Preventing Zoonoses: Testing an Intervention to Change Attitudes and Behaviors toward More Protective Actions
Abstract
:1. Introduction
1.1. Zoonoses
1.2. Risk Knowledge, Attitudes and Behaviors toward Protective Behaviors
1.3. The Present Research
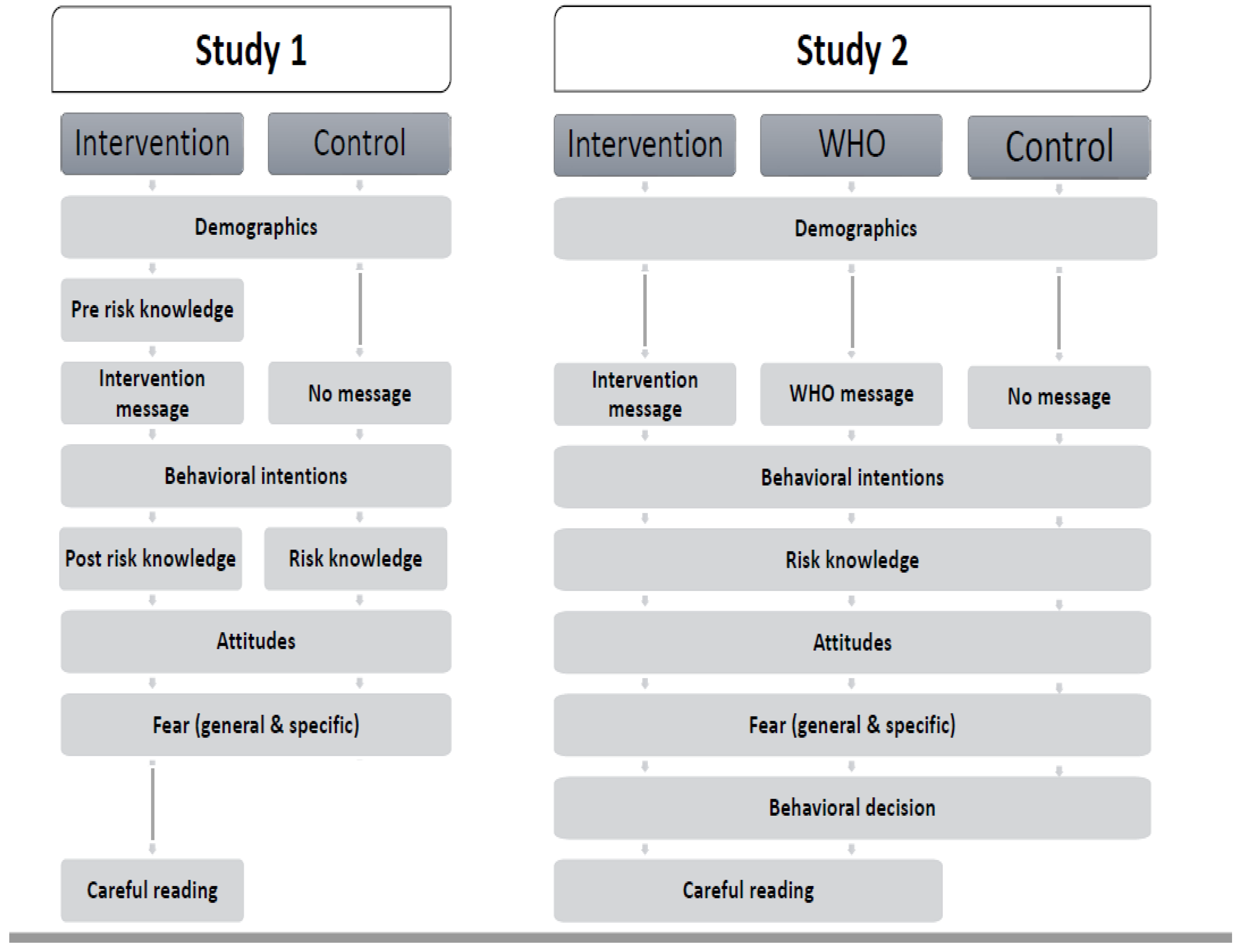
2. Study 1
2.1. Materials & Methods
2.1.1. Participants and Design
2.1.2. Procedure and Materials
2.2. Results
2.2.1. Manipulation Checks
2.2.2. Attitudes toward Protective Behavior
2.2.3. Intentions to Change Protective Behavior
2.2.4. Correlations between Risk Knowledge, Attitudes, Behavior, and Fear
3. Study 2
3.1. Materials & Methods
3.1.1. Participants and Design
3.1.2. Procedure and Materials
3.2. Results
3.2.1. Manipulation Checks
3.2.2. Attitudes toward Protective Behavior
3.2.3. Intentions to Change Protective Behavior
3.2.4. Behavioral Decision
3.2.5. Correlations
4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Coker, R.; Rushton, J.; Mounier-Jack, S.; Karimuribo, E.; Lutumba, P.; Kambarage, D.; Pfeiffer, D.U.; Stärk, K.; Rweyemamu, M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. 2011, 11, 326–331. [Google Scholar]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [PubMed]
- Bueno-Marí, R.; Almeida, A.; Navarro, J.C. Emerging zoonoses: Eco-epidemiology, involved mechanisms, and public health implications. Front. Public Health 2015, 3, 157. [Google Scholar]
- Hubálek, Z. Emerging human infectious diseases: Anthroponoses, zoonoses, and sapronoses. Emerg. Infect. Dis. 2003, 9, 403. [Google Scholar]
- Meslin, F.X.; Stöhr, K.; Heymann, D. Public health implications of emerging zoonoses. Rev. Sci. Tech. 2000, 19, 310–317. [Google Scholar] [CrossRef]
- Recht, J.; Schuenemann, V.J.; Sánchez-Villagra, M.R. Host diversity and origin of zoonoses: The ancient and the new. Animals 2020, 10, 1672. [Google Scholar]
- Tomori, O.; Oluwayelu, D.O. Domestic Animals as Potential Reservoirs of Zoonotic Viral Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 33–55. [Google Scholar]
- Leibler, L.H.; Otte, J.; Roland-Holst, D.; Pfeiffer, D.U.; Soares Magalhaes, R.; Rushton, J.; Graham, J.P.; Silbergeld, E.K. Industrial food animal production and global health risks: Exploring the ecosystems and economics of avian influenza. EcoHealth 2009, 6, 58–70. [Google Scholar]
- Espinosa, E.; Tago, D.; Treich, N. Infectious diseases and meat production. Environ. Resour. Econ. 2020, 76, 1019–1044. [Google Scholar]
