Effectiveness of Blood Flow Restriction on Functionality, Quality of Life and Pain in Patients with Neuromusculoskeletal Pathologies: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
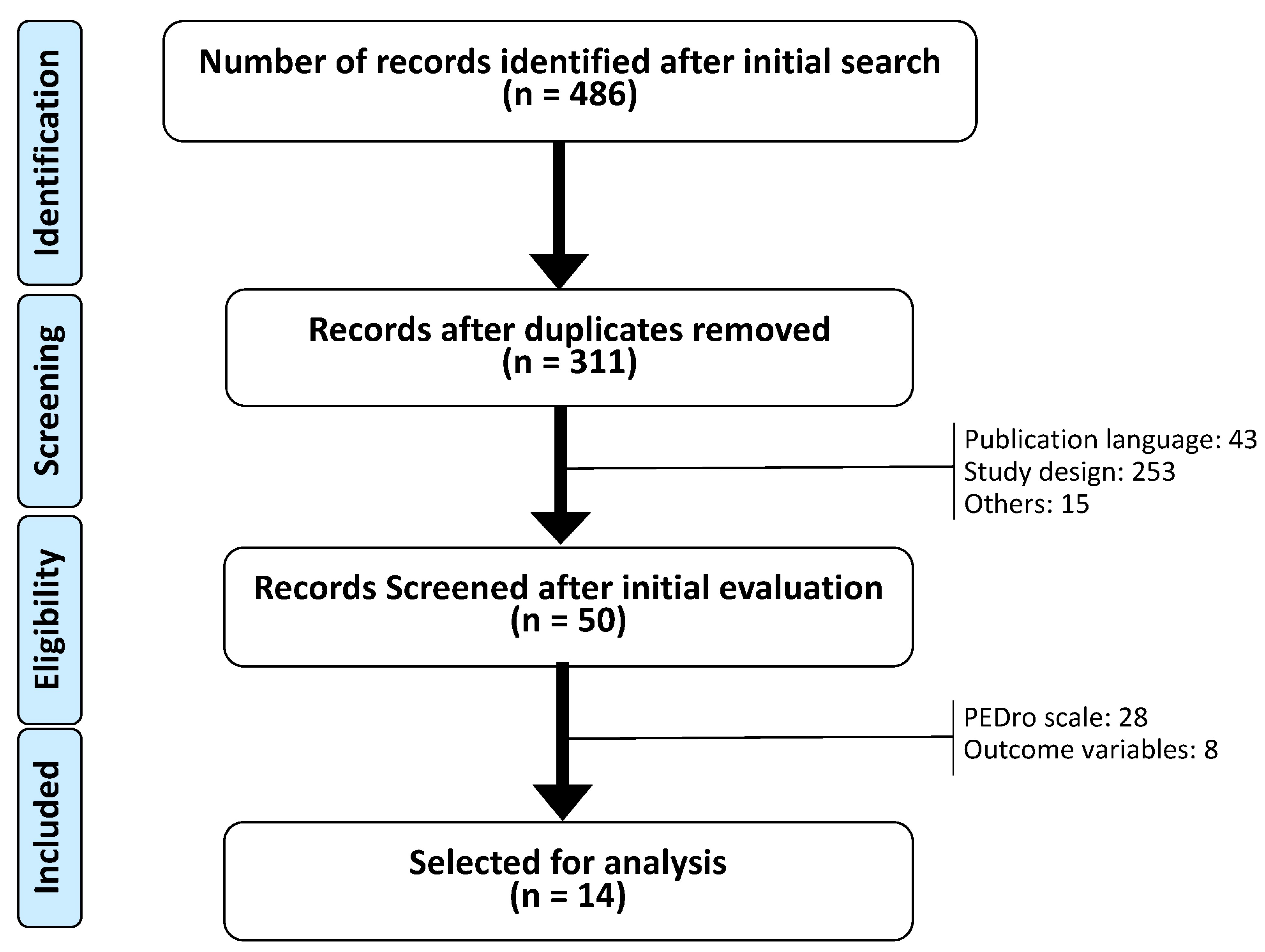
2.2. Selection of Documents
2.3. Selection Method
2.4. Methodological Quality
2.5. Outcomes
3. Results
3.1. Functionality (Objective Outcomes)
3.2. Functionality (Subjective Outcomes)
3.3. Pain
3.4. Quality of Life
4. Discussion
4.1. Functionality
4.1.1. Time
4.1.2. Speed
4.1.3. Distance
4.1.4. Other Objective Functional Outcomes
4.2. Subjective Functionality
4.3. Pain
4.4. Quality of Life
4.5. Physical Activity and BFR Intervention
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caru, M.; Levesque, A.; Lalonde, F.; Curnier, D. An overview of ischemic preconditioning in exercise performance: A systematic review. J. Sport Health Sci. 2019, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Castilla-López, C.; Molina-Mula, J.; Romero-Franco, N. Blood flow restriction during training for improving the aerobic capacity and sport performance of trained athletes: A systematic review and meta-analysis. J. Exerc. Sci. Fit. 2022, 20, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Slysz, J.; Stultz, J.; Burr, J.F. The efficacy of blood flow restricted exercise: A systematic review & meta-analysis. J. Sci. Med. Sports 2016, 19, 669–675. [Google Scholar] [CrossRef]
- Teixeira, E.L.; Barroso, R.; Silva-batista, C.; Laurentino, C.; Loenneke, J.P.; Roschel, H.; Ugrinowitsch, C.; Tricoli, V. Blood Flow Restriction Increases Metabolic Stress But Decreases Muscle Activation During High-Load Resistance Exercise. Muscle Nerve 2017, 57, 107–111. [Google Scholar] [CrossRef]
- Ganesan, G.; Cotter, J.A.; Reuland, W.; Cerussi, A.E.; Tromberg, B.J.; Galassetti, P. Effect of blood flow restriction on tissue oxygenation during knee extension. Med. Sci. Sports Exerc. 2015, 47, 185–193. [Google Scholar] [CrossRef]
- Manini, T.M.; Vincent, K.R.; Leeuwenburgh, C.L.; Lees, H.A.; Kavazis, A.N.; Borst, S.E.; Clark, B.C. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol. 2011, 201, 255–263. [Google Scholar] [CrossRef]
- Heitkamp, H.C. Training with blood flow restriction. Mechanisms, gain in strength and Safety. J. Sports Med. Phys. Fit. 2015, 55, 446–456. [Google Scholar]
- Kacin, A.; Rosenblatt, B.; Žargi, T.G.; Biswas, A. Safety Considerations With Blood Flow Restricted Resistance Training. Ann. Kinesiol. 2015, 6, 3–26. [Google Scholar]
- Yinghao, L.; Jing, Y.; Yongqi, W.; Jianming, Z.; Zeng, G.; Yiting, T.; Shuoqi, L. Effects of a blood flow restriction exercise under different pressures on testosterone, growth hormone, and insulin-like growth factor levels. J. Int. Med. Res. 2021, 49, 03000605211039564. [Google Scholar] [CrossRef]
- Clarkson, M.J.; May, A.K.; Warmington, S.A. Is there rationale for the cuff pressures prescribed for blood flow restriction exercise? A systematic review. Scand. J. Med. Sci. Sports 2020, 30, 1318–1336. [Google Scholar] [CrossRef]
- Centner, C.; Lauber, B. A Systematic Review and Meta-Analysis on Neural Adaptations Following Blood Flow Restriction Training: What We Know and What We Don’t Know. Front. Physiol. 2020, 11, 887. [Google Scholar] [CrossRef]
- Baker, B.S.; Stannard, M.S.; Duren, D.L.; Cook, J.L.; Stannard, J.P. Does Blood Flow Restriction Therapy in Patients Older Than Age 50 Result in Muscle Hypertrophy, Increased Strength, or Greater Physical Function? A Systematic Review. Clin. Orthop. Relat. Res. 2020, 478, 593–606. [Google Scholar] [CrossRef]
- Minniti, M.C.; Statkevich, A.P.; Kelly, R.L.; Rigsby, V.P.; Exline, M.M.; Rhon, D.I.; Clewley, D. The Safety of Blood Flow Restriction Training as a Therapeutic Intervention for Patients With Musculoskeletal Disorders: A Systematic Review. Am. J. Sports Med. 2020, 48, 1773–1785. [Google Scholar] [CrossRef]
- Vinolo-gil, M.J.; Rodr, M.; Martin-vega, F.J.; Garcia-munoz, C.; Lagares-franco, C.; Garcia-campanario, I. Effectiveness of Blood Flow Restriction in Neurological Disorders: A Systematic Review. Healthcare 2022, 10, 2407. [Google Scholar] [CrossRef]
- Van Cant, J.; Dawe-Coz, A.; Aoun, E.; Esculier, J.-F. Quadriceps strengthening with blood flow restriction for the rehabilitation of patients with knee conditions: A systematic review with meta-analysis. J. Back Musculoskelet. Rehabil. 2020, 1, 529–544. [Google Scholar] [CrossRef]
