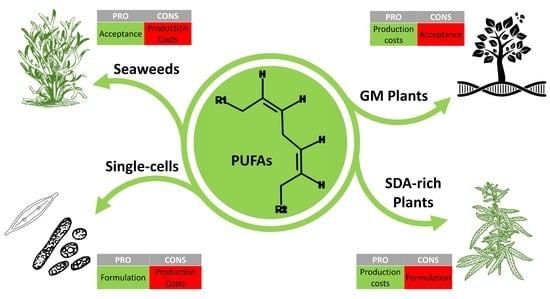
Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review
Abstract
1. Introduction
1.1. Chemical Structures of Essential Fatty Acids and Dietary Sources
1.2. Functions and Biological Activities of Essential Fatty Acids
1.3. Intakes and Requirements of Essential Fatty Acids
1.3.1. Omega-6 to Omega-3 Ratio
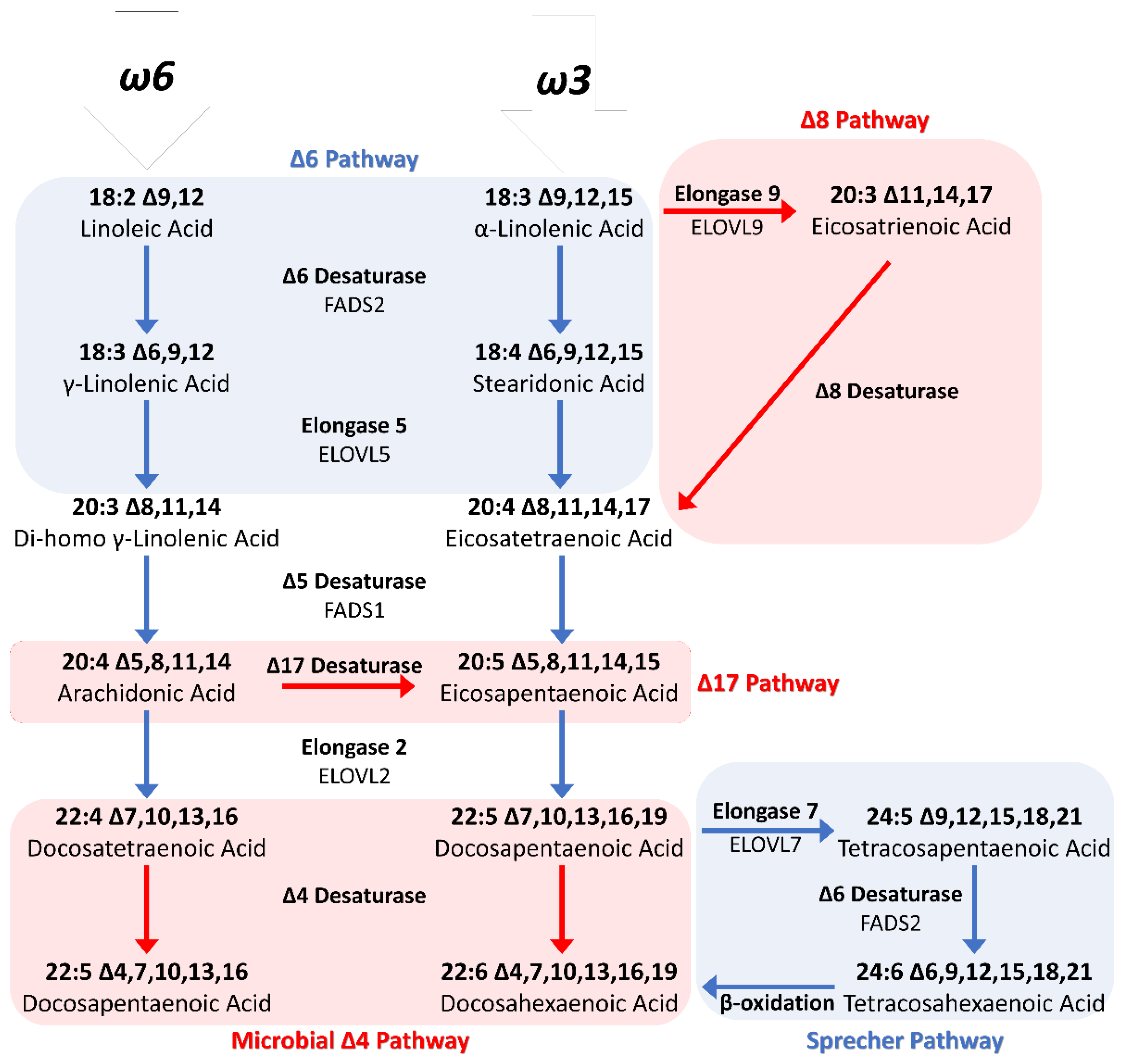
1.3.2. Biosynthetic Pathway
1.3.3. Pregnancy and Lactation: A Programming Window for Neurodevelopment
1.3.4. PUFAs Daily Requirements
1.4. Availability of Food Sources and Environmental Issues Related to the Supply of Essential Fatty Acids
1.4.1. Fishery
1.4.2. Aquaculture
1.4.3. Safety Concerns
1.4.4. Consumer Acceptability
1.4.5. Global Sustainability
1.4.6. Scope
2. Search Method
3. Algae
3.1. Macroalgae
- Phaeophyta or Brown Algae, including kelp; e.g., Analipus japonicus, Myagropsis myagroides, Padina australis, Sargassum polycytum, Sargassum thunbergii, Ecklonia cava, Ecklonia bicyclis (Arame), Ecklonia stolonifera, Sargassum fusiforme (Hijiki), Undaria pinnatifida (Wakame), Laminaria japonica (Konbu), Laminaria digitata, Laminaria saccharina, Himanthalia elongata (Sea spaghetti), Hizikia fusiforme, Ascophyllum nodosum, Fucus ssp., etc. They owe their color mainly to fucoxanthin;
- Rhodophyta or Red Algae; e.g., Laurencia undulata, Lithothamnion corallioides, Pyropia tenera (Nori), Pyropia yezonensis (Nori), Pyropia umbilicalis (Nori), Chondrus crispus (sea moss), Gracilaria verrucosa, Borentia secundiflora, Palmaria palmata, etc. They contain phycoerythrin, phycocyanin, lutein, zeaxanthin, beta carotene and phycobilin;
- Chlorophyta or Green Algae; e.g., Ulva conglobata, Ulva lactuca (Sea lettuce), Ulva pertusa, Enteromotpha compresa, Caulerpa racemosa, Codium reediae, etc. They owe their color to chlorophyll, lutein, beta carotene, neoxanthin, violaxanthin and zeaxanthin pigments.
3.2. Microalgae
4. Other Microorganisms
5. Plants Rich in Alpha Linolenic Acid
6. Plants Rich in Stearidonic Acid
7. Genetically Modified Plants
8. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AFSSA | Agence Française de Sécurité Sanitaire des Aliments |
| ALA | Alpha linolenic acid |
| BMI | Body mass index |
| cAMP | Cyclic adenosine monophosphate |
| CE | Cholesterol ester |
| COMA | Committee on Medical Aspects |
| COX | Cyclooxygenase |
| CYP P450 | Cytochrome P450/epoxygenase |
| DGLA | Dihomo-gamma linolenic acid |
| DHA | Docosahexaenoic acid |
| DPA | Docosapentaenoic acid |
| DW | Dry weight |
| EFAs | Essential fatty acids |
| EFSA | European food safety authority |
| EO | Echium oil |
| EPA | Eicosapentaenoic acid |
| FAO | Food and Agriculture Organization |
| FDA | Food and Drug Administration |
| FO | Fish oil |
| GLA | Gamma linolenic acid |
| GM | Genetically modified |
| GRAS | Generally recognized as safe |
| HDL-C | High-density lipoprotein cholesterol |
| HETE | Hydroxyeicosatetraenoic acid |
| HPDHA | Hydroperoxydocosahexaenoic acid |
| HPETE | Hydroperoxyeicosatetraenoic acid |
| iNOS | Inducible nitric oxide |
| IOM | Institute of Medicine |
| LA | Linoleic acid |
| LC-PUFAs | Long-chain fatty acids |
| LDL-C | Low-density lipoprotein cholesterol |
| LOX | Lipoxygenase |
| LT | Leukotriene |
| MAPKs | Mitogen-activated protein kinases |
| MAR | Maresin |
| NEFA | Not esterified fatty acids |
| NF-kB | Nuclear factor-kB |
| NNR | Nordic Nutrition Recommendations |
| PBMCs | Peripheral blood mononuclear cells |
| PC | Phosphatidylcholine |
| PD | Protectin |
| PG | Prostaglandin |
| PGI | Prostacyclin |
| PPAR | Peroxisomal proliferator-activated receptors |
| PUFAs | Polyunsaturated fatty acids |
| RBCs | Red blood cells |
| RCTs | Randomized controlled trials |
| RV | Resolvin |
| SACN | Scientific Advisory Committee on Nutrition |
| SDA | Stearidonic acid |
| SNPs | Single-nucleotide polymorphisms |
| TAG | Triacylglycerols |
| TC | Total cholesterol |
| TX | Thromboxane |
| WHO | World Health Organization |
References
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.O.; Burr, M.M. Nutrition Classics from The Journal of Biological Chemistry 82:345-67, 1929. A New Deficiency Disease Produced by the Rigid Exclusion of Fat from the Diet. Nutr. Rev. 1973, 31, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Essential Fatty Acids: The Work of George and Mildred Burr. J. Biol. Chem. 2012, 287, 35439–35441. [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and Flaxseed Oil: An Ancient Medicine & Modern Functional Food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Zamani Ghaleshahi, A.; Ezzatpanah, H.; Rajabzadeh, G.; Ghavami, M. Comparison and Analysis Characteristics of Flax, Perilla and Basil Seed Oils Cultivated in Iran. J. Food Sci. Technol. 2020, 57, 1258–1268. [Google Scholar] [CrossRef]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty Acid Profile of Edible Oils and Fats Consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef]
- Food Data Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/ (accessed on 24 October 2022).