- Hovi, M.; Sundrum, A.; Thamsborg, S.M. Animal health and welfare in organic livestock production in Europe: Current state and future challenges. Livest. Prod. Sci. 2003, 80, 41–53. [Google Scholar] [CrossRef]
- Garske, T.; Clarke, P.; Ghani, A.C. The transmissibility of highly pathogenic avian influenza in commercial poultry in industrialised countries. PLoS ONE 2007, 2, e349. [Google Scholar] [CrossRef] [PubMed]
- Stel, M.; Eggers, J.; Nagelmann, S. Accuracy of risk perception of zoonoses due to intensive animal farming and people’s willingness to change their animal product consumption. Sustainability 2022, 14, 589. [Google Scholar] [CrossRef]
- Stel, M.; Eggers, J.; Alonso, W.J. Mitigating zoonotic risks in intensive farming: Solutions for a sustainable change. EcoHealth 2022, 19, 324–328. [Google Scholar] [CrossRef]
- Dhingra, M.S.; Artois, J.; Dellicour, S.; Lemey, P.; Dauphin, G.; Von Dobschuetz, S.; Van Boeckel, T.P.; Castellan, D.M.; Morzaria, S.; Gilbert, M. Geographical and historical patterns in the emergences of novel highly pathogenic avian influenza (HPAI) H5 and H7 viruses in poultry. Front. Vet. Sci. 2018, 5, 84. [Google Scholar]
- El-Lethey, H.; Huber-Eicher, B.; Jungi, T.W. Exploration of stress-induced immunosuppression in chickens reveals both stress-resistant and stress-susceptible antigen responses. Vet. Immunol. Immunop. 2003, 95, 91–101. [Google Scholar] [CrossRef]
- Rostagno, M.H. Can stress in farm animals increase food safety risk? Foodborne Pathog. Dis. 2009, 6, 767–776. [Google Scholar] [CrossRef]
- Springbett, A.J.; MacKenzie, K.; Woolliams, J.A.; Bishop, S.C. The contribution of genetic diversity to the spread of infectious diseases in livestock populations. Genet 2003, 165, 1465–1474. [Google Scholar] [CrossRef]
- Schuck-Paim, C.; Alonso, W.J. Pandemics, Global Health and Consumer Choices; CriaEditora: Sao Paulo, Brazil, 2020. [Google Scholar]
- Paul, M.; King, L.; Carlin, E.P. Zoonoses of people and their pets: A US perspective on significant pet-associated parasitic diseases. Trends Parasitol. 2010, 26, 153–154. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Callaway, T.; McAllister, T. Farm fairs and petting zoos: A review of animal contact as a source of zoonotic enteric disease. Foodborne Pathog. Dis. 2017, 14, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Pencheva, M.S. Parasites in captive animals: A review of studies in some European zoos. Zoolog. Gart. 2013, 82, 60–71. [Google Scholar] [CrossRef]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfaal, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lázaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.; D’Agostino, M.; Santos, R.; Saiz, J.C.; Rzezutka, A.; et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 2011, 36, 786–814. [Google Scholar] [PubMed]
- Bakker, M.; Mertens, C. Gedrag Beïnvloeden Met Risicocommunicatie. 2019. Available online: https://www.ifv.nl/kennisplein/Documents/20191101-IFV-KP-Gedrag-beinvloeden-met-risicocommunicatie.pdf (accessed on 2 February 2022).
- Helsloot, I.; van’t Padje, B. Met Vertrouwen Communiceren over Potentiële Rampen en Crises: Een Onderzoek Naar Wat Burgers écht Verwachten van Risicocommunicatie Door de Overheid. 2011. Available online: https://crisislab.nl/wordpress/wp-content/uploads/definitief-eindrapport-10.pdf (accessed on 2 February 2022).
- Kievik, M.; Gutteling, J.M. Yes, we can: Motivate Dutch citizens to engage in self-protective behavior with regard to flood risks. Nat. Hazards 2011, 59, 1475–1490. [Google Scholar] [CrossRef]
- Hendrickx, L.; Vlek, C.; Oppewal, H. Relative importance of scenario information and frequency information in the judgement of risk. Acta Psychol. 1989, 72, 41–63. [Google Scholar] [CrossRef]
- Johnson, E.J.; Hershey, J.; Meszaros, J.; Kunreuther, H. Framing, probability distortions, and insurance decisions. J. Risk Uncertain. 1993, 7, 35–51. [Google Scholar] [CrossRef]
- Loewenstein, G.F.; Weber, E.U.; Hsee, C.K.; Welch, C.K.N. Risk as feelings. Psychol. Bull. 2011, 12, 267–286. [Google Scholar] [CrossRef]
- Paek, H.; Hove, T. Risk Perceptions and Risk Characteristics. Oxford Research Encyclopedia of Communication. 2017. Available online: https://oxfordre.com/communication/view/10.1093/acrefore/9780190228613.001.0001/acrefore-9780190228613-e-283 (accessed on 2 February 2022).
- Claassen, L.; Greven, F.; Reen, W.; Hall, E.F. Risicocommunicatie over Stralingsongevallen en de Verspreiding van Jodiumtabletten (RIVM Rapport Nr. 2016-0011). 2016. Available online: https://www.rivm.nl/publicaties/risicocommunicatie-over-stralingsongevallen-en-verspreiding-van-jodiumtabletten (accessed on 2 February 2022).