- Clarkson, M.J.; May, A.K.; Warmington, S.A. Chronic Blood Flow Restriction Exercise Improves Objective Physical Function: A Systematic Review. Front. Physiol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Centner, C.; Zdzieblik, D.; Roberts, L.; Gollhofer, A.; König, D. Effects of blood flow restriction training with protein supplementation on muscle mass and strength in older men. J. Sports Sci. Med. 2019, 18, 471–478. [Google Scholar]
- Bobes Álvarez, C.; Issa-khozouz Santamaría, P.; Fernández-Matías, R.; Pecos-martín, D.; Achalandabaso-Ochoa, A.; Fernández-Carnero, S.; Martínez-Amat, A.; Gallego-Izquierdo, T. Comparison of Blood Flow Restriction Training versus Non-Occlusive Training in Patients with Anterior Cruciate Ligament Reconstruction or Knee Osteoarthritis: A Systematic Review. J. Clin. Med. 2020, 10, E68. [Google Scholar] [CrossRef]
- Wortman, R.J.; Brown, S.M.; Savage-Elliott, I.; Finley, Z.J.; Mulcahey, M.K. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2021, 49, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Lixandrao, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Rev. Esp. Nutr. Hum. Diet 2014, 18, 172–181. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Vidal Fuentes, J. Versión actualizada de la definición de dolor de la IASP: Un paso adelante o un paso atrás. Rev. Soc. Esp. Dolor 2020, 27, 232–233. [Google Scholar] [CrossRef]
- Bouça-Machado, R.; Duarte, G.S.; Patriarca, M.; Castro Caldas, A.; Alarcão, J.; Fernandes, R.M.; Mestre, T.A.; Matias, R.; Ferreira, J.J. Measurement Instruments to Assess Functional Mobility in Parkinson’s Disease: A Systematic Review. Mov. Disord. Clin. Pract. 2020, 7, 129–139. [Google Scholar] [CrossRef]
- Haraldstad, K.; Wahl, A.; Andenæs, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef]
- Jørgensen, A.N.; Aagaard, P.; Frandsen, U.; Boyle, E.; Diederichsen, L.P. Blood-flow restricted resistance training in patients with sporadic inclusion body myositis: A randomized controlled trial. Scand. J. Rheumatol. 2018, 47, 400–409. [Google Scholar] [CrossRef]
- Giles, L.; Webster, K.E.; Mcclelland, J.; Cook, J.L. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: A double-blind randomised trial. Br. J. Sports Med. 2017, 51, 1688–1694. [Google Scholar] [CrossRef]
- Curran, M.T.; Bedi, A.; Mendias, C.L.; Wojtys, E.M.; Kujawa, M.V.; Palmieri-Smith, R.M. Blood Flow Restriction Training Applied with High-Intensity Exercise Does Not Improve Quadriceps Muscle Function after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Am. J. Sports Med. 2020, 48, 825–837. [Google Scholar] [CrossRef]
- Korakakis, V.; Whiteley, R.; Giakas, G. Low load resistance training with blood flow restriction decreases anterior knee pain more than resistance training alone. A pilot randomised controlled trial. Phys. Ther. Sport 2018, 34, 121–128. [Google Scholar] [CrossRef]
- Branco Ferraz, R.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; De Sa-Pinto, A.L.; Roschel, H. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef]
- Constantinou, A.; Mamais, I.; Papathanasiou, G. Comparing hip and knee focused exercises versus hip and knee focused exercises with the use of blood flow restriction training in adults with patellofemoral pain. Eur. J. Phys. Rehabil. Med. 2022, 58, 225–235. [Google Scholar] [CrossRef]
- Harper, S.; Roberts, L.; Layne, A.; Jaeger, B.; Gardner, A.; Sibille, K.; Wu, S.S.; Vincent, K.R.; Fillingim, R.B.; Manini, T.M.; et al. Blood-Flow Restriction Resistance Exercise for Older Adults with Knee Osteoarthritis: A Pilot Randomized Clinical Trial. J. Clin. Med. 2019, 8, 265. [Google Scholar] [CrossRef]
- Hughes, L.; Patterson, S.D.; Haddad, F.; Rosenblatt, B.; Gissane, C.; McCarthy, D.; Clarke, T.; Ferris, G.; Dawes, J.; Paton, B. Examination of the comfort and pain experienced with blood flow restriction training during post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: A UK National Health Service trial. Phys. Ther. Sport 2019, 39, 90–98. [Google Scholar] [CrossRef]
- Hughes, L.; Rosenblatt, B.; Haddad, F.; Gissane, C.; McCarthy, D.; Clarke, T.; Ferris, G.; Dawes, J.; Paton, B.; Patterson, S.D. Comparing the Effectiveness of Blood Flow Restriction and Traditional Heavy Load Resistance Training in the Post-Surgery Rehabilitation of Anterior Cruciate Ligament Reconstruction Patients: A UK National Health Service Randomised Controlled Trial. Sports Med. 2019, 49, 1787–1805. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Donadi, M.; Tanaka, H.; Basaglia, N.; Manfredini, F. Effectiveness of blood flow-restricted slow walking on mobility in severe multiple sclerosis: A pilot randomized trial. Scand. J. Med. Sci. Sports 2020, 30, 1999–2009. [Google Scholar] [CrossRef]