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-Metabolism, Absorption, Bioavailability and Health Benefits–A Review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Ian Givens, D.; Gibbs, R.A. Current Intakes of EPA and DHA in European Populations and the Potential of Animal-Derived Foods to Increase Them. Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef]
- Givens, D.I.; Gibbs, R.A. Very Long Chain N-3 Polyunsaturated Fatty Acids in the Food Chain in the UK and the Potential of Animal-Derived Foods to Increase Intake. Nutr. Bull. 2006, 31, 104–110. [Google Scholar] [CrossRef]
- Harwood, J.L.; Guschina, I.A. The Versatility of Algae and Their Lipid Metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n-3) Fatty Acids and Brain Development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef]
- Campoy, C.; Escolano-Margarit, M.V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 Fatty Acids on Child Growth, Visual Acuity and Neurodevelopment. Br. J. Nutr. 2012, 107 (Suppl. S2), S85–S106. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Niu, S.L.; Litman, B.J. Optimization of Receptor-G Protein Coupling by Bilayer Lipid Composition I: Kinetics of Rhodopsin-Transducin Binding. J. Biol. Chem. 2001, 276, 42801–42806. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S. The Link between Homocysteine and Omega-3 Polyunsaturated Fatty Acid: Critical Appraisal and Future Directions. Biomolecules 2020, 10, 219. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of Inflammation: An Integrated View. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and New Generation Lipid Mediators in Acute Inflammation and Resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef]
- Astarita, G.; Kendall, A.C.; Dennis, E.A.; Nicolaou, A. Targeted Lipidomic Strategies for Oxygenated Metabolites of Polyunsaturated Fatty Acids. Biochim. Biophys. Acta 2015, 1851, 456–468. [Google Scholar] [CrossRef]
- Kim, H.; Spector, A.A. N-Docosahexaenoylethanolamine: A Neurotrophic and Neuroprotective Metabolite of Docosahexaenoic Acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The Opposing Effects of N−3 and N−6 Fatty Acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lands, B. Historical Perspectives on the Impact of N-3 and N-6 Nutrients on Health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From Molecular Action to Physiological Outputs: Peroxisome Proliferator-Activated Receptors Are Nuclear Receptors at the Crossroads of Key Cellular Functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May Omega-3 Fatty Acid Dietary Supplementation Help Reduce Severe Complications in COVID-19 Patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef]
- Manuelli, M.; Della Guardia, L.; Cena, H. Enriching Diet with N-3 PUFAs to Help Prevent Cardiovascular Diseases in Healthy Adults: Results from Clinical Trials. Int. J. Mol. Sci. 2017, 18, 1552. [Google Scholar] [CrossRef]
- Bäck, M. Omega-3 Fatty Acids in Atherosclerosis and Coronary Artery Disease. Future Sci. OA 2017, 3, FSO236. [Google Scholar] [CrossRef]
- Fischer, R.; Konkel, A.; Mehling, H.; Blossey, K.; Gapelyuk, A.; Wessel, N.; von Schacky, C.; Dechend, R.; Muller, D.N.; Rothe, M.; et al. Dietary Omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-Epoxygenase Pathway. J. Lipid Res. 2014, 55, 1150–1164. [Google Scholar] [CrossRef]
- Johnson, G.H.; Fritsche, K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041.e15. [Google Scholar] [CrossRef]
- Mir, M. Echium Oil: A Valuable Source of n-3 and n-6 Fatty Acids. OCL 2008, 15, 252–256. [Google Scholar] [CrossRef]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary Supplementation with Gamma-Linolenic Acid Alters Fatty Acid Content and Eicosanoid Production in Healthy Humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish Oil and Omega-3 Fatty Acids in Cardiovascular Disease: Do They Really Work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y.; Gosslau, A.M.; Li, S. Are There Serious Adverse Effects of Omega-3 Polyunsaturated Fatty Acid Supplements? J. Food Bioact. 2019, 7, 1–6. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation Products from Omega-3 and Omega-6 Fatty Acids during a Simulated Shelf Life of Edible Oils. LWT 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of N-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Siddique, M.A.B.; Fathi, M. Vegetable Oils Rich in Polyunsaturated Fatty Acids: Nanoencapsulation Methods and Stability Enhancement. Food Rev. Int. 2022, 38, 32–69. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Li, D. Chemistry behind Vegetarianism. J. Agric. Food Chem. 2011, 59, 777–784. [Google Scholar] [CrossRef]
- Calder, P.C. Chapter 4—Omega-6 and Omega-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Diseases. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 55–79. ISBN 978-0-12-397154-8. [Google Scholar]
- Food and Agriculture Organization of the United Nations (Ed.) Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation: 10–14 November 2008, Geneva; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-92-5-106733-8. [Google Scholar]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Sharon, P.; Stenson, W.F. Enhanced Synthesis of Leukotriene B4 by Colonic Mucosa in Inflammatory Bowel Disease. Gastroenterology 1984, 86, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Eggersdorfer, M. Is the World Supply of Omega-3 Fatty Acids Adequate for Optimal Human Nutrition? Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Algae: Critical Sources of Very Long-Chain Polyunsaturated Fatty Acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Long Chain Omega-3 Fatty Acids and Cardiovascular Disease: A Systematic Review. Br. J. Nutr. 2012, 107 (Suppl. 2S), S201–S213. [Google Scholar] [CrossRef]
- Calder, P.C. Very Long Chain Omega-3 (n-3) Fatty Acids and Human Health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- Calder, P.C. Very Long-Chain n-3 Fatty Acids and Human Health: Fact, Fiction and the Future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma Phospholipid Long-Chain ω-3 Fatty Acids and Total and Cause-Specific Mortality in Older Adults. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and Therapeutics of Omega-3 Polyunsaturated Fatty Acids in Chronic Inflammatory Disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2012, 107, S214–S227. [Google Scholar] [CrossRef]
- Chen, C.; Yu, X.; Shao, S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis. PLoS ONE 2015, 10, e0139565. [Google Scholar] [CrossRef]
- Sanders, T.A.B. Protective Effects of Dietary PUFA against Chronic Disease: Evidence from Epidemiological Studies and Intervention Trials. Proc. Nutr. Soc. 2014, 73, 73–79. [Google Scholar] [CrossRef]
- Griel, A.E.; Kris-Etherton, P.M.; Hilpert, K.F.; Zhao, G.; West, S.G.; Corwin, R.L. An Increase in Dietary N-3 Fatty Acids Decreases a Marker of Bone Resorption in Humans. Nutr. J. 2007, 6, 2. [Google Scholar] [CrossRef]
- Storz, M.A. The Role of Vegan Diets in Lipotoxicity-Induced Beta-Cell Dysfunction in Type-2-Diabetes: A Narrative Review. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e22–e38. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids in Wild Plants, Nuts and Seeds. Asia Pac. J. Clin. Nutr. 2002, 11, S163–S173. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and How Meet N-3 PUFA Dietary Recommendations? Gastroenterol. Res. Pract. 2010, 2011, e364040. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, e539426. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W.L. Are All N-3 Polyunsaturated Fatty Acids Created Equal? Lipids. Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Rao, J.S.; Igarashi, M. Brain Metabolism of Nutritionally Essential Polyunsaturated Fatty Acids Depends on Both the Diet and the Liver. Prostaglandins Leukot Essent Fat. Acids 2007, 77, 251–261. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Chordates: Insights into the Evolution of Fads and Elovl Gene Repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Burdge, G.C. Metabolism of Alpha-Linolenic Acid in Humans. Prostaglandins Leukot Essent Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N. Physiological Compartmental Analysis of Alpha-Linolenic Acid Metabolism in Adult Humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Serini, S.; Chen, Y.Q.; Su, H.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed Res. Int. 2015, 2015, e143109. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and Docosapentaenoic Acids Are the Principal Products of α-Linolenic Acid Metabolism in Young Men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Childs, C.E.; Kew, S.; Finnegan, Y.E.; Minihane, A.M.; Leigh-Firbank, E.C.; Williams, C.M.; Calder, P.C. Increased Dietary α-Linolenic Acid Has Sex-Specific Effects upon Eicosapentaenoic Acid Status in Humans: Re-Examination of Data from a Randomised, Placebo-Controlled, Parallel Study. Nutr. J. 2014, 13, 113. [Google Scholar] [CrossRef]
- Harnack, K.; Andersen, G.; Somoza, V. Quantitation of Alpha-Linolenic Acid Elongation to Eicosapentaenoic and Docosahexaenoic Acid as Affected by the Ratio of N6/N3 Fatty Acids. Nutr. Metab. 2009, 6, 8. [Google Scholar] [CrossRef]
- Plourde, M.; Cunnane, S.C. Extremely Limited Synthesis of Long Chain Polyunsaturates in Adults: Implications for Their Dietary Essentiality and Use as Supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C.; International Society for the Study of Fatty Acids and Lipids, ISSFAL. Alpha-Linolenic Acid Supplementation and Conversion to n-3 Long-Chain Polyunsaturated Fatty Acids in Humans. Prostaglandins Leukot Essent Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Cunnane, S.C. Problems with Essential Fatty Acids: Time for a New Paradigm? Prog. Lipid Res. 2003, 42, 544–568. [Google Scholar] [CrossRef]
- Sayanova, O.; Napier, J.A. Transgenic Oilseed Crops as an Alternative to Fish Oils. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 253–260. [Google Scholar] [CrossRef]
- Meyer, A.; Kirsch, H.; Domergue, F.; Abbadi, A.; Sperling, P.; Bauer, J.; Cirpus, P.; Zank, T.K.; Moreau, H.; Roscoe, T.J.; et al. Novel Fatty Acid Elongases and Their Use for the Reconstitution of Docosahexaenoic Acid Biosynthesis. J. Lipid Res. 2004, 45, 1899–1909. [Google Scholar] [CrossRef]
- Delarue, J.; Guriec, N. Opportunities to Enhance Alternative Sources of Long-Chain n-3 Fatty Acids within the Diet. Proc. Nutr. Soc. 2014, 73, 376–384. [Google Scholar] [CrossRef]
- Burdge, G. α-Linolenic Acid Metabolism in Men and Women: Nutritional and Biological Implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes, M.A.; Wareham, N.J.; Khaw, K.-T. Dietary Intake and Status of n–3 Polyunsaturated Fatty Acids in a Population of Fish-Eating and Non-Fish-Eating Meat-Eaters, Vegetarians, and Vegans and the Precursor-Product Ratio of α-Linolenic Acid to Long-Chain n–3 Polyunsaturated Fatty Acids: Results from the EPIC-Norfolk Cohort. Am. J. Clin. Nutr. 2010, 92, 1040–1051. [Google Scholar] [CrossRef]
- Kuhnt, K.; Fuhrmann, C.; Köhler, M.; Kiehntopf, M.; Jahreis, G. Dietary Echium Oil Increases Long-Chain n–3 PUFAs, Including Docosapentaenoic Acid, in Blood Fractions and Alters Biochemical Markers for Cardiovascular Disease Independently of Age, Sex, and Metabolic Syndrome. J. Nutr. 2014, 144, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Blüher, M.; Dawczynski, C. Benefits of Foods Supplemented with Vegetable Oils Rich in α-Linolenic, Stearidonic or Docosahexaenoic Acid in Hypertriglyceridemic Subjects: A Double-Blind, Randomized, Controlled Trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Yurko-Mauro, K.; Dicklin, M.R.; Schild, A.L.; Geohas, J.G. A New, Microalgal DHA- and EPA-Containing Oil Lowers Triacylglycerols in Adults with Mild-to-Moderate Hypertriglyceridemia. Prostaglandins Leukot Essent Fat. Acids 2014, 91, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Symington, A.M. Algal-Oil Supplements Are a Viable Alternative to Fish-Oil Supplements in Terms of Docosahexaenoic Acid (22:6n-3; DHA). J. Funct. Foods 2015, 19, 852–858. [Google Scholar] [CrossRef]
- Khandelwal, S.; Kondal, D.; Chaudhry, M.; Patil, K.; Swamy, M.K.; Metgud, D.; Jogalekar, S.; Kamate, M.; Divan, G.; Gupta, R.; et al. Effect of Maternal Docosahexaenoic Acid (DHA) Supplementation on Offspring Neurodevelopment at 12 Months in India: A Randomized Controlled Trial. Nutrients 2020, 12, 3041. [Google Scholar] [CrossRef]
