. CON vs. MOD Comparisons for the Main Outcomes
3.3. Food and Insulin Intake and Background Physical Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moy, C.S.; Songer, T.J.; LaPorte, R.E.; Dorman, J.S.; Kriska, A.M.; Orchard, T.J.; Becker, D.J.; Drash, A.L. Insulin-dependent diabetes mellitus, physical activity, and death. Am. J. Epidemiol. 1993, 137, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Iacobellis, G.; Parisi, L.; Di Biase, N.; Calandriello, E.; Leonetti, F.; Fallucca, F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J. Diabetes Complicat. 2006, 20, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Waden, J.; Tikkanen, H.K.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Saraheimo, M.; Tolonen, N.; Rosengard-Barlund, M.; Gordin, D.; Tikkanen, H.O.; et al. Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: The FinnDiane Study. Diabetologia 2015, 58, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, A.S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef] [PubMed]
- Pinsker, J.E.; Kraus, A.; Gianferante, D.; Schoenberg, B.E.; Singh, S.K.; Ortiz, H.; Dassau, E.; Kerr, D. Techniques for exercise preparation and management in adults with type 1 diabetes. Can. J. Diabetes 2016, 40, 503–508. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Yardley, J.; Francescato, M.P.; Morrison, S.A. Fluid intake habits in type 1 diabetes individuals during typical training bouts. Ann. Nutr. Metab. 2018, 73, 10–18. [Google Scholar] [CrossRef]
- Johansen, K.; Svendsen, P.A.; Lorup, B. Variations in renal threshold for glucose in Type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1984, 26, 180–182. [Google Scholar] [CrossRef]
- Dhatariya, K. People with type 1 diabetes using short acting analogue insulins are less dehydrated than those with using human soluble insulin prior to onset of diabetic ketoacidosis. Med. Hypotheses 2008, 71, 706–708. [Google Scholar] [CrossRef]
- Simková, R.; Kazdová, L.; Karasová, L.; Simek, S.; Pelikánová, T. Effect of acute hyperglycaemia on sodium handling and excretion of nitric oxide metabolites, bradykinin, and cGMP in Type 1 diabetes mellitus. Diabet. Med. 2004, 21, 968–975. [Google Scholar] [CrossRef]
- Turner, G.; Coates, P.; Warren, S.; Woodhead, J.S.; Peters, J.R. Proximal tubular reabsorption of growth hormone and sodium/fluid in normo- and microalbuminuric insulin-dependent diabetes mellitus. Acta Diabetol. 1997, 34, 27–32. [Google Scholar] [CrossRef]
- Pun, K.K.; Ho, P.W. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res. Clin. Pract. 1989, 7, 163–167. [Google Scholar] [CrossRef]
- Moon, J. The effect of the heatwave on the morbidity and mortality of diabetes patients; a meta-analysis for the era of the climate crisis. Environ. Res. 2021, 195, 110762. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Sane, T.; Fyhrquist, F.; Pelkonen, R. Fuel and fluid homeostasis during long-term exercise in healthy subjects and type I diabetic patients. Diabetes Care 1992, 15, 1736–1741. [Google Scholar] [CrossRef]
- Boehncke, S.; Poettgen, K.; Maser-Gluth, C.; Reusch, J.; Boehncke, W.H.; Badenhoop, K. Endurance capabilities of triathlon competitors with type 1 diabetes mellitus. Dtsch. Med. Wochenschr. 2009, 134, 677–682. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Turksoy, K.; McGaugh, S.M.; Pooni, R.; Vienneau, T.; Ly, T.; Riddell, M.C. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 313–321. [Google Scholar] [CrossRef]