- Mason, J.S.; Crowell, M.S.; Brindle, R.A.; Dolbeer, J.A.; Miller, E.M.; Telemeco, T.A.; Goss, D.L. The Effect of Blood Flow Restriction Training on Muscle Atrophy following Meniscal Repair or Chondral Restoration Surgery in Active Duty Military: A Randomized Controlled Trial. J. Sport Rehabil. 2022, 31, 77–84. [Google Scholar] [CrossRef]
- Rodrigues, R.; Ferraz, R.B.; Kurimori, C.O.; Guedes, L.K.; Lima, F.R.; de Sá-Pinto, A.L.; Gualano, B.; Roschel, H. Low-load resistance training with blood flow restriction increases muscle function, mass and functionality in women with rheumatoid arthritis. Arthritis Care Res. 2019, 72, 787–797. [Google Scholar] [CrossRef]
- Segal, N.A.; Williams, G.N.; Davis, M.C.; Wallace, R.B.; Mikesky, A.E. Efficacy of Blood Flow-Restricted, Low-Load Resistance Training in Women with Risk Factors for Symptomatic Knee Osteoarthritis. PM&R 2015, 7, 376–384. [Google Scholar] [CrossRef]
- Segal, N.; Davis, M.D.; Mikesky, A.E. Efficacy of Blood Flow-Restricted Low-Load Resistance Training For Quadriceps Strengthening in Men at Risk of Symptomatic Knee Osteoarthritis. Geriatr. Orthop. Surg. Rehabil. 2015, 6, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Tennent, D.J.; Hylden, C.M.; Johnson, A.E.; Burns, T.C.; Wilken, J.M.; Owens, J.G. Blood flow restriction training after knee arthroscopy: A randomized controlled pilot study. Clin. J. Sport Med. 2017, 27, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, C.R.; Peiffer, J.J.; Meeusen, R.; Skorski, S. Role of Ratings of Perceived Exertion during Self-Paced Exercise: What are We Actually Measuring? Sports Med. 2015, 45, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood flow restriction exercise position stand: Considerations of methodology, application, and safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Helms, E.; Fitschen, P.J.; Aragon, A.; Cronin, J.; Schoenfeld, B.J. Recommendations for Natural Bodybuilding Contest Preparation: Resistance and Cardiovascular Training. J. Sports Med. Phys. Fit. 2013, 55, 164–178. [Google Scholar]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Hobart, J.; Blight, A.R.; Goodman, A.; Lynn, F.; Putzki, N. Timed 25-Foot Walk meaningful in MS. Neurology 2013, 80, 1509–1517. [Google Scholar] [CrossRef]
- Kirkland, M.C.; Chen, A.; Downer, M.B.; Holloway, B.J.; Wallack, E.M.; Lockyer, E.J.; Buckle, N.C.; Abbott, C.L.; Ploughman, M. Bipedal hopping timed to a metronome to detect impairments in anticipatory motor control in people with mild multiple sclerosis. Clin. Biomech. 2018, 55, 45–52. [Google Scholar] [CrossRef]
- Sayers, S.P.; Gibson, K.; Cook, C.R. Effect of high-speed power training on muscle performance, function, and pain in older adults with knee osteoarthritis: A pilot investigation. Arthritis Care Res. 2012, 64, 46–53. [Google Scholar] [CrossRef]
- Campbell, C.M.; Buenaver, L.F.; Finan, P.; Bounds, S.C.; Redding, M.; McCauley, L.; Robinson, M.; Edwards, R.R.; Smith, M.T. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care Res. 2015, 67, 1387–1396. [Google Scholar] [CrossRef]
- Brandner, C.R.; Warmington, S.A.; Kidgell, D.J. Corticomotor Excitability is Increased Following an Acute Bout of Blood Flow Restriction Resistance Exercise. Front. Hum. Neurosci. 2015, 9, 652. [Google Scholar] [CrossRef]
- Urbach, D.; Awiszus, F. Impaired Ability of Voluntary Quadriceps Activation Bilaterally Interferes with Function Testing after Knee Injuries. A Twitch Interpolation Study. Int. J. Sports Med. 2002, 23, 231–236. [Google Scholar] [CrossRef]
- Zult, T.; Gokeler, A.; Van Raay, J.J.; Brouwer, R.W.; Zijdewind, I.; Farthing, J.P. Cross-education does not accelerate the rehabilitation of neuromuscular functions after ACL reconstruction: A randomized controlled clinical trial. Eur. J. Appl. Physiol. 2018, 118, 1609–1623. [Google Scholar] [CrossRef]
- Glasgow, P.; Phillips, N.; Bleakley, C. Optimal loading: Key variables and mechanisms. Br. J. Sports Med. 2015, 49, 278–279. [Google Scholar] [CrossRef]
- Hurkmans, E.; van der Giesen, F.J.; Vlieland, T.P.V.; Schoones, J.; Van den Ende, E.C. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis (Review). Cochrane Database Syst. Rev. 2009, 4. [Google Scholar] [CrossRef]
- Hughes, L.; Patterson, S.D. The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J. Appl. Physiol. 2020, 128, 914–924. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Thiebaud, R.S.; Mattocks, K.T.; Abe, T.; Bemben, M.G. Blood flow restriction pressure recommendations: A tale of two cuffs. Front. Physiol. 2013, 4, 249. [Google Scholar] [CrossRef]