- Dewell, A.; Marvasti, F.F.; Harris, W.S.; Tsao, P.; Gardner, C.D. Low- and High-Dose Plant and Marine (n-3) Fatty Acids Do Not Affect Plasma Inflammatory Markers in Adults with Metabolic Syndrome. J. Nutr. 2011, 141, 2166–2171. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar Effects on Infants of N-3 and n-6 Fatty Acids Supplementation to Pregnant and Lactating Women. Pediatrics 2001, 108, e82. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal Supplementation with Very-Long-Chain n-3 Fatty Acids during Pregnancy and Lactation Augments Children’s IQ at 4 Years of Age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Simmer, K.; Dixon, G.; Prescott, S.L. Cognitive Assessment of Children at Age 2(1/2) Years after Maternal Fish Oil Supplementation in Pregnancy: A Randomised Controlled Trial. Arch. Dis. Child Fetal. Neonatal Ed. 2008, 93, F45–F50. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; DOMInO Investigative Team. Effect of DHA Supplementation During Pregnancy on Maternal Depression and Neurodevelopment of Young Children: A Randomized Controlled Trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Tofail, F.; Kabir, I.; Hamadani, J.D.; Chowdhury, F.; Yesmin, S.; Mehreen, F.; Huda, S.N. Supplementation of Fish-Oil and Soy-Oil during Pregnancy and Psychomotor Development of Infants. J. Health Popul. Nutr. 2006, 24, 48–56. [Google Scholar]
- Smithers, L.G.; Gibson, R.A.; McPhee, A.; Makrides, M. Effect of Long-Chain Polyunsaturated Fatty Acid Supplementation of Preterm Infants on Disease Risk and Neurodevelopment: A Systematic Review of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 87, 912–920. [Google Scholar] [CrossRef]
- Judge, M.P.; Harel, O.; Lammi-Keefe, C.J. A Docosahexaenoic Acid-Functional Food during Pregnancy Benefits Infant Visual Acuity at Four but Not Six Months of Age. Lipids 2007, 42, 117–122. [Google Scholar] [CrossRef]
- Innis, S.M.; Friesen, R.W. Essential N-3 Fatty Acids in Pregnant Women and Early Visual Acuity Maturation in Term Infants. Am. J. Clin. Nutr. 2008, 87, 548–557. [Google Scholar] [CrossRef]
- Gould, J.F.; Smithers, L.G.; Makrides, M. The Effect of Maternal Omega-3 (n-3) LCPUFA Supplementation during Pregnancy on Early Childhood Cognitive and Visual Development: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2013, 97, 531–544. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Horvath, A.; Szajewska, H. Effects of N-3 Long-Chain Polyunsaturated Fatty Acid Supplementation during Pregnancy and/or Lactation on Neurodevelopment and Visual Function in Children: A Systematic Review of Randomized Controlled Trials. J. Am. Coll. Nutr. 2010, 29, 443–454. [Google Scholar] [CrossRef]
- Lo, A.; Sienna, J.; Mamak, E.; Djokanovic, N.; Westall, C.; Koren, G. The Effects of Maternal Supplementation of Polyunsaturated Fatty Acids on Visual, Neurobehavioural, and Developmental Outcomes of the Child: A Systematic Review of the Randomized Trials. Obstet. Gynecol. Int. 2012, 2012, e591531. [Google Scholar] [CrossRef]
- Larqué, E.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Koletzko, B. Omega 3 Fatty Acids, Gestation and Pregnancy Outcomes. Br. J. Nutr. 2012, 107, S77–S84. [Google Scholar] [CrossRef]
- Aranceta, J.; Pérez-Rodrigo, C. Recommended Dietary Reference Intakes, Nutritional Goals and Dietary Guidelines for Fat and Fatty Acids: A Systematic Review. Br. J. Nutr. 2012, 107, S8–S22. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and Docosahexaenoic Acid Derived Specialised Pro-Resolving Mediators: Concentrations in Humans and the Effects of Age, Sex, Disease and Increased Omega-3 Fatty Acid Intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Mandal, C.C. Omega-3 Fatty Acids in Pathological Calcification and Bone Health. J. Food Biochem. 2020, 44, e13333. [Google Scholar] [CrossRef]
- Martinez, M. Tissue Levels of Polyunsaturated Fatty Acids during Early Human Development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The Essentiality of Long Chain N-3 Fatty Acids in Relation to Development and Function of the Brain and Retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Carlson, S.E. Docosahexaenoic Acid Supplementation in Pregnancy and Lactation. Am. J. Clin. Nutr. 2009, 89, 678S–684S. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A. Long-Chain Polyunsaturated Fatty Acid Requirements during Pregnancy and Lactation. Am. J. Clin. Nutr. 2000, 71, 307S–311S. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.M.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current Information and Asian Perspectives on Long-Chain Polyunsaturated Fatty Acids in Pregnancy, Lactation, and Infancy: Systematic Review and Practice Recommendations from an Early Nutrition Academy Workshop. ANM 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.-P.; Puigrredon, C.; Valenzuela, A. The Impact of Maternal Diet during Pregnancy and Lactation on the Fatty Acid Composition of Erythrocytes and Breast Milk of Chilean Women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef]
- Fougère, H.; Bilodeau, J.-F.; Lavoie, P.M.; Mohamed, I.; Rudkowska, I.; Pronovost, E.; Simonyan, D.; Berthiaume, L.; Guillot, M.; Piedboeuf, B.; et al. Docosahexaenoic Acid-Rich Algae Oil Supplementation on Breast Milk Fatty Acid Profile of Mothers Who Delivered Prematurely: A Randomized Clinical Trial. Sci. Rep. 2021, 11, 21492. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, Regional, and National Consumption Levels of Dietary Fats and Oils in 1990 and 2010: A Systematic Analysis Including 266 Country-Specific Nutrition Surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef]
- World Health Origanization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation, Geneva, 28 January–1 February 2002; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- SACN. Advice on Fish Consumption: Benefits & Risks; TSO: London, UK, 2004; ISBN 978-0-11-243083-4. [Google Scholar]
- EFSA. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine; The National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. J. Am. Diet Assoc. 2005, 102, 1621–1630. [Google Scholar] [CrossRef]
- Opinion of the French Food Safety Agency on the Update of French Population Reference Intakes (ANCs) for Fatty Acids. Available online: https://www.anses.fr/en/content/opinion-french-food-safety-agency-update-french-population-reference-intakes-ancs-fatty (accessed on 26 September 2022).
- D-A-CH-Referenzwerte für die Nährstoffzufuhr. Available online: https://www.dge-medienservice.de/d-a-ch-referenzwerte-fur-die-nahrstoffzufuhr.html (accessed on 27 October 2022).
- TSO. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom: Report; Britain, G., Ed.; Report on Health and Social Subjects; 18. Impression.; TSO: London, UK, 1991; ISBN 978-0-11-321397-9. [Google Scholar]
- Nordic Nutrition Recommendations. 2004. Available online: https://www.norden.org/en/publication/nordic-nutrition-recommendations-2004 (accessed on 27 October 2022).
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are Fish What They Eat? A Fatty Acid’s Perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture—2016 (SOFIA): Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; ISBN 978-92-5-109185-2. [Google Scholar]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Myers, R.A.; Worm, B. Rapid Worldwide Depletion of Predatory Fish Communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective Fisheries Management Instrumental in Improving Fish Stock Status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Jackson, J.B.C. The Future of the Oceans Past. Philos. Trans. R. Soc. Biol. Sci. 2010, 365, 3765–3778. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Reynolds, J.D. Marine Fish Population Collapses: Consequences for Recovery and Extinction Risk. BioScience 2004, 54, 297–309. [Google Scholar] [CrossRef]
- Tocher, D. Issues Surrounding Fish as a Source of Omega-3 Long-Chain Polyunsaturated Fatty Acids. Lipid Technol. 2009, 21, 13–16. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards Sustainable Sources for Omega-3 Fatty Acids Production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of Sustainable Feeds on Omega-3 Long-Chain Fatty Acid Levels in Farmed Atlantic Salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Fish to 2030: Prospects for Fisheries and Aquaculture; World Bank: Washington, DC, USA, 2013. [Google Scholar]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Li, X. Bioaccumulation of Heavy Metals in Fish Species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T.; et al. Aquatic Food Security: Insights into Challenges and Solutions from an Analysis of Interactions between Fisheries, Aquaculture, Food Safety, Human Health, Fish and Human Welfare, Economy and Environment. Fish Fish. 2016, 17, 893–938. [Google Scholar] [CrossRef]
- Costa, L. Contaminants in Fish: Risk-Benefit Considerations. Arch. Ind. Hyg. Toxicol. 2007, 58, 367–374. [Google Scholar] [CrossRef]
- Hites, R.A.; Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Global Assessment of Organic Contaminants in Farmed Salmon. Science 2004, 303, 226–229. [Google Scholar] [CrossRef]
- Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Risk-Based Consumption Advice for Farmed Atlantic and Wild Pacific Salmon Contaminated with Dioxins and Dioxin-like Compounds. Environ. Health Perspect. 2005, 113, 552–556. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Health and Ecological Implications of Fish Consumption: A Deeper Insight. Mediterr. J. Nutr. Metab. 2016, 9, 7–22. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2985 (accessed on 26 September 2022).
- Commission Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs.|UNEP Law and Environment Assistance Platform. Available online: https://leap.unep.org/countries/eu/national-legislation/commission-regulation-ec-no-18812006-setting-maximum-levels (accessed on 26 September 2022).
- Storelli, M.M.; Stuffler, R.G.; Marcotrigiano, G.O. Total and Methylmercury Residues in Tuna-Fish from the Mediterranean Sea. Food Addit. Contam. 2002, 19, 715–720. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Salerno, M.; Berlino, M.; Mangano, M.C.; Sarà, G. Microplastics and the Functional Traits of Fishes: A Global Meta-Analysis. Glob. Chang. Biol. 2021, 27, 2645–2655. [Google Scholar] [CrossRef]
- Pennino, M.G.; Bachiller, E.; Lloret-Lloret, E.; Albo-Puigserver, M.; Esteban, A.; Jadaud, A.; Bellido, J.M.; Coll, M. Ingestion of Microplastics and Occurrence of Parasite Association in Mediterranean Anchovy and Sardine. Mar. Pollut. Bull. 2020, 158, 111399. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and Functional Effects of Plant-Derived Omega-3 Fatty Acids in Humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Maki, K.C.; Rains, T.M. Stearidonic Acid Raises Red Blood Cell Membrane Eicosapentaenoic Acid. J. Nutr. 2012, 142, 626S–629S. [Google Scholar] [CrossRef]
- Chan, R.L.; Olshan, A.F.; Savitz, D.A.; Herring, A.H.; Daniels, J.L.; Peterson, H.B.; Martin, S.L. Maternal Influences on Nausea and Vomiting in Early Pregnancy. Matern. Child Health J. 2011, 15, 122–127. [Google Scholar] [CrossRef]
- McCarthy, F.P.; Lutomski, J.E.; Greene, R.A. Hyperemesis Gravidarum: Current Perspectives. Int. J. Womens Health 2014, 6, 719–725. [Google Scholar] [CrossRef]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.B.; Allen, N.E.; Key, T.J. Long-Chain n-3 Polyunsaturated Fatty Acids in Plasma in British Meat-Eating, Vegetarian, and Vegan Men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef]
- Burdge, G.C.; Tan, S.; Henry, C.J. Long-Chain n-3 PUFA in Vegetarian Women: A Metabolic Perspective. J. Nutr. Sci. 2017, 6, e58. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan Diets: Practical Advice for Athletes and Exercisers. J. Int. Soc. Sport. Nutr. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, Are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association. Nutrition Committee Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global Survey of the Omega-3 Fatty Acids, Docosahexaenoic Acid and Eicosapentaenoic Acid in the Blood Stream of Healthy Adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and Genetically Engineered Oils as Replacements for Fish Oil in Aquaculture Feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Omega-3: Global Product Trends and Opportunities: Market Research Report. Available online: https://www.packagedfacts.com/Omega-Global-Product-6385341/ (accessed on 28 September 2022).