- Hansen, J.; Holm, L.; Frewer, L.; Robinson, P.; Sandoe, P. Beyond the knowledge deficit: Recent research into lay and expert attitudes to food risks. Appetite 2003, 41, 111–121. [Google Scholar] [CrossRef]
- Hegglin, D.; Bontadina, F.; Gloor, S.; Romig, T.; Deplazes, P.; Kern, P. Survey of public knowledge about Echinococcus multilocularis in four European countries: Need for proactive information. BMC Public Health 2008, 8, 247. [Google Scholar] [CrossRef]
- Katagiri, S.; Oliveira-Sequeira, T.C. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in Sao Paulo State, Brazil. Zoonoses Public Health 2008, 33, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Mengele, I.J.; Garcia-Gonzalez, D.; Mekonnen, G.; Arenas-Gamboa, A. Bovine brucellosis seroprevalence, farmers’ awareness, practices and animal health extension services inputs in Mpwapwa district, Tanzania. Tanzan. Vet. Assoc. Proceed. 2018, 3636, 135–144. [Google Scholar]
- Verhoeven, F.; Karreman, J.; Bosma, A.R.; Hendrix, R.; van Gemert-Pijnen, J.E.W. Toward improved education of the public about methicillin-resistant Staphylococcus aureus: A Mental Models Approach. Internat. J. Infect. Control 2010, 6. [Google Scholar]
- Davis, T.; Goldwater, M.B.; Ireland, M.E.; Gaylord, N.; Van Allen, J. Can you catch Ebola from a stork bite? Inductive reasoning influences generalization of perceived zoonosis risk. PLoS ONE 2017, 12, e0186969. [Google Scholar] [CrossRef]
- Davis, T.; LaCour, M.; Goldwater, M.; Hughes, B.; Ireland, M.E.; Worthy, D.A.; Gaylord, N.; Van Allen, J. Communicating about diseases that originate in animals: Lessons from the psychology of inductive reasoning. Behav. Sci. Policy 2020, 6, 1–11. [Google Scholar] [CrossRef]
- LaCour, M.; Hughes, B.; Goldwater, M.; Ireland, M.; Worthy, D.; Van Allen, J.; Gaylord, N.; Van-Hoosier, G.; Davis, T. The double bind of communicating about zoonotic origins: Describing exotic animal sources of COVID-19 increases both healthy and discriminatory avoidance intentions. Risk Anal. 2022, 42, 506–521. [Google Scholar] [CrossRef]
- Fischhoff, B. Risk perception and communication unplugged: Twenty years of process. Risk Anal. 1995, 15, 137–146. [Google Scholar] [CrossRef]
- Kasperson, R.E.; Stallen, P.J.M. Risk communication: The evolution of attempts. In Communicating Risks to the Public: International Perspectives; Kasperson, R.E., Stallen, J.M., Eds.; Kluwer: Dordrecht, The Netherlands, 1991; pp. 1–14. [Google Scholar]
- Witte, K.; Allen, M. A meta-analysis of fear appeals: Implications for effective public health campaigns. Health Educ. Beh. 2000, 27, 591–615. [Google Scholar] [CrossRef]
- Ruiter, R.A.; Abraham, C.; Kok, G. Scary warnings and rational precautions: A review of the psychology of fear appeals. Psychol. Health 2001, 16, 613–630. [Google Scholar] [CrossRef]
- Maddux, J.E.; Rogers, R.W. Protection motivation and self-efficacy: A revised theory of fear appeals and attitude change. J. Exp. Soc. Psychol. 1983, 19, 469–479. [Google Scholar] [CrossRef]
- Rogers, R.W. A Protection Motivation Theory of fear appeals and attitude change. J. Psychol. 1975, 91, 93–114. [Google Scholar] [CrossRef]
- Floyd, D.L.; Prentice-Dunn, S.; Rogers, R.W. A meta-analysis of research on protection motivation theory. J. Appl. Soc. Psychol. 2000, 30, 407–429. [Google Scholar] [CrossRef]
- Witte, K. Putting the fear back into fear appeals: The extended parallel process model. Commun. Monogr. 1992, 59, 329–349. [Google Scholar] [CrossRef]