- Gundermann, D.M.; Fry, C.S.; Dickinson, J.M.; Walker, D.K.; Timmerman, K.L.; Drummond, M.J.; Volpi, E.; Rasmussen, B.B. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J. Appl. Physiol. 2012, 112, 1520–1528. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Iida, H. Effect of low-load resistance exercise with and without blood flow restriction to volitional fatigue on muscle swelling. Eur. J. Appl. Physiol. 2014, 115, 919–926. [Google Scholar] [CrossRef]
- Jones, F.; Harris, P.; Waller, H.; Coggins, A. Adherence to an exercise prescription scheme: The role of expectations, self-efficacy, stage of change and psychological well-being. Br. J. Health Psychol. 2005, 10, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Lauver, J.D.; Cayot, T.E.; Rotarius, T.R.; Scheuermann, B.W. Acute Neuromuscular and Microvascular Responses to Concentric and Eccentric Exercises with Blood Flow Restriction. J. Strength Cond. Res. 2020, 34, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Andersen, C.H.; Sundstrup, E.; Jakobsen, M.D.; Mortensen, O.S.; Zebis, M.K. Central Adaptation of Pain Perception in Response to Rehabilitation of Musculoskeletal Pain: Randomized Controlled Trial. Pain Physician 2012, 15, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.B.; Reeves, G.V.; Clavier, J.D.; Francois, M.R.; Thomas, C.; Kraemer, R.R. Partial Occlusion during resistance exercise alters effort sense and pain. J. Strength Cond. Res. 2010, 24, 235–243. [Google Scholar] [CrossRef]
- Van der Heijden, R.A.; Lankhorst, N.E.; van Linschoten, R.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst. Rev. 2015, 1, CD010387. [Google Scholar] [CrossRef]
- Ferlito, J.V.; Pecce, S.A.P.; Oselame, L.; De Marchi, T. The blood flow restriction training effect in knee osteoarthritis people: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1378–1390. [Google Scholar] [CrossRef]
- Lu, Y.; Patel, B.H.; Kym, C.; Nwachukwu, B.U.; Beletksy, A.; Forsythe, B.; Chahla, J. Perioperative Blood Flow Restriction Rehabilitation in Patients Undergoing ACL Reconstruction: A Systematic Review. Orthop. J. Sports Med. 2020, 8, 2325967120906822. [Google Scholar] [CrossRef]
- Sallis, J.F.; Kraft, K.; Linton, L.S. How the environment shapes physical activity. A transdisciplinary research agenda. Am. J. Prev. Med. 2002, 22, 208. [Google Scholar] [CrossRef]
| Random Allocation | Concealed Allocation | Baseline Comparability | Blind Subjects | Blind Therapists | Blinding Assessors | Adequate Follow-Up | Intention to Treat | Between-Group Comparisons | Variability Outcome | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Branco Ferraz et al. [33] | • | - | • | • | - | - | - | • | • | • | 6 |
| Constantinou et al. [34] | • | • | • | - | • | • | • | - | • | • | 8 |
| Giles et al. [30] | • | • | • | - | • | • | • | • | • | • | 9 |
| Harper et al. [35] | • | - | • | • | - | - | - | • | • | • | 6 |
| Hughes et al. [36] | • | • | • | • | - | - | • | - | • | • | 7 |
| Hughes et al. [37] | • | • | • | • | - | - | • | - | • | • | 7 |
| Jorgensen et al. [29] | • | - | - | • | - | - | • | • | • | • | 6 |
| Korakakis et al. [32] | • | • | • | • | - | - | • | • | • | • | 8 |
| Lamberti et al. [38] | • | • | • | • | - | - | • | • | • | • | 8 |
| Mason et al. [39] | • | • | • | - | - | • | • | - | • | • | 7 |
| Rodrigues et al. [40] | • | - | • | • | - | - | • | • | • | • | 7 |
| Segal et al. [41] | • | • | • | • | - | - | • | - | • | • | 7 |
| Segal et al. [42] | • | • | • | • | - | - | • | - | • | • | 7 |
| Tennent et al. [43] | • | • | • | • | - | - | • | - | • | • | 7 |
| Author | Size | Age | Pressure Cuff | Interventions | Frequency Sessions | Development Interventions | Pathology |
|---|---|---|---|---|---|---|---|
| Branco Ferraz et al. [33] | n = 48 EG₁: 16 EG₂: 16 BFR: 16 | EG₁: 59.9 ± 4 | EG₁: High-intensity workout | 20 min/ss 2 ss/w Time: 12 w | EG₁: 1 week (4 s, 10 reps, 50% 1RM), 2 week (4 s, 10 reps, 80% 1RM), 5 week (5 s, 10 reps, 80% 1RM). | Knee osteoarthritis | |
| EG₂: 60.7 ± 4 | EG₂: Low-intensity workout | EG₂: 1 week (4 s, 15 reps, 25% 1RM), 2 week (4 s, 15 reps, 30% 1RM), 5 week (5 s, 15 reps, 30% 1RM) | |||||
| BFR: 60.3 ± 3 | 70% LOP | BFR: EG₂ + BFR. | BFR: EG₂ + BFR. | ||||
| Constantinou et al. [34] | n = 60 CON: 30 BFR: 30 | CON: 30.5 (18–40) | CON: High-load workout | 3 ss/w Time: 4 w F/U: 8 w | CON: Hip and knee exercise program 70% 1RM, 3 s, 10 reps, 30 s rest/s. Rep: 1 s concentric–2 s eccentric. | Patellofemoral pain | |
| BFR: 25.5 (18–40) | 70% LOP | BFR: BFR + Low-load workout | BFR: Hip and knee exercise program + BFR 30% 1RM, 4 s (reps: 30,15,15,15), 30 s rest/s, 2 min rest/ex. | ||||