- Omega 3 Market Size & Share Report, 2020–2028. Available online: https://www.grandviewresearch.com/industry-analysis/omega-3-market (accessed on 26 September 2022).
- Omega-3 Market by Type (DHA, EPA, and ALA), Application (Dietary Supplements, Functional Foods & Beverages, Pharmaceuticals, Infant Formula, and Pet Food & Feed), Source (Marine and Plant), and Region—Global Forecasts to 2025. Available online: https://www.marketresearch.com/MarketsandMarkets-v3719/Omega-Type-DHA-EPA-ALA-12837793/ (accessed on 26 September 2022).
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Bocanegra, A.; Macho-González, A.; Garcimartín, A.; Benedí, J.; Sánchez-Muniz, F.J. Whole Alga, Algal Extracts, and Compounds as Ingredients of Functional Foods: Composition and Action Mechanism Relationships in the Prevention and Treatment of Type-2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3816. [Google Scholar] [CrossRef]
- Brown, E.S.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol B Suppresses Inflammatory Responses by Down-Regulating Nuclear Factor ΚB Activation via Akt, ERK, and JNK in LPS-Stimulated Microglial Cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Hannon, B.A.; Fairfield, W.D.; Adams, B.; Kyle, T.; Crow, M.; Thomas, D.M. Use and Abuse of Dietary Supplements in Persons with Diabetes. Nutr. Diabetes 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- European Commission. Directorate General for Maritime Affairs and Fisheries; Analisis de Especies, Ed.; EUMOFA Publications Office: Luxemburg, 2019. [Google Scholar]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N. Long-Chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges. Biomolecules 2019, 9, 485. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G., Jr.; Flecker, A.S. Highly Unsaturated Fatty Acids in Nature: What We Know and What We Need to Learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Kabeya, N.; Fonseca, M.M.; Ferrier, D.E.K.; Navarro, J.C.; Bay, L.K.; Francis, D.S.; Tocher, D.R.; Castro, L.F.C.; Monroig, Ó. Genes for de Novo Biosynthesis of Omega-3 Polyunsaturated Fatty Acids Are Widespread in Animals. Sci. Adv. 2018, 4, eaar6849. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Rodríguez, J.; Macías-Sánchez, M.D.; Cerón-García, M.C.; Alarcón, F.J.; Molina-Grima, E. Microalgae as a Potential Ingredient for Partial Fish Meal Replacement in Aquafeeds: Nutrient Stability under Different Storage Conditions. J. Appl. Phycol. 2018, 30, 1049–1059. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–Nutritious, Sustainable Aqua- and Animal Feed Source. J. Funct. Food 2019, 62, 103545. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.K.; Malde, M.K.; Eilertsen, K.-E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendez, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, H.J.; Lee, H.; Kang, M.; Park, Y.K. Six-Week Supplementation with Chlorella Has Favorable Impact on Antioxidant Status in Korean Male Smokers. Nutrition 2010, 26, 175–183. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Dartsch, P.C. Antioxidant Potential of Selected Spirulina Platensis Preparations. Phytother. Res. 2008, 22, 627–633. [Google Scholar] [CrossRef]
- Fleurence, J.; Gutbier, G.; Mabeau, S.; Leray, C. Fatty Acids from 11 Marine Macroalgae of the French Brittany Coast. J. Appl. Phycol. 1994, 6, 527–532. [Google Scholar] [CrossRef]
- van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated Fatty Acids in Various Macroalgal Species from North Atlantic and Tropical Seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Vazhappilly, R.; Chen, F. Eicosapentaenoic Acid and Docosahexaenoic Acid Production Potential of Microalgae and Their Heterotrophic Growth. J. Am. Oil Chem. Soc. 1998, 75, 393–397. [Google Scholar] [CrossRef]
- Diraman, H.; Koru, E.; Dibeklioglu, H. Fatty Acid Profile of Spirulina Platensis Used as a Food Supplement. Isr. J. Aquac. 2009, 61, 2019. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular-Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Winwood, R.J. Recent Developments in the Commercial Production of DHA and EPA Rich Oils from Micro-Algae. OCL 2013, 20, D604. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of Temperature on Growth, Chemical Composition and Fatty Acid Composition of Tropical Australian Microalgae Grown in Batch Cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Ackman, R.G.; Tocher, C.S.; McLachlan, J. Marine Phytoplankter Fatty Acids. J. Fish Res. Bd. Can. 1968, 25, 1603–1620. [Google Scholar] [CrossRef]
- Hixson, S.M.; Arts, M.T. Climate Warming Is Predicted to Reduce Omega-3, Long-Chain, Polyunsaturated Fatty Acid Production in Phytoplankton. Glob. Chang. Biol. 2016, 22, 2744–2755. [Google Scholar] [CrossRef]
- Colombo, S.M.; Rodgers, T.F.M.; Diamond, M.L.; Bazinet, R.P.; Arts, M.T. Projected Declines in Global DHA Availability for Human Consumption as a Result of Global Warming. Ambio 2020, 49, 865–880. [Google Scholar] [CrossRef]
- Fuschino, J.R.; Guschina, I.A.; Dobson, G.; Yan, N.D.; Harwood, J.L.; Arts, M.T. Rising Water Temperatures Alter Lipid Dynamics and Reduce N-3 Essential Fatty Acid Concentrations in Scenedesmus Obliquus (Chlorophyta)1. J. Phycol. 2011, 47, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Seong, T.; Matsutani, H.; Haga, Y.; Kitagima, R.; Satoh, S. First Step of Non-Fish Meal, Non-Fish Oil Diet Development for Red Seabream, (Pagrus major), with Plant Protein Sources and Microalgae Schizochytrium sp. Aquac. Res. 2019, 50, 2460–2468. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of Microalgal Biomasses as Functional Food Ingredients: Focus on the Composition of Cell Wall Related Polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the Potential of 9 Nannochloropsis Strains for Biodiesel Production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Metherel, A.H.; Bazinet, R.P. Updates to the N-3 Polyunsaturated Fatty Acid Biosynthesis Pathway: DHA Synthesis Rates, Tetracosahexaenoic Acid and (Minimal) Retroconversion. Prog. Lipid Res. 2019, 76, 101008. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional Evaluation of Microalgae Oils Rich in Omega-3 Long Chain Polyunsaturated Fatty Acids as an Alternative for Fish Oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Warwick, J.; Terry, A.; Allen, M.J.; Napier, J.A.; Sayanova, O. Towards the Industrial Production of Omega-3 Long Chain Polyunsaturated Fatty Acids from a Genetically Modified Diatom Phaeodactylum Tricornutum. PLoS ONE 2015, 10, e0144054. [Google Scholar] [CrossRef]
- Barclay, W.R.; Meager, K.M.; Abril, J.R. Heterotrophic Production of Long Chain Omega-3 Fatty Acids Utilizing Algae and Algae-like Microorganisms. J. Appl. Phycol. 1994, 6, 123–129. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal Biofactories: A Promising Approach towards Sustainable Omega-3 Fatty Acid Production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef]
- Byreddy, A.R. Thraustochytrids as an Alternative Source of Omega-3 Fatty Acids, Carotenoids and Enzymes. Lipid Technol. 2016, 28, 68–70. [Google Scholar] [CrossRef]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 Biotechnology: Thraustochytrids as a Novel Source of Omega-3 Oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of Thraustochytrids Aurantiochytrium Sp., Schizochytrium Sp., Thraustochytrium Sp., and Ulkenia Sp. for Production of Biodiesel, Long-Chain Omega-3 Oils, and Exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; Fernández de Castro, L.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Yeiser, M.; Harris, C.L.; Kirchoff, A.L.; Patterson, A.C.; Wampler, J.L.; Zissman, E.N.; Berseth, C.L. Growth and Tolerance of Infants Fed Formula with a New Algal Source of Docosahexaenoic Acid: Double-Blind, Randomized, Controlled Trial. Prostaglandins Leukot Essent Fat. Acids 2016, 115, 89–96. [Google Scholar] [CrossRef]
- Mühlroth, A.; Li, K.; Røkke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of Lipid Metabolism in Marine Algae, Co-Expression Network, Bottlenecks and Candidate Genes for Enhanced Production of EPA and DHA in Species of Chromista. Mar. Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Wheaton, D.K.H.; James, K.J.; Tuazon, M.; Diersen-Schade, D.A.; Harris, C.L.; Stolz, S.; Berseth, C.L. Docosahexaenoic Acid in Red Blood Cells of Term Infants Receiving Two Levels of Long-Chain Polyunsaturated Fatty Acids. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 287–292. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Birch, E.E.; Birch, D.G.; Uauy, R.; Castañeda, Y.S.; Lapus, M.G.; Wheaton, D.H. Impact of Early Dietary Intake and Blood Lipid Composition of Long-Chain Polyunsaturated Fatty Acids on Later Visual Development. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 540–553. [Google Scholar] [CrossRef]
- Birch, E.E.; Hoffman, D.R.; Uauy, R.; Birch, D.G.; Prestidge, C. Visual Acuity and the Essentiality of Docosahexaenoic Acid and Arachidonic Acid in the Diet of Term Infants. Pediatr. Res. 1998, 44, 201–209. [Google Scholar] [CrossRef]
- Birch, E.E.; Castañeda, Y.S.; Wheaton, D.H.; Birch, D.G.; Uauy, R.D.; Hoffman, D.R. Visual Maturation of Term Infants Fed Long-Chain Polyunsaturated Fatty Acid-Supplemented or Control Formula for 12 Mo. Am. J. Clin. Nutr. 2005, 81, 871–879. [Google Scholar] [CrossRef]
- Innis, S.M.; Adamkin, D.H.; Hall, R.T.; Kalhan, S.C.; Lair, C.; Lim, M.; Stevens, D.C.; Twist, P.F.; Diersen-Schade, D.A.; Harris, C.L.; et al. Docosahexaenoic Acid and Arachidonic Acid Enhance Growth with No Adverse Effects in Preterm Infants Fed Formula. J. Pediatr. 2002, 140, 547–554. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Van Aerde, J.E.; Merkel, K.L.; Harris, C.L.; Springer, M.A.; Hansen, J.W.; Diersen-Schade, D.A. Growth and Development of Preterm Infants Fed Infant Formulas Containing Docosahexaenoic Acid and Arachidonic Acid. J. Pediatr. 2005, 146, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Ziegler, E.; Mitmesser, S.H.; Harris, C.L.; Diersen-Schade, D.A. Soy-Based Infant Formula Supplemented with DHA and ARA Supports Growth and Increases Circulating Levels of These Fatty Acids in Infants. Lipids 2008, 43, 29–35. [Google Scholar] [CrossRef] [PubMed]
- GRAS Notices GRN No. 844 Algal Oil (55% Docosahexaenoic Acid) from Schizochytrium Sp. Strain FCC-3204. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=844&sort=GRN_No&order=DESC&startrow=1&type=basic&search=844 (accessed on 27 September 2022).
- GRAS Notices GRN No. 677 Docosahexaenoic Acid Oil Produced in Schizochytrium sp. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=677&sort=GRN_No&order=DESC&startrow=1&type=basic&search=677 (accessed on 27 September 2022).