- Ruiter, R.A.C.; Kessels, L.T.E.; Peters, G.Y.; Kok, G. Sixty years of fear appeal research: Current state of the evidence. Int. J. Psychol. 2014, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, M.B.; Hepler, J.; Zimmerman, R.S.; Saul, L.; Jacobs, S.; Wilson, K.; Albarracin, D. Appealing to fear: A meta-analysis of fear appeal effectiveness and theories. Psychol. Bull. 2015, 141, 1178–1204. [Google Scholar] [CrossRef] [PubMed]
- Verroen, S.; Gutteling, J.M.; De Vries, P.W. Enhancing self-protective behavior: Efficacy beliefs and peer feedback in risk communication. Risk Anal. 2012, 33, 1252–1264. [Google Scholar] [CrossRef]
- De Hoog, N.; Stroebe, W.; de Wit, J.B.F. The impact of vulnerability to and severity of a health risk on processing and acceptance of fear-arousing communications: A meta-analysis. Rev. Gen. Psychol. 2007, 11, 258–285. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Van ’t Veld, A.; Beerepoot, R.; Kanne, P.; Stel, M.; de Vries, P.W.; Kuttschreuter, M. Het Corona-en Het Eenzaamheidsvirus. 2020. Available online: https://www.ioresearch.nl/wp-content/uploads/2020/04/Het-corona-en-het-eenzaamheidsvirus-uitgebreid-rapport.pdf (accessed on 2 February 2022).
- Macpherson, C.N.L. Human behavior and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef]
- Bekedam, H.; Stegeman, A.; de Boer, F.; Fouchier, R.; Kluytmans, J.; Koenraadt, S.; Kuiken, T.; van der Poel, W.; Reis, R.; van Schaik, G.; et al. Zoönosen in Het Vizier. Rapport van de Expertgroep Zoönosen. 2021. Available online: https://www.rijksoverheid.nl/documenten/rapporten/2021/06/01/rapport-expertgroep-zoonosen (accessed on 2 February 2022).
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 00863. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.J.; Conner, M. Efficacy of the theory of planned behaviour: A meta-analytic review. Br. J. Soc. Psychol. 2001, 40, 471–499. [Google Scholar] [CrossRef] [PubMed]
- McEachan, R.R.C.; Conner, M.; Taylor, N.J.; Lawton, R.J. Prospective prediction of health-related behaviors with the theory of planned behavior: A meta-analysis. Health Psychol. Rev. 2011, 5, 97–144. [Google Scholar] [CrossRef]
- McEachan, R.; Taylor, N.; Harrison, R.; Lawton, R.; Gardner, P.; Conner, M. Meta-analysis of the Reasoned Action Approach (RAA) to understanding health behaviors. Ann. Behav. Med. 2016, 50, 592–612. [Google Scholar] [CrossRef] [PubMed]
- Pornpitakpan, C. The persuasiveness of source credibility: A critical review of five decades’ evidence. J. Appl. Soc. Psychol. 2004, 34, 243–281. [Google Scholar] [CrossRef]
- Chen, L.; Dong, C.; Zhang, Y. An Online Experiment Evaluating the Effects of Social Endorsement Cues, Message Source, and Responsibility Attribution on Young Adults’ COVID-19 Vaccination Intentions. SAGE Open 2022, 12, 21582440221093046. [Google Scholar] [CrossRef]
- Sun, R.; Meng, J. Looking at young millennials’ risk perception and purchase intention toward GM foods: Exploring the role of source credibility and risk attitude. Health Mark. Q. 2022, 39, 263–279. [Google Scholar] [CrossRef]
- Umeh, K. Does a credible source also need a fearful audience? J. Appl. Soc. Psychol. 2012, 42, 1716–1744. [Google Scholar] [CrossRef]
- Algan, Y.; Cohen, D.; Davoine, E.; Foucault, M.; Stantcheva, S. Trust in scientists in times of pandemic: Panel evidence from 12 countries. Proc. Natl. Acad. Sci. USA 2021, 118, e2108576118. [Google Scholar] [CrossRef]
- Koch, A.; Blohm, M. Nonresponse Bias. In GESIS Survey Guidelines; GESIS—Leibniz Institute for the Social Sciences: Mannheim, Germany, 2016. [Google Scholar] [CrossRef]