| Curran et al. [31] | n = 34 EG₁: 8 EG₂: 8 BFR: 9 EG₃: 9 | EG₁: 16.1 ± 2.6 | EG₁: Concentrics. | 2 ss/w Time: 8 w | EG₁: 1 s 20% 1RM (PC) + 4 s leg press 70% 1RM concentric–20% 1RM eccentric. | Anterior cruciate ligament reconstruction | |
| EG₂: 18.8 ± 3.9 | EG₂: Eccentrics. | EG₂: PC+ 4 s leg press 20% 1RM concentric-70% 1RM eccentric. | |||||
| BFR: 15.3 ± 0.9 | 80% LOP | BFR: Concentrics + BFR | BFR: PC + 4 s leg press 70% 1RM concentric-20% 1RM eccentric + BFR. | ||||
| EG₃: 16.0 ± 1.7 | EG₃: Eccentrics + BFR | EG₃: PC+ 4 s leg press 20% 1RM concentric-70% 1RM eccentric + BFR. | |||||
| Giles et al. [30] | n = 79 EG₁: 39 BFR: 40 | EG₁: 26.7 ± 5.5 | EG₁: Strength training | Trt: 3 ss/w, 8 w (6 individual ss/1–3 w) F/U: 16 w | EG1: 5 min bicycle, leg press 0–60°, y knee extension 45–90°; VAS +2/10 > ↓ 20% load (PC) + 3 s, 7–10 reps, 70% 1RM, placebo BFR (2 fingers skin/cuff). | Patellofemoral pain | |
| BFR: 28.5 ± 5.2 | 60% LOP | BFR: EG₁ + BFR | BFR: PC + 1 s (30 reps or volitive fatigue), 3 s (15 reps), 30% 1RM, 30 s rest. | ||||
| Harper et al. [35] | n = 35 EG₁: 19 BFR: 16 | EG₁: 69.1 ± 7.1 | EG₁: Moderate-resistance training | 3 ss/w Time: 12 w | EG₁: wmup + leg press, leg extension, leg curl, and calf flexion at 60% 1RM + Flexibility–Balance ex. | Knee osteoarthritis | |
| BFR: 67.2 ± 5.2 | Pressure mm Hg = 0.5 (SBP) + 2(thigh circumference) + 5 | BFR: EG₁ + BFR | BFR: EG₁ + BFR 20% 1RM (↓ pression/s). | ||||
| Hughes et al. [36] | n = 28 EG₁: 14 BFR: 14 | EG₁: 29 ± 7 | EG₁: High-resistance training | 2 ss/w (48 h rest/ss) Time: 8 w | EG₁: 5 min bicycle no resistance and 10 reps unilateral leg press-low load, 5 min rest (PC) + unilateral leg press 70% 1RM, 3 s, 10 reps, 30 s rest. | Anterior cruciate ligament reconstruction | |
| BFR: 29 ± 7 | 80% LOP | BFR: EG₁ + BFR | BFR: PC + EG₁ + BFR 30% 1RM, 4 s (reps: 30, 15, 15, 15). | ||||
| Hughes et al. [37] | n = 28 EG₁: 14 BFR: 14 | EG₁: 29 ± 7 | EG₁: High resistance training | 2 ss/w (48 h rest/ss) Time: 8 w | EG₁: 5 min bicycle no resistance and 10 reps unilateral leg press-low load, 5 min rest (PC) + unilateral leg press 70% 1RM, 3 s, 10 reps, 30 s rest. | Anterior cruciate ligament reconstruction | |
| BFR: 29 ± 7 | 80% LOP | BFR: EG₁ + BFR | BFR: PC + EG₁ + BFR 30% 1RM, 4 s (reps: 30, 15, 15, 15). | ||||
| Jørgensen et al. [29] | n = 22 CON: 11 BFR: 11 | CON: 69.8 ± 4.8 | CON: No workout. | 2 ss/w Time: 12 w F/U: 12 w | CON: Nothing. | Sporadic inclusion body myositis | |
| BFR: 68.1 ± 6.4 | 110 mm Hg | BFR: Strength training + BFR | BFR: leg press, knee extension, knee flexion (4 w), calf raise, and dorsal flexion. 3 s × 25 reps (9 w: 4 s) | ||||
| Korakakis et al. [32] | n = 40 EG₁: 20 BFR: 20 | EG₁: 29.7 ± 7.6 | EG₁: Low-resistance training | 1 session | EG₁: Knee extension open-kinetic chain. 4 s (reps: max reps, 15, 15, 15), 30 s rest. Max load 5 kg, VAS 4/10. Rep: 2 s concentric, 2 s eccentric metronome. | Anterior knee pain | |
| BFR: 29.1 ± 6.6 | 80% LOP | BFR: EG₁ + BFR | BFR: EG₁ + BFR | ||||
| Lamberti et al. [38] | n = 22 CON: 11 BFR: 11 | CON: 56 ± 10 | CON: Physiotherapy assisted walking | 2 ss/w Time: 6 w F/U: 6 w | CON: PC + 40 min physiotherapy assisted walking-60 m corridor. Rest: 8/10 RPE on chair. | Severe multiple sclerosis | |
| BFR: 54 ± 11 | 30% systolic blood pressure | BFR: Walking interval-metronome + BFR | BFR: 10 min wmup (PC) + 5 cycles (3 reps: 1 min work y 1 min rest. 3 min rest cycle deflated BFR) low velocity-walking (60 steps/min-metronome) + 10 min cool down and stretching CORE (PC). | ||||
| Mason et al. [39] | n = 17 CON: 9 BFR: 8 | CON: 24 (20–28) | CON: Resistance exercises | 2–3 ss/w Time: 12 w F/U: 12 w | CON: 4 s (reps: 30, 15, 15, 15), plus 5 lb if 75 reps in less than 5 min. Ph 1: Isometric quadriceps, and flex-ext and abd-add hip straight leg raises; Ph 2: Ph 1 + knee extension 45°–90°; Ph 3: Ph 1 + Ph 2 + hamstring curls; Ph 4: Full weight-bearing, and squats and single leg press up to 60° knee flexion. | Meniscal repair surgery | |
| BFR: 23 (20–26) | 80% LOP | BFR: CON + BFR | BFR: CON + BFR | ||||
| Rodrigues et al. [40] | n = 48 CON: 16 EG₁: 16 BFR: 16 | CON: 58.1 ± 5.9 | CON: No workout | 2 ss/w Time: 12 w | CON: Activities of daily living. | Rheumatoid arthritis | |
| EG₁: 58.0 ± 6.6 | EG₁: High-load workout | EG₁: Bilateral leg press and knee extension. 1 Week: 4 s, 10 reps, 50% 1RM; 2 Week: 4 s, 10 reps, 70% 1RM; 5 Week: 5 s, 10 reps, 70% 1RM. | |||||
| BFR: 59.6 ± 3.9 | 70% LOP | BFR: Low-load workout + BFR | BFR: EG₁. (1 Week: 4 s, 15 reps, 20% 1RM; 2 Week: 4 s, 15 reps, 30% 1RM; 5 Week: 5 s, 15 reps, 30% 1RM) | ||||