- GRAS Notices GRN No. 860 Algal Oil (36% Docosahexaenoic Acid) from Schizochytrium Sp. Strain DHF. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=860&sort=GRN_No&order=DESC&startrow=1&type=basic&search=860 (accessed on 27 September 2022).
- GRAS Notices GRN No. 553 Algal Oil (40% Docosahexaenoic Acid) Derived from Schizochytrium sp. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=553&sort=GRN_No&order=DESC&startrow=1&type=basic&search=553 (accessed on 27 September 2022).
- Safety of Schizochytrium Sp. Oil as a Novel Food Pursuant to Regulation (EU) 2015/2283|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6242 (accessed on 27 September 2022).
- Canada, H. List of Non-Novel Determinations for Food and Food Ingredients. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/requesting-novelty-determination/list-non-novel-determinations.html (accessed on 27 September 2022).
- Commission Implementing Decision (EU) 2015/545 of 31 March 2015 Authorising the Placing on the Market of Oil from the Micro-Algae Schizochytrium sp. (ATCC PTA-9695) as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified under Document C(2015) 2082); Official Journal of the European Union, European Union. 2015, Volume 90, pp. 7–10. Available online: https://faolex.fao.org/docs/pdf/eur142925.pdf (accessed on 27 September 2022).
- Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-on Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding (Text with EEA Relevance); Official Journal of the European Union, European Union. 2015, Volume 25, pp. 1–29. Available online: https://faolex.fao.org/docs/pdf/eur151710.pdf. (accessed on 27 September 2022).
- GRAS Notices GRN No. 732 Docosahexaenoic Acid Oil Produced in Schizochytrium sp. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=732&sort=GRN_No&order=DESC&startrow=1&type=basic&search=732 (accessed on 27 September 2022).
- Gonzalez Casanova, I.; Schoen, M.; Tandon, S.; Stein, A.D.; Barraza Villarreal, A.; DiGirolamo, A.M.; Demmelmair, H.; Ramirez Silva, I.; Feregrino, R.G.; Rzehak, P.; et al. Maternal FADS2 Single Nucleotide Polymorphism Modified the Impact of Prenatal Docosahexaenoic Acid (DHA) Supplementation on Child Neurodevelopment at 5 Years: Follow-up of a Randomized Clinical Trial. Clin. Nutr. 2021, 40, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, U.; Stein, A.D.; Parra-Cabrera, S.; Wang, M.; Imhoff-Kunsch, B.; Juárez-Márquez, S.; Rivera, J.; Martorell, R. Effects of Docosahexaenoic Acid Supplementation during Pregnancy on Gestational Age and Size at Birth: Randomized, Double-Blind, Placebo-Controlled Trial in Mexico. Food Nutr. Bull. 2010, 31, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA Supplementation and Pregnancy Outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Shaddy, D.J.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) Clinical Trial: Long-Term Behavioral Follow-up of the Effects of Prenatal DHA Supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.A.; Kerling, E.H.; Shaddy, D.J.; Li, S.; Thodosoff, J.M.; Colombo, J.; Carlson, S.E. Docosahexaenoic Acid (DHA) Supplementation in Pregnancy Differentially Modulates Arachidonic Acid and DHA Status across FADS Genotypes in Pregnancy. Prostaglandins Leukot Essent Fat. Acids 2015, 94, 29–33. [Google Scholar] [CrossRef]
- Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic Acid for Reading, Cognition and Behavior in Children Aged 7–9 Years: A Randomized, Controlled Trial (the DOLAB Study). PLoS ONE 2012, 7, e43909. [Google Scholar] [CrossRef]
- Montgomery, P.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Richardson, A.J. Fatty Acids and Sleep in UK Children: Subjective and Pilot Objective Sleep Results from the DOLAB Stud—A Randomized Controlled Trial. J. Sleep Res. 2014, 23, 364–388. [Google Scholar] [CrossRef]
- Montgomery, P.; Spreckelsen, T.F.; Burton, A.; Burton, J.R.; Richardson, A.J. Docosahexaenoic Acid for Reading, Working Memory and Behavior in UK Children Aged 7-9: A Randomized Controlled Trial for Replication (the DOLAB II Study). PLoS ONE 2018, 13, e0192909. [Google Scholar] [CrossRef]
- Muthayya, S.; Dwarkanath, P.; Thomas, T.; Ramprakash, S.; Mehra, R.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Bhat, S.; Vaz, M.; et al. The Effect of Fish and ω-3 LCPUFA Intake on Low Birth Weight in Indian Pregnant Women. Eur. J. Clin. Nutr. 2009, 63, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; Kondal, D.; Chaudhry, M.; Patil, K.; Swamy, M.K.; Pujeri, G.; Mane, S.B.; Kudachi, Y.; Gupta, R.; Ramakrishnan, U.; et al. Prenatal Maternal Docosahexaenoic Acid (DHA) Supplementation and Newborn Anthropometry in India: Findings from DHANI. Nutrients 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Ding, E.L.; Willett, W.C.; Rimm, E.B. A Meta-Analysis Shows That Docosahexaenoic Acid from Algal Oil Reduces Serum Triglycerides and Increases HDL-Cholesterol and LDL-Cholesterol in Persons without Coronary Heart Disease. J. Nutr. 2012, 142, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Yip, E.L.; Reghunathan, R.; Mohan, S.; Sabaté, J. Effect of Altering Dietary N-6:N-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults. Nutrients 2017, 9, 1162. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic Acid in the Treatment of Rheumatoid Arthritis: A Double-Blind, Placebo-Controlled, Randomized Cross-over Study with Microalgae vs. Sunflower Oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- López-Neyra, A.; Suárez, L.; Muñoz, M.; de Blas, A.; Ruiz de Valbuena, M.; Garriga, M.; Calvo, J.; Ribes, C.; Girón Moreno, R.; Máiz, L.; et al. Long-Term Docosahexaenoic Acid (DHA) Supplementation in Cystic Fibrosis Patients: A Randomized, Multi-Center, Double-Blind, Placebo-Controlled Trial. Prostaglandins Leukot Essent Fat. Acids 2020, 162, 102186. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef]
- Gema, H.; Kavadia, A.; Dimou, D.; Tsagou, V.; Komaitis, M.; Aggelis, G. Production of γ-Linolenic Acid by Cunninghamella Echinulata Cultivated on Glucose and Orange Peel. Appl. Microbiol. Biotechnol. 2002, 58, 303–307. [Google Scholar] [CrossRef]
- Xie, D.; Jackson, E.N.; Zhu, Q. Sustainable Source of Omega-3 Eicosapentaenoic Acid from Metabolically Engineered Yarrowia Lipolytica: From Fundamental Research to Commercial Production. Appl. Microbiol. Biotechnol. 2015, 99, 1599–1610. [Google Scholar] [CrossRef]
- Gemperlein, K.; Dietrich, D.; Kohlstedt, M.; Zipf, G.; Bernauer, H.S.; Wittmann, C.; Wenzel, S.C.; Müller, R. Polyunsaturated Fatty Acid Production by Yarrowia Lipolytica Employing Designed Myxobacterial PUFA Synthases. Nat. Commun. 2019, 10, 4055. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.-M. Yarrowia Lipolytica as a Biotechnological Chassis to Produce Usual and Unusual Fatty Acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef]
- Cerone, M.; Smith, T.K. A Brief Journey into the History of and Future Sources and Uses of Fatty Acids. Front. Nutr. 2021, 8, 429. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, Y.J.; Wenning, L.; Liu, Q.; Krivoruchko, A.; Siewers, V.; Nielsen, J.; David, F. Metabolic Engineering of Saccharomyces Cerevisiae for Production of Very Long Chain Fatty Acid-Derived Chemicals. Nat. Commun. 2017, 8, 15587. [Google Scholar] [CrossRef]
- Ji, X.-J.; Huang, H. Engineering Microbes to Produce Polyunsaturated Fatty Acids. Trends Biotechnol. 2019, 37, 344–346. [Google Scholar] [CrossRef]
- Ratledge, C. 19—Microbial Production of Polyunsaturated Fatty Acids as Nutraceuticals. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 531–558. ISBN 978-0-85709-343-1. [Google Scholar]
- Couto, S.R.; Sanromán, M.Á. Application of Solid-State Fermentation to Food Industry—A Review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- CRC. Nutraceutical and Specialty Lipids and Their Co-Products; Shahidi, F., Ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-13540-8. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids, Olive Oil and Health Status: A Systematic Review and Meta-Analysis of Cohort Studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in Fatty Acid and Mineral Composition of Rapeseed (Brassica napus ssp. oleifera L.) Oil with Seed Sizes. Ind. Crops Prod. 2019, 129, 10–14. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Sakuma, M.; Taketani, Y.; Takeda, E. Flaxseed Oil Intake Reduces Serum Small Dense Low-Density Lipoprotein Concentrations in Japanese Men: A Randomized, Double Blind, Crossover Study. Nutr. J. 2015, 14, 39. [Google Scholar] [CrossRef]
- Hodson, L.; Crowe, F.L.; McLachlan, K.J.; Skeaff, C.M. Effect of Supplementation with Flaxseed Oil and Different Doses of Fish Oil for 2 Weeks on Plasma Phosphatidylcholine Fatty Acids in Young Women. Eur. J. Clin. Nutr. 2018, 72, 832–840. [Google Scholar] [CrossRef]
- Joris, P.J.; Draijer, R.; Fuchs, D.; Mensink, R.P. Effect of α-Linolenic Acid on Vascular Function and Metabolic Risk Markers during the Fasting and Postprandial Phase: A Randomized Placebo-Controlled Trial in Untreated (Pre-)Hypertensive Individuals. Clin. Nutr. 2020, 39, 2413–2419. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vlassopoulos, A.; Gatzieva, A.; Farmaki, A.-E.; Katsiougiannis, S.; Panagiotakos, D.B.; Kalogeropoulos, N.; Skopouli, F.N. Flaxseed Oil Does Not Affect Inflammatory Markers and Lipid Profile Compared to Olive Oil, in Young, Healthy, Normal Weight Adults. Metabolism 2013, 62, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Taghizadeh, M.; Mirhosseini, N.; Akbari, E.; Bahmani, F.; Memarzadeh, M.R.; Sharifi, N.; Jafarnejad, S.; Banikazemi, Z.; Asemi, Z. A Comparison between the Effects of Flaxseed Oil and Fish Oil Supplementation on Cardiovascular Health in Type 2 Diabetic Patients with Coronary Heart Disease: A Randomized, Double-Blinded, Placebo-Controlled Trial. Phytother. Res. 2019, 33, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Marineli, R.D.S.; Moraes, É.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R., Jr. Chemical Characterization and Antioxidant Potential of Chilean Chia Seeds and Oil (Salvia Hispanica L.). LWT—Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia Hispanica L.): An Overview—Phytochemical Profile, Isolation Methods, and Application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion on the Safety of ‘Chia Seeds (Salvia hispanica L.) and Ground Whole Chia Seeds’ as a Food Ingredient [1]. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/996 (accessed on 10 January 2023).