- Moriyama, M.; Hayashi, N.; Ohyabu, C.; Mukai, M.; Kawano, S.; Kumagai, S. Performance evaluation and cross-reactivity from insulin analogs with the ARCHITECT insulin assay. Clin. Chem. 2006, 52, 1423–1426. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Ferrannini, E. Learning from glycosuria. Diabetes 2011, 60, 695–696. [Google Scholar] [CrossRef]
- Simerville, J.A.; Maxted, W.C.; Pahira, J.J. Urinalysis: A comprehensive review. Am. Fam. Physician 2005, 71, 1153–1162. [Google Scholar] [PubMed]
- Akarsu, E.; Buyukhatipoglu, H.; Aktaran, S.; Geyik, R. The value of urine specific gravity in detecting diabetes insipidus in a patient with uncontrolled diabetes mellitus: Urine specific gravity in differential diagnosis. J. Gen. Intern. Med. 2006, 21, C1–C2. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A. Assessing hydration status. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Popowski, L.A.; Oppliger, R.A.; Patrick Lambert, G.; Johnson, R.F.; Kim Johnson, A.; Gisolf, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sport. Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 2011, 22, 104–112. [Google Scholar] [CrossRef]
- Harmer, A.R.; Ruell, P.A.; McKenna, M.J.; Chisholm, D.J.; Hunter, S.K.; Thom, J.M.; Morris, N.R.; Flack, J.R. Effects of sprint training on extrarenal potassium regulation with intense exercise in Type 1 diabetes. J. Appl. Physiol. 2006, 100, 26–34. [Google Scholar] [CrossRef]
- Kjeldsen, K.P.; Schmidt, T.A. Potassium homoeostasis and pathophysiology of hyperkalaemia. Eur. Heart J. Suppl. 2019, 21, A2–A5. [Google Scholar] [CrossRef]
- Yang, L.; Frindt, G.; Palmer, L.G. Magnesium modulates ROMK channel-mediated potassium secretion. J. Am. Soc. Nephrol. 2010, 21, 2109–2116. [Google Scholar] [CrossRef]
- Liamis, G.; Liberopoulos, E.; Barkas, F.; Elisaf, M. Diabetes mellitus and electrolyte disorders. World. J. Clin. Cases 2014, 2, 488–496. [Google Scholar] [CrossRef]
- Fort, P.; Lifshitz, F. Magnesium status in children with insulin-dependent diabetes mellitus. J. Am. Coll. Nutr. 1986, 5, 69–78. [Google Scholar] [CrossRef]
- Sjögren, A.; Florén, C.H.; Nilsson, A. Magnesium deficiency in IDDM related to level of glycosylated hemoglobin. Diabetes 1986, 35, 459–463. [Google Scholar] [CrossRef]
- Driziene, Z.; Stakisaitis, D.; Balsiene, J. Magnesium urinary excretion in diabetic adolescents. Acta Med. (Hradec Kral.) 2005, 48, 157–161. [Google Scholar] [CrossRef]
- Wongdee, K.; Krishnamra, N.; Charoenphandhu, N. Derangement of calcium metabolism in diabetes mellitus: Negative outcome from the synergy between impaired bone turnover and intestinal calcium absorption. J. Physiol. Sci. 2017, 67, 71–81. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Joborn, H.; Akerström, G.; Ljunghall, S. Effects of exogenous catecholamines and exercise on plasma magnesium concentrations. Clin. Endocrinol. 1985, 23, 219–226. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Scholten, R.R.; van Duijnhoven, N.T.; Thijssen, D.H.; Hopman, M.T. Sex difference in fluid balance responses during prolonged exercise. Scand. J. Med. Sci. Sport. 2013, 23, 198–206. [Google Scholar] [CrossRef]
- Riddell, M.C.; Zaharieva, D.P.; Tansey, M.; Tsalikian, E.; Admon, G.; Li, Z.; Kollman, C.; Beck, R.W. Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: The higher they start, the harder they fall. Pediatr. Diabetes 2019, 20, 99–106. [Google Scholar] [CrossRef]