| Segal et al. [41] | n = 42 CON: 22 BFR: 20 | CON: 56.1 ± 7.7 | CON: Low-load workout | 3 ss/w Time: 4 w F/U: 3 d | CON: Leg press 30% 1RM: 4 s (reps: 30, 15, 15, 15), 30 s rest. Rep: 2 s concentric and 2 s eccentric. | Knee osteoarthritis | |
| BFR: 58.4 ± 8.7 | 1 Week: 160 mm Hg 2 Week: 180 mm Hg 3 Week: 200 mm Hg | BFR: CON + BFR. | BFR: CON + BFR. | ||||
| Segal et al. [42] | n = 45 CON: 24 BFR: 21 | CON: 54.6 ± 6.9 | CON: Low-load workout | 3 ss/w Time: 4 w F/U: 3 d | CON: Leg press 30% 1RM: 4 s (reps: 30, 15, 15, 15), 30 s rest. Rep: 2 s concentric–2 s eccentric. | Knee osteoarthritis | |
| BFR: 56.1 ± 5.9 | 1 Week: 160 mm Hg 2 Week: 180 mm Hg 3 Week: 200 mm Hg | BFR: CON + BFR. | BFR: CON + BFR. | ||||
| Tennent et al. [43] | n = 24 CON: 13 BFR: 11 | CON: 37.0 (32–47) | CON: Physiotherapy | 12 ss Time: 6 w | CON: Immediate weight loading, immediate formal physiotherapy and no range of motion restrictions. | Non-reconstructive knee arthroscopy | |
| BFR: 37.0 (30–46.2) | 80% LOP | BFR: Physiotherapy + (Strength training + BFR) | BFR: CON + 4 sets (reps: 30, 15, 15, 15), 30% 1RM, 30 s rest-1 min rest/ex. (leg press, leg extension, and reverse press). 5 min max. occlusion/ex. |
| Measurement Tool | Article | Group | Baseline | Measurements (sd/ci 95%) | Follow-Up (sd/ci 95%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–6 Week | 6–12 Weeks | 3–6 Months | 1–3 Months | 3–6 Months | >6 Months | |||||
| TIME | Time Up and Go Test (s) | Branco Ferraz et al. [33] | EG₁ | 6.75 ± 1.5 * | - | 6.5 ± 1 * | - | - | - | - |
| EG₂ | 7 ± 0.8 * | - | 6.85 ± 0.7 * | - | - | - | - | |||
| BFR | 6.9 ± 0.6 * | - | 6.65 ± 0.35 * | - | - | - | - | |||
| Rodrigues et al. [40] | EG₁ | 7.15 ± 0.7 * | - | 6.4 ± 0.6 * | - | - | - | - | ||
| BFR | 7.25 ± 1.4 * | - | 6.7 ± 0.95 * | - | - | - | - | |||
| CON | 7.35 ± 1.1 * | - | 7.3 ± 1.2 * | - | - | - | - | |||
| TSA (s) | Tennent et al. [43] | BFR | 9.50 (5.9–12.9) | 5.11 (4.5–8.0) | - | - | - | - | - | |
| CON | 5.84 (4.5–8.0) | 4.92 (4.0–7.1) | - | - | - | - | - | |||
| FSST | Tennent et al. [43] | BFR | 7.39 (6.5–10.0) | 5.89 (5.6–6.8) | - | - | - | - | - | |
| CON | 8.45 (7.2–9.4) | 6.36 (5.9–7.6) | - | - | - | - | - | |||
| STS5 (s) | Tennent et al. [43] | BFR | 10.62 (9.6–12.7) | 7.77 (6.5–9.3) | - | - | - | - | - | |
| CON | 11.27 (10.0–13.0) | 7.98 (7.6–10.1) | - | - | - | - | - | |||
| Lamberti et al. [38] | BFR | 24 (8–40) | 18 (7–28) | - | - | 20 (5–35) | - | - | ||
| CON | 27 (1–53) | 23 (3–44) | - | - | 24 (2–46) | - | - | |||
| SPEED | 400 m walk gait speed (m/s) | Harper et al. [35] | EG₁ | 1.05 * | 1.05 ± 0.035 * | 1.02 ± 0.045 * | - | - | - | - |
| BFR | 1.05 * | 0.955 ± 0.03 * | 1.005 ± 0.4 * | - | - | - | - | |||
| T25FW (m/s) | Lamberti et al. [38] | BFR | 0.78 (0.54–1.03) | 0.90 (0.64–1.16) | - | - | 0.87 (0.62–1.12) | - | - | |
| CON | 0.76 (0.51–0.99) | 0.79 (0.54–1.03) | - | - | 0.76 (0.49–1.02) | - | - | |||
| SSWV (m/s) | Tennent et al. [43] | BFR | 1.31 (0.9–1.6) | 1.80 (1.5–2.0) | - | - | - | - | - | |
| CON | 1.45 (1.6–1.3) | 1.91 (1.6–1.4) | - | - | - | - | - | |||
| DISTANCE | 6-Min Walk Test (m) | Lamberti et al. [38] | BFR | 215 (153–278) | 264 (188–340) | - | 266 (186–345) | - | - | |
| CON | 183 (120–245) | 218 (152–285) | - | 223 (155–291) | - | - | ||||
| Modified SEBT (%LL) | Hughes et al. (2019) [36] | EG₁ | - | ANT-N: 7.5 ± 8.0 ANT-I: 9.0 ± 3.5 PM-N: 8.5 ± 7.2 PM-I: 5.5 ± 5.2 PL-N: 9.8 ± 9.7 PL-I: 5.8 ± 8.0 | ANT-N: 10.5 ± 9.2 ANT-I: 17.5 ± 6.7 PM-N: 12.8 ± 9.1 PM-I: 13.9 ± 7.7 PL-N: 14.5 ± 10.1 PL-I: 13.2 ± 10.3 | - | - | - | - | |
| BFR | - | ANT-N: 8.4 ± 5.1 ANT-I: 22.3 ± 5.2 PM-N: 11.6 ± 8.1 PM-I: 19.1 ± 9.2 PL-N: 13.0 ± 15.6 PL-I: 23.3 ± 12.5 | ANT-N: 18.7 ± 9.3 ANT-I: 32.9 ± 9.7 PM-N: 22.4 ± 13.7 PM-I: 32.1 ± 15.1 PL-N: 23.8 ± 17.8 PL-I: 34.8 ± 15.3 | - | - | - | - | |||
| REPETITIONS | Timed Stand Test (reps) | Branco Ferraz et al. [33] | EG₁ | 14.25 ± 3.75 * | - | 16.5 ± 4.5 * | - | - | - | - |
| EG₂ | 13 ± 2.5 * | - | 14 ± 2.5 * | - | - | - | - | |||
| BFR | 13.5 ± 2.5 * | - | 15 ± 2 * | - | - | - | - | |||
| Rodrigues et al. [40] | EG₁ | 13.25 ± 2.5 * | - | 15.25 ± 2.65 * | - | - | - | - | ||
| BFR | 14.5 ± 3.25 * | - | 16 ± 2.8 * | - | - | - | - | |||
| CON | 13.75 ± 3.75 * | - | 13.5 ± 2.6 * | - | - | - | - | |||
| POWER | Stair Climb Power (W) | Segal et al. (2015) [41] | BFR | 364.3 ± 71.2 | - | - | - | 29.3 ± 11.6 ¨ | - | - |
| CON | 404.3 ± 118.4 | - | - | - | 53.4 ± 11.0 ¨ | - | - | |||
| Measurement Tool | Article | Group | Baseline | Measurements (sd/ci 95%) | Follow-Up (sd/ci 95%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–6 Week | 6–12 Weeks | 3–6 Months | 1–3 Months | 3–6 Months | >6 Months | |||||