- Valenzuela, R.; Bascuñán, K.A.; Chamorro, R.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Parraguez, G.; Orellana, P.; Gonzalez, V.; Valenzuela, A. Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing. Nutrients 2015, 7, 6405–6424. [Google Scholar] [CrossRef] [PubMed]
- Vollmann, J.; Eynck, C. Camelina as a Sustainable Oilseed Crop: Contributions of Plant Breeding and Genetic Engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef]
- Manninen, S.; Lankinen, M.; de Mello, V.; Ågren, J.; Laaksonen, D.; Schwab, U.; Erkkilä, A. The Effect of Camelina Sativa Oil and Fish Intakes on Fatty Acid Compositions of Blood Lipid Fractions. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 51–61. [Google Scholar] [CrossRef]
- Schwab, U.S.; Lankinen, M.A.; de Mello, V.D.; Manninen, S.M.; Kurl, S.; Pulkki, K.J.; Laaksonen, D.E.; Erkkilä, A.T. Camelina Sativa Oil, but Not Fatty Fish or Lean Fish, Improves Serum Lipid Profile in Subjects with Impaired Glucose Metabolism-A Randomized Controlled Trial. Mol. Nutr. Food Res. 2018, 62, 1700503. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C.; Villegas, L. Exposure of Fatty Acids after a Single Oral Administration of Sacha Inchi (Plukenetia volubilis L.) and Sunflower Oil in Human Adult Subjects. Toxicol. Mech. Methods 2014, 24, 60–69. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Physicochemical Characterization of Basil (Ocimum basilicum L.) Seeds. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100295. [Google Scholar] [CrossRef]
- Kim, D.-E.; Shang, X.; Assefa, A.D.; Keum, Y.-S.; Saini, R.K. Metabolite Profiling of Green, Green/Red, and Red Lettuce Cultivars: Variation in Health Beneficial Compounds and Antioxidant Potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Phytochemical Composition and Nutritional Value of Different Plant Parts in Two Cultivated and Wild Purslane (Portulaca oleracea L.) Genotypes. Food Chem. 2020, 320, 126621. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical Composition and Yield of Six Genotypes of Common Purslane (Portulaca oleracea L.): An Alternative Source of Omega-3 Fatty Acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of Alpha-Linolenic Acid to Longer-Chain Polyunsaturated Fatty Acids in Human Adults. Reprod Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Prasad, P.; Sreedhar, R.V. Identification and Functional Characterization of Buglossoides Arvensis Microsomal Fatty Acid Desaturation Pathway Genes Involved in Polyunsaturated Fatty Acid Synthesis in Seeds. J. Biotechnol. 2020, 308, 130–140. [Google Scholar] [CrossRef]
- Harris, W.S. Stearidonic Acid as a ‘pro-Eicosapentaenoic Acid’. Curr. Opin. Lipidol. 2012, 23, 30–34. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jaudszus, A.; Jahreis, G. Searching for Health Beneficial N-3 and n-6 Fatty Acids in Plant Seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 153–160. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L. Matthäus Oil Content, Tocopherol Composition and Fatty Acid Patterns of the Seeds of 51 Cannabis Sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of Hemp (Cannabis sativa L.) Seed Oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-Based Stearidonic Acid as Sustainable Source of Omega-3 Fatty Acid with Functional Outcomes on Human Health. Crit. Rev. Food Sci. Nutr. 2021, 61, 1725–1737. [Google Scholar] [CrossRef]
- James, M.J.; Ursin, V.M.; Cleland, L.G. Metabolism of Stearidonic Acid in Human Subjects: Comparison with the Metabolism of Other N−3 Fatty Acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Weiß, S.; Kiehntopf, M.; Jahreis, G. Consumption of Echium Oil Increases EPA and DPA in Blood Fractions More Efficiently Compared to Linseed Oil in Humans. Lipids Health Dis. 2016, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary Intake of Stearidonic Acid–Enriched Soybean Oil Increases the Omega-3 Index: Randomized, Double-Blind Clinical Study of Efficacy and Safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef]
- Surette, M.E.; Edens, M.; Chilton, F.H.; Tramposch, K.M. Dietary Echium Oil Increases Plasma and Neutrophil Long-Chain (n-3) Fatty Acids and Lowers Serum Triacylglycerols in Hypertriglyceridemic Humans. J. Nutr. 2004, 134, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Lemke, S.L.; Hansen, S.N.; Goldstein, D.A.; DiRienzo, M.A.; Su, H.; Nemeth, M.A.; Taylor, M.L.; Ahmed, G.; George, C. Stearidonic Acid-Enriched Soybean Oil Increased the Omega-3 Index, an Emerging Cardiovascular Risk Marker. Lipids 2008, 43, 805–811. [Google Scholar] [CrossRef]
- Krul, E.S.; Lemke, S.L.; Mukherjea, R.; Taylor, M.L.; Goldstein, D.A.; Su, H.; Liu, P.; Lawless, A.; Harris, W.S.; Maki, K.C. Effects of Duration of Treatment and Dosage of Eicosapentaenoic Acid and Stearidonic Acid on Red Blood Cell Eicosapentaenoic Acid Content. Prostaglandins Leukot Essent Fat. Acids 2012, 86, 51–59. [Google Scholar] [CrossRef]
- Goyens, P.L.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of Alpha-Linolenic Acid in Humans Is Influenced by the Absolute Amounts of Alpha-Linolenic Acid and Linoleic Acid in the Diet and Not by Their Ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; Rincón-Cervera, M.Á.; Venegas-Venegas, E. Restricted-Range Boraginaceae Species Constitute Potential Sources of Valuable Fatty Acids. J. Am. Oil Chem. Soc. 2014, 91, 301–308. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Substantiation of Health Claims Related to Echium Oil and Maintenance of Normal Blood Concentrations of Triglycerides (ID 548) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/1256 (accessed on 27 September 2022).
- Cumberford, G.; Hebard, A. Ahiflower Oil: A Novel Non-GM Plant-Based Omega-3 + 6 Source. Lipid Technol. 2015, 27, 207–210. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Prasad, P.; Reddy, L.P.A.; Rajasekharan, R.; Srinivasan, M. Unravelling a Stearidonic Acid-Rich Triacylglycerol Biosynthetic Pathway in the Developing Seeds of Buglossoides Arvensis: A Transcriptomic Landscape. Sci. Rep. 2017, 7, 10473. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety of Refined Buglossoides Oil as a Novel Food Ingredient. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/4029 (accessed on 27 September 2022).
- GRAS Notices GRN No. 486 Oil from the Seeds of Buglossoides Arvensis. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=486 (accessed on 27 September 2022).
- Lefort, N.; LeBlanc, R.; Giroux, M.-A.; Surette, M.E. Consumption of Buglossoides Arvensis Seed Oil Is Safe and Increases Tissue Long-Chain n-3 Fatty Acid Content More than Flax Seed Oil—Results of a Phase I Randomised Clinical Trial. J. Nutr. Sci. 2016, 5, e2. [Google Scholar] [CrossRef]
- Lefort, N.; LeBlanc, R.; Surette, M.E. Dietary Buglossoides Arvensis Oil Increases Circulating N-3 Polyunsaturated Fatty Acids in a Dose-Dependent Manner and Enhances Lipopolysaccharide-Stimulated Whole Blood Interleukin-10—A Randomized Placebo-Controlled Trial. Nutrients 2017, 9, 261. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Fabrikov, D.; Rincón-Cervera, M.Á.; Urrestarazu, M.; Gómez-Mercado, F. γ-Linolenic and Stearidonic Acids from Boraginaceae of Diverse Mediterranean Origin. Chem. Biodivers 2020, 17, e2000627. [Google Scholar] [CrossRef]
- Piskernik, S.; Vidrih, R.; Demšar, L.; Koron, D.; Rogelj, M.; Žontar, T.P. Fatty Acid Profiles of Seeds from Different Ribes Species. LWT 2018, 98, 424–427. [Google Scholar] [CrossRef]
- Schulze, M.B.; Minihane, A.M.; Saleh, R.N.M.; Risérus, U. Intake and Metabolism of Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Nutritional Implications for Cardiometabolic Diseases. Lancet Diabetes Endocrinol. 2020, 8, 915–930. [Google Scholar] [CrossRef]
- Hall, M.N.; Campos, H.; Li, H.; Sesso, H.D.; Stampfer, M.J.; Willett, W.C.; Ma, J. Blood Levels of Long-Chain Polyunsaturated Fatty Acids, Aspirin, and the Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 314–321. [Google Scholar] [CrossRef]
- Walker, C.G.; Jebb, S.A.; Calder, P.C. Stearidonic Acid as a Supplemental Source of ω-3 Polyunsaturated Fatty Acids to Enhance Status for Improved Human Health. Nutrition 2013, 29, 363–369. [Google Scholar] [CrossRef]
- Calder, P.C. N−3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Whelan, J. Dietary Stearidonic Acid Is a Long Chain (n-3) Polyunsaturated Fatty Acid with Potential Health Benefits. J. Nutr. 2009, 139, 5–10. [Google Scholar] [CrossRef]
- Ohnishi, H.; Saito, Y. Eicosapentaenoic Acid (EPA) Reduces Cardiovascular Events: Relationship with the EPA/Arachidonic Acid Ratio. J. Atheroscler. Thromb. 2013, 20, 861–877. [Google Scholar] [CrossRef]
- Greupner, T.; Koch, E.; Kutzner, L.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Single-Dose SDA-Rich Echium Oil Increases Plasma EPA, DPAn3, and DHA Concentrations. Nutrients 2019, 11, 2346. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Banerjee, T.; Calder, P.C. The Influence of Different Combinations of Gamma-Linolenic, Stearidonic and Eicosapentaenoic Acids on the Fatty Acid Composition of Blood Lipids and Mononuclear Cells in Human Volunteers. Prostaglandins Leukot Essent Fat. Acids 2004, 70, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, Interconversion, and Dose Response of n-3 Fatty Acids in Humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Reid, K.J.; Windsor, S.L.; Harris, W.S. The Impact of Age, Body Mass Index, and Fish Intake on the EPA and DHA Content of Human Erythrocytes. Lipids 2005, 40, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Maes, A.; Goemaere, S.; Rottey, S.; Foubert, I.; et al. Echium Oil Is Not Protective against Weight Loss in Head and Neck Cancer Patients Undergoing Curative Radio(Chemo)Therapy: A Randomised-Controlled Trial. BMC Complement Altern. Med. 2014, 14, 382. [Google Scholar] [CrossRef]
- Pieters, D.J.M.; Mensink, R.P. Effects of Stearidonic Acid on Serum Triacylglycerol Concentrations in Overweight and Obese Subjects: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2015, 69, 121–126. [Google Scholar] [CrossRef]
- Ursin, V.M. Modification of Plant Lipids for Human Health: Development of Functional Land-Based Omega-3 Fatty Acids. J. Nutr. 2003, 133, 4271–4274. [Google Scholar] [CrossRef]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The Impact of Polyunsaturated Fatty Acid-Based Dietary Supplements on Disease Biomarkers in a Metabolic Syndrome/Diabetes Population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef]
- Diwakar, B.T.; Dutta, P.K.; Lokesh, B.R.; Naidu, K.A. Physicochemical Properties of Garden Cress (Lepidium Sativum L.) Seed Oil. J. Am. Oil Chem. Soc. 2010, 87, 539–548. [Google Scholar] [CrossRef]
- Amjad Khan, W.; Chun-Mei, H.; Khan, N.; Iqbal, A.; Lyu, S.-W.; Shah, F. Bioengineered Plants Can Be a Useful Source of Omega-3 Fatty Acids. BioMed Res. Int. 2017, 2017, e7348919. [Google Scholar] [CrossRef]
- Ohlrogge, J.B. Design of New Plant Products: Engineering of Fatty Acid Metabolism. Plant Physiol. 1994, 104, 821–826. [Google Scholar] [CrossRef]
- Napier, J.A.; Usher, S.; Haslam, R.P.; Ruiz-Lopez, N.; Sayanova, O. Transgenic Plants as a Sustainable, Terrestrial Source of Fish Oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1317–1324. [Google Scholar] [CrossRef]
- Ruiz-López, N.; Haslam, R.P.; Venegas-Calerón, M.; Li, T.; Bauer, J.; Napier, J.A.; Sayanova, O. Enhancing the Accumulation of Omega-3 Long Chain Polyunsaturated Fatty Acids in Transgenic Arabidopsis Thaliana via Iterative Metabolic Engineering and Genetic Crossing. Transgenic. Res. 2012, 21, 1233–1243. [Google Scholar] [CrossRef]
- Kajikawa, M.; Matsui, K.; Ochiai, M.; Tanaka, Y.; Kita, Y.; Ishimoto, M.; Kohzu, Y.; Shoji, S.; Yamato, K.T.; Ohoyama, K.; et al. Production of Arachidonic and Eicosapentaenoic Acids in Plants Using Bryophyte Fatty Acid Δ6-Desaturase, Δ6-Elongase, and Δ5-Desaturase Genes. Biosci. Biotechnol. Biochem. 2008, 72, 435–444. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC85709 (accessed on 27 September 2022).