- Jenni, S.; Oetliker, C.; Allemann, S.; Ith, M.; Tappy, L.; Wuerth, S.; Egger, A.; Boesch, C.; Schneiter, P.; Diem, P.; et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus--a prospective single-blinded randomised crossover trial. Diabetologia 2008, 51, 1457–1465. [Google Scholar] [CrossRef]
| Characteristic | T1D Participants | Control Participants | p-Value |
|---|---|---|---|
| M/F (n) | 2/10 | 2/10 | n/a |
| Age (yrs) | 29.0 ± 7.8 | 29.3 ± 7.9 | 0.93 |
| Height (cm) | 165.3 ± 7.3 | 169.1 ± 8.0 | 0.24 |
| Weight (kg) | 69.4 ± 7.9 | 68.7 ± 8.4 | 0.82 |
| BMI (kg/m2) | 25.4 ± 2.0 | 24 ± 2.5 | 0.16 |
| VO2peak (mL·kg−1·min−1) | 37.9 ± 7.7 | 38.4 ± 7.7 | 0.86 |
| CON (n = 12) | T1D MOD (n = 12) | T1D HI (n = 12) | p (CON vs. MOD) | p (MOD vs. HI) | ||
|---|---|---|---|---|---|---|
| 0–6 h post-exercise | Mean CGM glucose (mmol/L) | 5.7 (5.2–6.2) | 8.7 (6.8–10.1) | 9.8 (8.4–11.5) | <0.0001 * | 0.08 |
| SD (mmol/L) | 0.5 (0.3–0.6) | 1.8 (0.9–2.6) | 2.1 (1.3–2.8) | <0.0001 * | 0.51 | |
| CV (%) | 8.8 (5.9–11.1) | 21.6 (13.3–28.6) | 22.2 (13.2–25.7) | 0.0002 * | 0.85 | |
| Time in range (%) | 100 (100–100) | 70.3 (59.0–95.4) | 52.2 (30.2–84.0) | <0.0001 * | 0.07 | |
| Time in level 1 hypoglycemia (%) | 0.0 (0.0–0.0) | 0.2 (0.0–0.0) | 2.0 (0.0–0.0) | >0.99 | 0.5 | |
| Time in level 2 hypoglycemia (%) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.7 (0.0–0.0) | n/a | 0.5 | |
| Time in level 1 hyperglycemia (%) | 0.0 (0.0–0.0) | 24.4 (4.5–39.6) | 36.7 (16.0–63.5) | <0.0001 * | 0.18 | |
| Time in level 2 hyperglycemia (%) | 0.0 (0.0–0.0) | 4.9 (0.0–5.2) | 8.3 (0.0–18.3) | 0.09 | 0.29 | |
| Frequency of level 1 hypoglycemia | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.16 (0.0–0.0) | n/a | 0.5 | |
| Frequency of level 2 hypoglycemia | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.08 (0.0–0.0) | n/a | >0.99 | |
| Frequency of level 1 hyperglycemia | 0.0 (0.0–0.0) | 1.1 (0.2–2) | 1.4 (1–2) | 0.0003 * | 0.44 | |
| Frequency of level 2 hyperglycemia | 0.0 (0.0–0.0) | 0.3 (0.0–1) | 0.5 (0–1) | 0.09 | 0.68 | |
| Overnight (00:00 to 06:00) | Mean CGM glucose (mmol/L) | 5.5 (5.1–5.9) | 8.7 (7.0–9.9) | 8 (7.2–9.0) | <0.0001 * | 0.44 |
| SD (mmol/L) | 0.27 (0.15–0.29) | 1.7 (0.8–2.7) | 1.6 (1.3–2.1) | 0.0001 * | 0.82 | |
| CV (%) | 5.0 (2.8–5.2) | 19.2 (11.6–27.1) | 20.6 (16.0–24.9) | <0.0001 * | 0.48 | |
| Time in range (%) | 100 (100–100) | 66.3 (30.6–100.0) | 65.8 (43.0–85.7) | 0.001 * | 0.86 | |
| Time in level 1 hypoglycemia (%) | 0.0 (0.0–0.0) | 2.3 (0.0–0.0) | 0.46 (0.0–0.0) | 0.21 | 0.34 | |
| Time in level 2 hypoglycemia (%) | 0.0 (0.0–0.0) | 8.8 (0.0–0.0) | 7.3 (0.0–0.0) | 0.47 | 0.73 | |
| Time in level 1 hyperglycemia (%) | 0.0 (0.0–0.0) | 17.4 (0.0–26.4) | 21.5 (7.6–32.6) | 0.004 * | 0.43 | |
| Time in level 2 hyperglycemia (%) | 0.0 (0.0–0.0) | 5.1 (0.0–0.0) | 4.86 (0.0–3.1) | 0.21 | 0.82 | |
| Frequency of level 1 hypoglycemia | 0.0 (0.0–0.0) | 0.2 (0.0–0.0) | 0.08 (0.0–0.0) | 0.21 | 0.59 | |
| Frequency of level 2 hypoglycemia | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.08 (0.0–0.0) | n/a | >0.99 | |
| Frequency of level 1 hyperglycemia | 0.0 (0.0–0.0) | 0.7 (0.0–1) | 0.9 (1–1) | 0.001 * | 0.40 | |
| Frequency of level 2 hyperglycemia | 0.0 (0.0–0.0) | 0.1 (0.0–0.0) | 0.2 (0.0–0.7) | 0.47 | 0.59 | |
| 0–24 h post-exercise | Mean CGM glucose (mmol/L) | 5.6 (5.2–5.8) | 7.8 (6.7–8.8) | 8.7 (7.4–9.9) | 0.002 * | 0.29 |