| FUNCTIONALITY | Short Physical Performance Battery (0–12) | Harper et al. [35] | EG₁ | 10.2 ± 1.9 | - | 0.2 ± 0.3 * | - | - | - | - |
| BFR | 10.4 ± 1.9 | - | 0.8 ± 0.5 * | - | - | - | - | |||
| LLFDI (30–80) | Harper et al. [35] | EG₁ | - | - | TF: 0.2 ± 2 * TL: 1 ± 3.75 * | - | - | - | - | |
| BFR | - | - | TF: -0.5 ± 1.65 * TL: 7.5 ± 2.7 * | - | - | - | - | |||
| IKDC (0–100) | Hughes et al. [36] | EG₁ | - | 13.50 ± 7.42 | 23.33 ± 8.76 | - | - | - | - | |
| BFR | - | 22.44 ± 5.27 | 35.63 ± 7.06 | - | - | - | - | |||
| Curran et al. [31] | EG₁ | - | - | 19.98 ± 17.30 | - | - | - | - | ||
| EG₂ | - | - | 15.81 ± 18.02 | - | - | - | - | |||
| BFR | - | - | 9.97 ± 15.96 | - | - | - | - | |||
| EG₃ | - | - | 13.69 ± 18.12 | - | - | - | - | |||
| LEFS (0–80) | Hughes et al.) [36] | EG₁ | - | 14.69 ± 7.76 | 21.83 ± 7.06 | - | - | - | - | |
| BFR | - | 21.46 ± 10.68 | 31.08 ± 12.22 | - | - | - | - | |||
| Mason et al. [39] | CON | - | - | 9 ± 15 ^ | - | - | 19 ± 6 ^ | - | ||
| BFR | - | - | 20 ± 12^ | - | - | 8 ± 10 ^ | - | |||
| KOOS (0–100) | Hughes et al. [36] | EG₁ | - | P: 11.67 ± 6.11 S: 12.17 ± 5.91 ADL: 11.17 ± 6.28 QOL: 12.50 ± 13.85 | P: 22.00 ± 7.48 S: 24.50 ± 7.62 ADL: 21.75 ± 6.90 QOL: 20.31 ± 12.82 | - | - | - | - | |
| BFR | - | P: 30.25 ± 9.29 S: 22.17 ± 11.65 ADL: 21.83 ± 8.35 QOL: 15.10 ± 10.81 | P: 39.75 ± 11.74 S: 33.33 ± 13.60 ADL: 32.33 ± 10.37 QOL: 29.58 ± 14.81 | - | - | - | - | |||
| Lysholm Knee- Scoring Scale (0–100) | Hughes et al. [36] | EG₁ | - | 17.25 ± 9.96 | 29.50 ± 12.07 | - | - | - | - | |
| BFR | - | 29.75 ± 12.86 | 44.58 ± 14.75 | - | - | - | - | |||
| Tegner Activity Scale (0–10) | Hughes et al. [36] | EG₁ | 7.42 ± 1.24 | - | - | - | - | - | - | |
| BFR | 6.83 ± 1.80 | - | - | - | - | - | - | |||
| IBMFRS (0–40) | Jørgensen et al. [29] | CON | 10-I: 30.4 ± 4.4 5-I: 29.7 ± 4.9 | - | 10-I: - 5-I: - | - | 10-I: 13.0 ± 3.4 5-I: 13.2 ± 3.3 | - | - | |
| BFR | 10-I: 31.6 ± 5.7 5-I: 14.0 ± 3.9 | - | 10-I: - 5-I: - | - | 10-I: 32.5 ± 4.9 5-I: 14.6 ± 3.5 | - | - | |||
| HAQ (0–3) | Jørgensen et al. [29] | CON | 1.05 ± 0.85 | - | - | - | 1.02 ± 0.79 | - | - | |
| BFR | 0.77 ± 0.58 | - | - | - | 0.89 ± 0.73 | - | - | |||
| Rodrigues et al. [40] | EG₁ | 0.38 ” | - | 0.23 ” | - | - | - | - | ||
| BFR | 0.36 ” | - | 0.16 ” | - | - | - | - | |||
| CON | 0.38 ” | - | 0.23 ” | - | - | - | - | |||
| BBS (0–56) | Lamberti et al. [38] | BFR | 48 (43–54) | 50 (45–54) | - | - | 48 (42–54) | - | - | |
| CON | 44 (39–50) | 46 (39–54) | - | - | 45 (37–53) | - | - | |||
| Objective Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement Tool | Article | Group | Baseline | Measurements (sd/ci 95%) | Follow-Up (sd/ci 95%) | ||||
| 0–6 Week | 6–12 Weeks | 3–6 Months | 1–3 Months | 3–6 Months | >6 Months | ||||
| KOOS (0-100) | Segal et al. [41] | BFR | 83.3 ± 15.4 | 2.9 ± 10.0 ¨ | - | - | 4.9 ± 3.3 ¨^ | - | - |
| CON | 76.6 ± 22.1 | 5.6 ± 11.7 ¨ | - | - | 14.2 ± 7.2 ¨^ | - | - | ||
| Segal et al. [42] | BFR | 80.5 ± 16.9 | - | - | - | 2.0 ± 2.8 ¨ | - | - | |
| CON | 76.0 ± 20.0 | - | - | - | 1.8 ± 2.7 ¨ | - | - | ||
| Tennent et al. [43] | BFR | P: 52.8 (40.3–61.8) S: 47.10 (42.0–64.3) ADL: 58.08 (44.5–72.1) QOL: 31.3 (15.6–46.9) SP: 10.00 (0–33.75) | P: 75.0 (58.3–84.7) S: 76.8 (58.9–89.3) ADL: 88.24 (50.4–95.2) QOL: 59.34 (46.9–70.3) SP: 47.5 (37.5–71.25) | - | - | - | - | - | |
| CON | P: 69.40 (66.7–72.2) S: 67.90 (39.3–75) ADL: 73.50 (66.2–75.0) QOL: 43.80 (31.25–50) SP: 35.00 (10.0–45.0) | P: 77.80 (61.1–91.7) S: 71.40 (46.4–89.3) ADL: 75.00 (63.2–98.5) QOL: 62.50 (37.5–81.25) SP: 70.00 (10.0–90.0) | - | - | - | - | - | ||
| VAS (0–100 mm) | Giles et al. [41] | EG₁ | WP: 51.4 ± 15.3 P-ADL: 42.5 ± 22.8 | - | WP: 29.2 ± 25.6 P-ADL: 23.5 ± 24.1 | - | - | WP: 25.8 ± 27.1 P-ADL: 23.9 ± 25.4 | - |
| BFR | WP: 55.7 ± 13.9 P-ADL: 58.2 ± 17.5 | - | WP: 27.4 ± 20.1 P-ADL: 21.6 ± 25.0 | - | - | WP: 28.1 ± 25.5 P-ADL: 31.7 ± 26.6 | - | ||
| Rodrigues et al. [40] | EG₁ | 3.22 ” | - | 3.15 | - | - | - | - | |
| BFR | 4.73 | - | 2.30 | - | - | - | - | ||
| CON | 2.59 | - | 2.81 | - | - | - | - | ||
| DAS-28 (0–10) | Rodrigues et al. [40] | EG₁ | 2.76 ± 0.79 | - | - | - | - | - | - |
| BFR | 2.72 ± 1.0 | - | - | - | - | - | - | ||
| CON | 2.66 ± 0.8 | - | - | - | - | - | - | ||
| Kujala Patellofemoral Score (0–100) | Giles et al. [41] | EG₁ | 72.6 ± 10.5 | - | 83.2 ± 12.3 | - | - | 85.9 ± 13.3 | - |
| BFR | 73.6 ± 9.9 | - | 86.5 ± 10.5 | - | - | 84.4 ± 12.0 | - | ||
| Constantinou et al. [34] | CON | 74.1 (71.66- 76.54) | 94.1 (92.25–96.09) | - | - | 98.7 (97.38–99.95) | - | - | |
| BFR | 72.7 (69.89- 75.57) | 94.9 (93.19–96.61) | - | - | 98.9 (97.81–99.99) | - | - | ||