- Feednavigator.com ‘Alltech Has Not Exited Algae’. Available online: https://www.feednavigator.com/Article/2018/08/29/Alltech-has-not-exited-algae (accessed on 27 September 2022).
- Napier, J.A.; Olsen, R.-E.; Tocher, D.R. Update on GM Canola Crops as Novel Sources of Omega-3 Fish Oils. Plant Biotechnol. J. 2019, 17, 703–705. [Google Scholar] [CrossRef]
- Mansour, M.P.; Shrestha, P.; Belide, S.; Petrie, J.R.; Nichols, P.D.; Singh, S.P. Characterization of Oilseed Lipids from “DHA-Producing Camelina Sativa”: A New Transformed Land Plant Containing Long-Chain Omega-3 Oils. Nutrients 2014, 6, 776–789. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic Engineering Camelina Sativa with Fish Oil-Like Levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful High-Level Accumulation of Fish Oil Omega-3 Long-Chain Polyunsaturated Fatty Acids in a Transgenic Oilseed Crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Zhou, X.-R.; Mansour, M.P.; Liu, Q.; Belide, S.; Nichols, P.D.; Singh, S.P. Metabolic Engineering Plant Seeds with Fish Oil-like Levels of DHA. PLoS ONE 2012, 7, e49165. [Google Scholar] [CrossRef]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Han, L.; Sayanova, O.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Postprandial Incorporation of EPA and DHA from Transgenic Camelina Sativa Oil into Blood Lipids Is Equivalent to That from Fish Oil in Healthy Humans. Br. J. Nutr. 2019, 121, 1235–1246. [Google Scholar] [CrossRef]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Han, L.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Dietary Supplementation with Seed Oil from Transgenic Camelina Sativa Induces Similar Increments in Plasma and Erythrocyte DHA and EPA to Fish Oil in Healthy Humans. Br. J. Nutr. 2020, 124, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic Engineering of the Omega-3 Long Chain Polyunsaturated Fatty Acid Biosynthetic Pathway into Transgenic Plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Desaint, N.; Varbanova, M. The Use and Value of Polling to Determine Public Opinion on GMOs in Europe. GM Crops Food 2013, 4, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lucht, J.M. Public Acceptance of Plant Biotechnology and GM Crops. Viruses 2015, 7, 4254–4281. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, N.; Haslam, R.P.; Venegas-Calerón, M.; Larson, T.R.; Graham, I.A.; Napier, J.A.; Sayanova, O. The Synthesis and Accumulation of Stearidonic Acid in Transgenic Plants: A Novel Source of ‘Heart-Healthy’ Omega-3 Fatty Acids. Plant Biotechnol. J. 2009, 7, 704–716. [Google Scholar] [CrossRef]
- Eckert, H.; LaVallee, B.; Schweiger, B.J.; Kinney, A.J.; Cahoon, E.B.; Clemente, T. Co-Expression of the Borage Δ6 Desaturase and the Arabidopsis Δ15 Desaturase Results in High Accumulation of Stearidonic Acid in the Seeds of Transgenic Soybean. Planta 2006, 224, 1050–1057. [Google Scholar] [CrossRef]
- Harris, W.S. Stearidonic Acid-Enhanced Soybean Oil: A Plant-Based Source of (n-3) Fatty Acids for Foods. J. Nutr. 2012, 142, 600S–604S. [Google Scholar] [CrossRef]
- Lee, K.R.; Kim, K.H.; Kim, J.B.; Hong, S.B.; Jeon, I.; Kim, H.U.; Lee, M.H.; Kim, J.K. High Accumulation of γ-Linolenic Acid and Stearidonic Acid in Transgenic Perilla (Perilla Frutescens Var. Frutescens) Seeds. BMC Plant Biol. 2019, 19, 120. [Google Scholar] [CrossRef]
- Lemke, S.L.; Maki, K.C.; Hughes, G.; Taylor, M.L.; Krul, E.S.; Goldstein, D.A.; Su, H.; Rains, T.M.; Mukherjea, R. Consumption of Stearidonic Acid-Rich Oil in Foods Increases Red Blood Cell Eicosapentaenoic Acid. J. Acad. Nutr. Diet. 2013, 113, 1044–1056. [Google Scholar] [CrossRef]
- GRAS Notices GRN No. 283 Stearidonic Acid Soybean Oil. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=283&sort=GRN_No&order=DESC&startrow=1&type=basic&search=soybean (accessed on 27 September 2022).
- EFSA. Statement Complementing the EFSA Scientific Opinion on Application (EFSA-GMO-NL-2010-85) for Authorisation of Food and Feed Containing, Consisting of and Produced from Genetically Modified Soybean MON 87769 × MON 89788. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/6589 (accessed on 27 September 2022).
- EFSA. Petition for the Determination of Nonregulated Status for EPA+DHA Canola Event LBFLFK. Available online: https://www.regulations.gov/document/APHIS-2018-0014-0002 (accessed on 7 January 2023).
- Eck, C. BASF Petition (17-321-01p) for Determination of Nonregulated Status of EPA + DHA Canola Event LBFLFK. Available online: https://www.aphis.usda.gov/brs/aphisdocs/17_32101p_fpra.pdf (accessed on 7 January 2023).
- Abbadi, A.; Domergue, F.; Bauer, J.; Napier, J.A.; Welti, R.; Zähringer, U.; Cirpus, P.; Heinz, E. Biosynthesis of Very-Long-Chain Polyunsaturated Fatty Acids in Transgenic Oilseeds: Constraints on Their Accumulation. Plant Cell 2004, 16, 2734–2748. [Google Scholar] [CrossRef]
- Cheng, B.; Wu, G.; Vrinten, P.; Falk, K.; Bauer, J.; Qiu, X. Towards the Production of High Levels of Eicosapentaenoic Acid in Transgenic Plants: The Effects of Different Host Species, Genes and Promoters. Transgenic. Res. 2010, 19, 221–229. [Google Scholar] [CrossRef]