- Groves, R.M.; Presser, S.; Dipko, S. The role of topic interest in survey participation decisions. Public Opin. Q. 2004, 68, 2–31. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Park, M.C. How to randomize. In A statistical Methods for Rates and Proportion; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ringold, D.J. Bomerang effects in response to public health interventions: Some unintended consequences in the alcoholic beverage market. J. Cons. Policy 2002, 25, 27–63. [Google Scholar] [CrossRef]
| Risk Communication | Pre Risk Knowledge | Post Risk Knowledge | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Yes | 4.69 a | 0.53 | 4.88 b | 0.70 |
| No | 4.63 a | 0.55 | ||
| Risk Communication | Intentions to Change Behavior | Attitudes toward Protective Behavior | Fear | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Intervention | 3.00 a | 0.84 | 4.77 a | 0.62 | 4.98 a | 1.32 |
| Control | 2.85 b | 0.72 | 4.53 b | 0.52 | 4.58 b | 1.37 |
| Attitude | Behavioral Intentions | Post Risk Knowledge | |
|---|---|---|---|
| Behavioral intentions | rs = 0.12, p = 0.017 | ||
| Post risk knowledge | rs = 0.64, p < 0.001 | rs = 0.02, p = 0.69 | |
| General fear | r = 0.39, p < 0.001 | rs = 0.08, p = 0.11 | rs = 0.26, p = 0.002 |
| Risk Communication | Intentions to Change Behavior | Attitudes toward Protective Behavior | Post Risk Knowledge | General Fear | Specific Fear | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Intervention | 3.09 a | 0.66 | 5.22 a | 0.82 | 5.69 a | 0.76 | 2.98 a | 1.53 | 4.41 a | 1.44 |
| WHO | 3.00 a | 0.62 | 5.01 b | 0.74 | 5.55 b | 0.71 | 2.84 a | 1.50 | 4.21 a,b | 1.44 |
| Control | 2.87 b | 0.63 | 4.61 c | 0.67 | 4.96 c | 0.76 | 2.89 a | 1.50 | 4.13 b | 1.41 |
| Attitude | Behavioral Intentions | Behavioral Decision | Post risk knowledge | General Fear | |
|---|---|---|---|---|---|
| Behavioral intentions | rs = 0.35 (p < 0.001) | ||||
| Behavioral decision | rs = 0.12 (p = 0.001) | rs = 0.16 (p < 0.001) | |||
| Post risk knowledge | rs = 0.66 (p < 0.001) | r = 0.24 (p < 0.001) | rs = 0.09 (p = 0.024) | ||
| General fear | rs = 0.25 (p < 0.001) | r = 0.23 (p < 0.001) | rs = 0.05 (p = 0.20) | r = 0.11 (p = 0.002) | |
| Specific fear | rs = 0.31 (p < 0.001) | r = 0.17 (p < 0.001) | rs = 0.03 (p = 0.43) | r = 0.16 (p < 0.001) | r = 0.54 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stel, M.; Banach, N. Preventing Zoonoses: Testing an Intervention to Change Attitudes and Behaviors toward More Protective Actions. Int. J. Environ. Res. Public Health 2023, 20, 6987. https://doi.org/10.3390/ijerph20216987
Stel M, Banach N. Preventing Zoonoses: Testing an Intervention to Change Attitudes and Behaviors toward More Protective Actions. International Journal of Environmental Research and Public Health. 2023; 20(21):6987. https://doi.org/10.3390/ijerph20216987
Chicago/Turabian StyleStel, Marielle, and Nicole Banach. 2023. "Preventing Zoonoses: Testing an Intervention to Change Attitudes and Behaviors toward More Protective Actions" International Journal of Environmental Research and Public Health 20, no. 21: 6987. https://doi.org/10.3390/ijerph20216987
APA StyleStel, M., & Banach, N. (2023). Preventing Zoonoses: Testing an Intervention to Change Attitudes and Behaviors toward More Protective Actions. International Journal of Environmental Research and Public Health, 20(21), 6987. https://doi.org/10.3390/ijerph20216987