| SD (mmol/L) | 0.5 (0.46–0.53) | 2.2 (1.2–3.1) | 2.5 (1.6–3.2) | <0.0001 * | 0.5 | |
| CV (%) | 9.3 (8.4–10.0) | 29.6 (19.3–39.0) | 29 (20.1–36.7) | <0.0001 * | 0.84 | |
| Time in range (%) | 100 (100–100) | 73.3 (61.5–94.2) | 63.8 (49.0–81.3) | <0.0001 * | 0.31 | |
| Time in level 1 hypoglycemia (%) | 0.0 (0.0–0.0) | 1.7 (0.0–3.4) | 2.3 (0.0–2.8) | 0.004 * | 0.68 | |
| Time in level 2 hypoglycemia (%) | 0.0 (0.0–0.0) | 0.35 (0.0–0.0) | 2.4 (0.0–0.3) | >0.99 | 0.32 | |
| Time in level 1 hyperglycemia (%) | 0.0 (0.0–0.0) | 21.5 (4.2–29.5) | 23.2 (10.4–31.8) | <0.0001 * | 0.49 | |
| Time in level 2 hyperglycemia (%) | 0.0 (0.0–0.0) | 3 (0.0–7.1) | 8.3 (0.0–16.3) | 0.03 * | 0.24 | |
| Frequency of level 1 hypoglycemia | 0.0 (0.0–0.0) | 0.5 (0.0–1.0) | 0.5 (0.0–1.0) | 0.013 * | 0.75 | |
| Frequency of level 2 hypoglycemia | 0.0 (0.0–0.0) | 0.08 (0.0–0.0) | 0.2 (0.0–0.0) | >0.99 | 0.59 | |
| Frequency of level 1 hyperglycemia | 0.0 (0.0–0.0) | 2.5 (1.2–3.0) | 3.0 (2.0–4.0) | <0.0001 * | 0.24 | |
| Frequency of level 2 hyperglycemia | 0.0 (0.0–0.0) | 0.0 (0.0-1.0) | 1.3 (0.0–3.0) | 0.03 * | 0.24 |
| CON (n = 12) | T1D MOD (n = 12) | T11D HI (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| Fat intake (g) | 54.1 ± 8.8 | 66.6 ± 8.9 | 55.5 ± 7.5 | 57.6 ± 13.3 | 50.0 ± 4.3 * | 44.9 ± 6.4 | 52.5 ± 8.8 | 53.9 ± 5.2 | 47.3 ± 8.2 |
| Carbohydrate intake (g) | 157.6 ± 19.1 | 200.7 ± 24.1 | 187.2 ± 20.7 | 120.6 ± 19.2 | 127.8 ± 15.4 * | 102.2 ± 17.8 * | 112.8 ± 17.1 | 140.3 ± 18.5 | 141.8 ± 28.3 |
| Protein intake (g) | 68.4 ± 11.1 | 85.7 ± 12 | 72.2 ± 10.4 | 67.4 ± 14.3 | 83.9 ± 13.7 | 64.7 ± 15.7 | 63.3 ± 14.6 | 87.5 ± 14.1 | 73.4 ± 7.4 |
| Daily energy intake (kcal) | 1368 ± 179 | 1717 ± 204 | 1521 ± 162 | 1240 ± 208 | 1274 ± 110 | 1069 ± 156 | 1165 ± 176 | 1377 ± 107 | 1248 ± 170 |
| Total daily insulin (U) | n/a | n/a | n/a | 29.9 ± 6.9 (n = 11) | 28.9 ± 4.9 (n = 11) | 25.8 ± 3.1 (n = 11) | 24.9 ± 4.3 | 25.5 ± 4.5 | 25.5 ± 4.3 |
| Steps per day | 5176 ± 1071 | 7099 ± 1134 | 6317 ± 1251 | 6633 ± 1157 | 8623 ± 1301 | 7421 ± 1198 | 6289 ± 659 | 9581 ± 931 | 7648 ± 1604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momeni, Z.; Boulé, N.G.; Prado, C.M.; Hinz, H.A.; Yardley, J.E. The Effect of Starting Blood Glucose Levels on Serum Electrolyte Concentrations during and after Exercise in Type 1 Diabetes. Int. J. Environ. Res. Public Health 2023, 20, 2109. https://doi.org/10.3390/ijerph20032109
Momeni Z, Boulé NG, Prado CM, Hinz HA, Yardley JE. The Effect of Starting Blood Glucose Levels on Serum Electrolyte Concentrations during and after Exercise in Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2023; 20(3):2109. https://doi.org/10.3390/ijerph20032109
Chicago/Turabian StyleMomeni, Zeinab, Normand G. Boulé, Carla M. Prado, Heather A. Hinz, and Jane E. Yardley. 2023. "The Effect of Starting Blood Glucose Levels on Serum Electrolyte Concentrations during and after Exercise in Type 1 Diabetes" International Journal of Environmental Research and Public Health 20, no. 3: 2109. https://doi.org/10.3390/ijerph20032109
APA StyleMomeni, Z., Boulé, N. G., Prado, C. M., Hinz, H. A., & Yardley, J. E. (2023). The Effect of Starting Blood Glucose Levels on Serum Electrolyte Concentrations during and after Exercise in Type 1 Diabetes. International Journal of Environmental Research and Public Health, 20(3), 2109. https://doi.org/10.3390/ijerph20032109