- Robert, S.S.; Singh, S.P.; Zhou, X.R.; Petrie, J.R.; Blackburn, S.I.; Mansour, P.M.; Nichols, P.D.; Liu, Q.; Green, A.G. Metabolic Engineering of Arabidopsis to Produce Nutritionally Important DHA in Seed Oil. Funct. Plant Biol. 2005, 32, 473–479. [Google Scholar] [CrossRef]
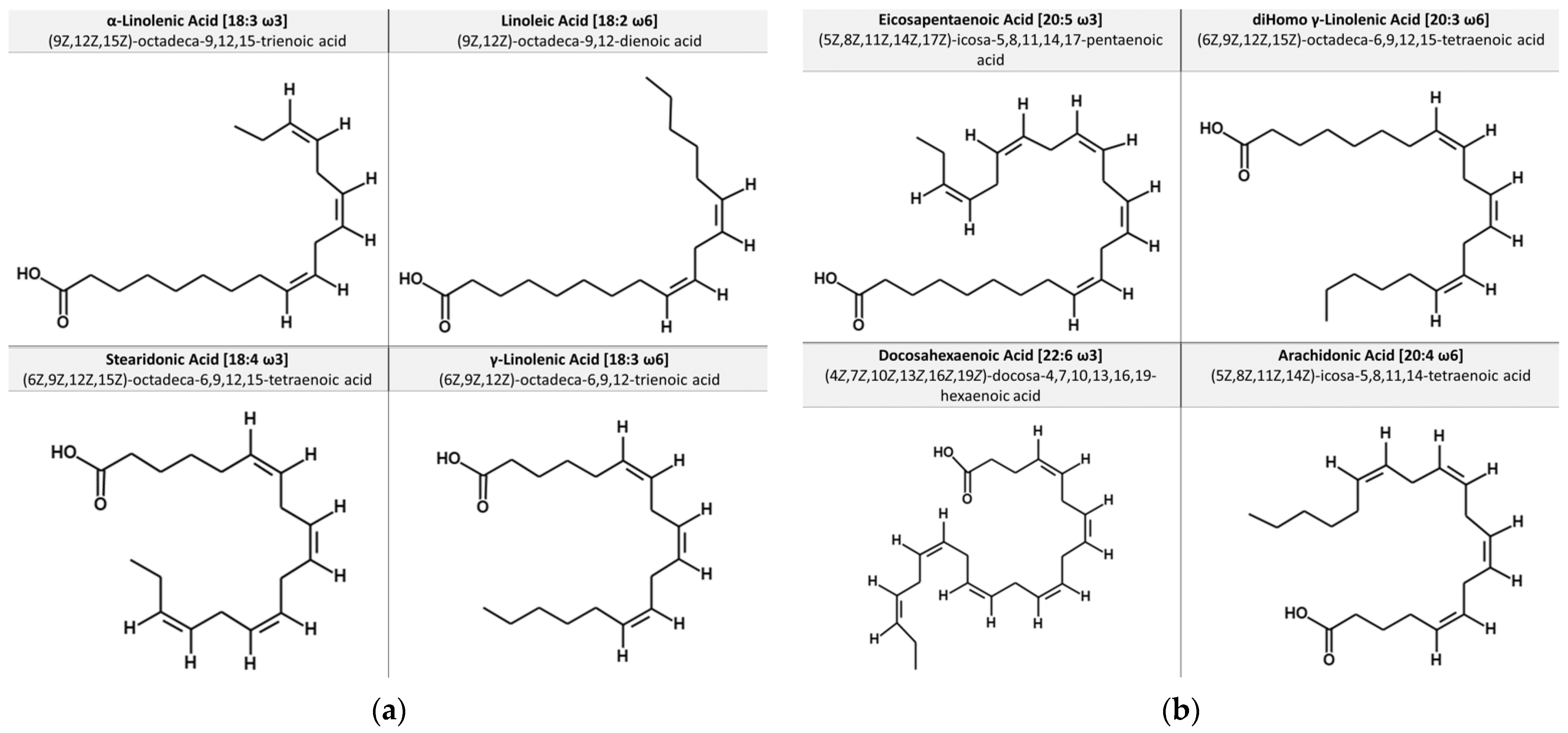
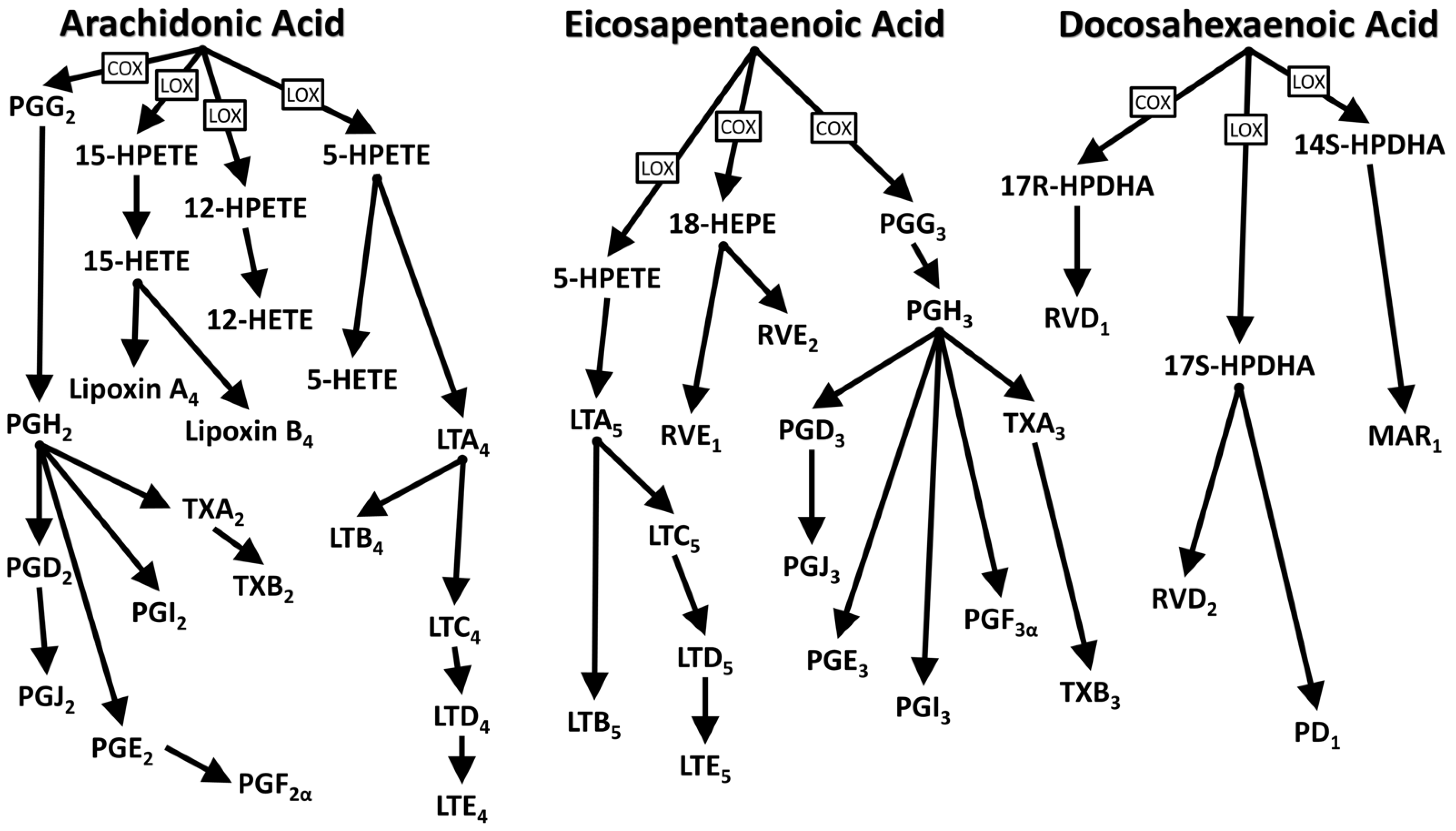
| Food | 18:2 n-6 (LA) | 18:3 n-3 (ALA) | 18:3 n-6 GLA | 18:4 n-3 SDA | 20:4 n-6 AA | 20:5 n-3 EPA | 22:6 n-3 DHA |
|---|---|---|---|---|---|---|---|
| Almond | 12.3 | 0.003 | 0 | 0 | 0 | 0 | 0 |
| Brazil nut | 23.9 | 0.018 | 0.018 | 0 | 0 | 0 | 0 |
| Canola oil | 18.6 | 9.14 | 0 | 0 | 0 | 0 | 0 |
| Cashew nut | 7.78 | 0.062 | NA | 0 | 0 | 0 | 0 |
| Chestnut | 0.78 | 0.093 | NA | 0 | 0 | 0 | 0 |
| Chia seed | 5.84 | 17.8 | NA | 0 | NA | 0 | 0 |
| Coconut oil | 1.68 | 0.019 | 0 | 0 | 0 | 0 | 0 |
| Extra virgin Olive oil | 8.4 | 0.65 | NA | NA | 0 | NA | NA |
| Flaxseed | 5.26 | 19.4 | NA | 0 | NA | 0 | 0 |
| Flaxseed oil | 14.2 | 53.4 | 0 | 0 | 0.015 | 0 | 0 |
| hemp seed | 27.4 | 8.68 | 1.34 | 0.617 | NA | NA | NA |
| Irish moss | NA | NA | NA | 0 | NA | 0.046 | 0 |
| Kelp | NA | NA | NA | 0.004 | NA | 0.004 | 0 |
| Macadamia nut | 1.3 | 0.21 | NA | 0 | 0 | 0 | 0 |
| Mustard | 0.43 | 0.444 | NA | 0 | 0 | 0 | 0 |
| Peanut | 9.72 | 0.026 | NA | 0 | 0 | 0 | 0 |
| Peanut oil | 19.7 | 0.318 | NA | 0 | 0.003 | 0.001 | 0 |
| Pecan nut | 20.6 | 0.99 | NA | 0 | 0 | 0 | 0 |
| Pine nut | 33.2 | 0.112 | 0.052 | 0 | 0 | 0 | 0 |
| Pistachio | 14.1 | 0.29 | NA | 0 | 0 | 0 | 0 |
| Pumpkin seed | 20.7 | 0.12 | 0 | 0 | 0 | 0 | 0 |
| Sea lettuce | NA | NA | NA | 0 | NA | 0.08 | 0 |
| Soy nut | NA | NA | NA | 0 | NA | 0 | 0 |
| Soybean oil | 50.9 | 6.62 | 0 | 0 | 0 | 0 | 0 |
| Spirulina | NA | NA | NA | 0 | NA | 0 | 0 |
| Wakame | NA | NA | NA | 0 | NA | 0.186 | 0 |
| Walnut | 33.8 | 2.68 | 0 | 0 | NA | 0 | 0 |
| Action | Biomolecules |
|---|---|
| Pro-inflammatory | PGE2, LTB4, LTC4 |
| Anti-inflammatory | PGI2, RVE1, RVD1, PD1 |
| Pro-aggregatory | PGE2, TXA2, TXA3 |
| Anti-aggregatory | PGI2, PGD2, PGE3, PGI3 |
| Immunostimulant | LTB4, 12-HETE, Lipoxin A |
| Immunosuppressive | PGE3, Lipoxin B |
| Vasodilatory | PGE2, PGI2, PGD2 |
| Vasoconstrictor | TXA2, PGF2α, LTC4, LTD4, PGE3 |
| Organization | Recommendation/Advice |
|---|---|
| Joint WHO/FAO 2003 [116] | PUFAs: 6–10% n6 PUFAs: 5–8% n3 PUFAs: 1–2% 200–500 mg/d of EPA+DHA |
| FAO 2010 [43] | PUFAs: 6–11% n6 PUFAs: 2.5–9% n3 PUFAs: 0.5–2% 250–2000 mg/d of EPA+DHA >300 mg/d of EPA-DHA (pregnancy and lactation) >200 mg/d of DHA (pregnancy and lactation) |
| SACN 2004 [117] | 450 mg/d of n3 LC PUFAs |
| COMA 1991 [122] | PUFAs: 6% LA: 1% ALA: 0.5% |
| EFSSA 2010 [118] | LA: 4% ALA: 0.5% 250 mg/d of EPA+DHA 100 mg/d of DHA (6–24 months) Additional 100–200 mg/d of DHA (pregnancy and lactation) |
| IOM 2005 [119] | n6 PUFAs: 5–10% n3 PUFAs: 0.6–1.2% LC PUFAs: 10% of total n3 and n6 PUFAs, respectively |
| AFSA 2010 [120] | LA: 4% ALA: 1% 500 mg/d of EPA+DHA (1:1) |
| D-A-CH 2021 [121] | LA: 2.5% ALA: 0.5% 250 mg/d of EPA+DHA |
| NNR 2004 [123] | n6 PUFAs: 2.5% n3 PUFAs: 0.5% n6 PUFAs: 4% (pregnancy, lactation and 6–11 months) n3 PUFAs: 1% (pregnancy, lactation and 6–11 months) |
| Species | 18:2 n6 (LA) | 18:3 n3 (ALA) | 18:4 n3 (SDA) | 20:4 n6 (AA) | 20:5 n3 (EPA) | 22:6 n3 (DHA) | Reference |
|---|---|---|---|---|---|---|---|
| C. sativa | 18.3 | 13.4 | 1 | 2.0 | 23.1 | 0 | [322] |
| C. sativa | 19.2 | 11.7 | 3.4 | 2.4 | 10.7 | 6.2 | [322] |
| B. napus (canola) | NA | NA | 0.26 | 2.26 | 7.21 | 1.02 | [336] |
| B. napus (canola) | 2–12 | 4–25 | 0–4 | NA | 0–4 | 6–15 | [337] |
| N. tabacum | 43.6 | 29.3 | 0 | 1.5 | 0 | NA | [338] |
| L. usitatissimum (flaseed) | 5.6 | 16.8 | 11.4 | 1.0 | 0.8 | NA | [338] |
| G. max | 15–30 | 9–12 | 15–30 | NA | NA | NA | [334] |
| B. carinata | 4.2 | 2.0 | 5.4 | 5.7 | 20.4 | NA | [339] |
| B. juncea | 18.8 | 6.2 | 2.2 | 4.3 | 5.0 | NA | [339] |
| A thaliana | 26.0 | 13.2 | 0.7 | 0.4 | 1.1 | 2.6 | [340] |
| A thaliana | 25.9 | 15.0 | 1.5 | 1.0 | 2.4 | 5.3 | [340] |
| A thaliana | 26.4 | 11.7 | 1.8 | 1.6 | 3.2 | 0 | [340] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Baroni, L.; Lombardo, M. Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 1683. https://doi.org/10.3390/ijerph20031683
Rizzo G, Baroni L, Lombardo M. Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review. International Journal of Environmental Research and Public Health. 2023; 20(3):1683. https://doi.org/10.3390/ijerph20031683
Chicago/Turabian StyleRizzo, Gianluca, Luciana Baroni, and Mauro Lombardo. 2023. "Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review" International Journal of Environmental Research and Public Health 20, no. 3: 1683. https://doi.org/10.3390/ijerph20031683
APA StyleRizzo, G., Baroni, L., & Lombardo, M. (2023). Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review. International Journal of Environmental Research and Public Health, 20(3), 1683. https://doi.org/10.3390/ijerph20031683