| MDI (0–38) | Jørgensen et al. [29] | CON | GD: 0.17 ± 0.04 PGD: 55.2 ± 17.8 PHGD: 52.8 ± 8.5 | - | - | - | GD: 0.17 ± 0.07 PGD: 46.9 ± 15.7 PHGD: 35.8 ± 9.7 | - | - |
| BFR | GD: 0.18 ± 0.05 PGD: 48.5 ± 12.1 PHGD: 45.0 ± 18.2 | - | - | - | GD: 0.19 ± 0.06 PGD: 28.3 ± 10.7 PHGD: 33.0 ± 19.0 | - | - | ||
| NPRS (0–10) | Korakakis et al. [32] | CON | SLS-S: 3.8 ± 2.3 SLS-D: 5.1 ± 1.8 SDT: 4.1 ± 2.6 | SLS-S: 2.6 ± 2.7/2.5 ± 2.3 SLS-D: 4.2 ± 2.2/4.0 ± 2.2 SDT: 2.2 ± -2.2/2.9 ± 2.2 | - | - | - | - | - |
| BFR | SLS-S: 4.6 ± 2.3 SLS-D: 5.6 ± 2.6 SDT: 4.2 ± 2.4 | SLS-S: 2.0 ± 1.6/2.0 ± 1.5 SLS-D: 2.9 ± 2.3/3.7 ± 2.3 SDT: 3.0 ± 2.5/2.2 ± 2.1 | - | - | - | - | - | ||
| Pain Börg Scale (0–11) | Hughes et al. [37] | EG₁ | - | MP: 0.7 ± 0.4 (I)/1.6 ± 0.6 (NI) * KP: 2.6 ± 1 (I)/3.3 ± 1 (NI) * | MP: 0.8 ± 0.4 (I)/0.9 ± 0.5 (NI) * KP: 1.3 ± 0.95 (I)/0.3 ± 0.3(NI) * | - | - | - | - |
| BFR | - | MP: 4 ± 1.5 (I)/4.75 ± 1 (NI) * KP: 0.4 ± 0.5 (I)/0.3 ± 0.2 (NI) * | MP: 3.75 ± 1.5 (I)/4.3 ± 0.9 (NI) * KP: 0.1 ± 0.2 (I)/0.05 ± 0.1 (NI) * | - | - | - | - | ||
| WOMAC Pain Subscale (0–20) | Harper et al. [35] | EG₁ | 7.23 ± 4.87 | - | 0.3 ± 1.4 | - | - | - | - |
| BFR | 6.19 ± 3.04 | - | 0.9 ± 1.05 | - | - | - | - | ||
| Measurement Tool | Article | Group | Baseline | Measurements (sd/ci 95%) | Follow-Up (sd/ci 95%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–6 Week | 6–12 Weeks | 3–6 Months | 1–3 Months | 3–6 Months | >6 Months | ||||
| SF-36 (0–100) | Branco Ferraz et al. [33] | EG₁ | MH: 65.4 ± 20.7 PH: 55.7 ± 16.9 | - | MH: 71.1 ± 23.1 PH: 64.8 ± 15.5 | - | - | - | - |
| EG₂ | MH: 69.0 ± 15.7 PH: 57.0 ± 15.9 | - | MH: 78.5 ± 19.8 PH: 66.0 ± 20.3 | - | - | - | - | ||
| BFR | MH: 68.0 ± 23.8 PH: 60.4 ± 16.1 | - | MH: 79.3 ± 12.0 PH: 73.4 ± 13.5 | - | - | - | - | ||
| Jørgensen et al. [29] | CON | 36.4 ± 21.7 | - | - | - | 32.3 ± 20.4 | - | - | |
| BFR | 54.5 ± 11.4 | - | - | - | 57.8 ± 17.6 | - | - | ||
| Rodrigues et al. [40] | EG₁ | PHF: 73.44 ” RPH: 85.71 ” BP: 68.94 ” GH: 57.50 ” V: 71.56 ” SF: 83.75 ” RE: 83.81 ” MH: 74.75 ” | - | PHF: 83.67 ” RPH: 100.0 ” BP: 70.73 ” GH: 62.53 ” V: 81.33 ” SF: 93.40 ” RE: 88.87 ” MH: 79.73 ” | - | - | - | - | |
| BFR | PHF: 73.13 ” RPH: 60.71 ” BP: 56.44 ” GH: 51.25 ” V: 69.06 ” SF: 86.81 ” RE: 83.38 ” MH: 77.75 ” | - | PHF: 83.33 ” RPH: 88.46 ” BP: 69.13 ” GH: 56.53 ” V: 75.33 ” SF: 91.80 ” RE: 91.07 ” MH: 81.33 ” | - | - | - | - | ||
| CON | PHF: 72.81 ” RPH: 71.43 ” BP: 72.75 ” GH: 60.38 ” V: 73.44 ” SF: 82.88 ” RE: 87.50 ” MH: 79.00 ” | - | PHF: 77.33 ” RPH: 82.69 ” BP: 71.20 ” GH: 57.93 ” V: 76.33 ” SF: 83.47 ” RE: 80.00 ” MH: 77.87 ” | - | - | - | - | ||
| Lamberti et al. [38] | BFR | PHF: 43 (31–54) RPH: 57 (41–72) BP: 60 (41–79) GH: 37 (26–47) V: 53 (44–62) SF: 54 (41–66) RE: 70 (44–95) MH: 64 (49–79) | PHF: 48 (36–60) RPH: 86 (66–107) BP: 66 (45–87) GH: 43 (29–58) V: 54 (44–65) SF: 64 (51–76) RE: 76 (53–98) MH: 63 (51–75) | - | - | PHF: 45 (32–59) RPH: 70 (49–91) BP: 64 (41–87) GH: 36 (26–46) V: 52 (43–60) SF: 64 (48–80) RE: 82 (59–105) MH: 67 (55–80) | - | - | |
| CON | PHF: 36 (21–51) RPH: 56 (34–78) BP: 62 (41–84) GH: 40 (33–47) V: 46 (38–54) SF: 49 (37–61) RE: 73 (53–92) MH: 66 (54–77) | PHF: 46 (29–64) RPH: 84 (62–106) BP: 75 (59–92) GH: 44 (33–54) V: 50 (39–60) SF: 62 (49–74) RE: 91 (81–101) MH: 75 (63–87) | - | - | PHF: 43 (27–59) RPH: 77 (53–101) BP: 75 (59–93) GH: 40 (30–50) V: 49 (37–61) SF: 60 (45–76) RE: 88 (73–103) MH: 71 (55–85) | - | - | ||
| MSIS-29 (0–100) | Lamberti et al. [38] | BFR | m: 62 (51–72) p: 24 (19–29) | m: 58 (48–68) p: 21 (16–26) | - | - | m: 57 (46–67) p: 22 (16–27) | - | - |
| CON | m: 61 (51–71) p: 21 (17–25) | m: 51 (42–61) p: 18 (14–22) | - | - | m: 53 (42–65) p: 19 (14–23) | - | - | ||
| VR-12 (0–100) | Tennent et al. [43] | BFR | PCS: 30.86 (22.4–39.4) MCS: 51.20 (41.2–59.5) | PCS: 46.3 (38.2–52.1) MCS: 60.24 (55.5–63.9) | - | - | - | - | - |
| CON | PCS: 36.50 (25.3–40.1) MCS: 57.60 (54.2–63.9) | PCS: 47.70 (35.6–50.5) MCS: 56.20 (50.4–61.5) | - | - | - | - | |||
| WOMAC (0–98) | Branco Ferraz et al. [33] | EG₁ | 36.6 ± 11.1 | - | 21.2 ± 13.2 | - | - | - | - |
| EG₂ | 35.1 ± 16.2 | - | 18.4 ± 11.5 | - | - | - | - | ||
| BFR | 31.5 ± 12.0 | - | 17.1 ± 11.2 | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reina-Ruiz, Á.J.; Martínez-Cal, J.; Molina-Torres, G.; Romero-Galisteo, R.-P.; Galán-Mercant, A.; Carrasco-Vega, E.; González-Sánchez, M. Effectiveness of Blood Flow Restriction on Functionality, Quality of Life and Pain in Patients with Neuromusculoskeletal Pathologies: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1401. https://doi.org/10.3390/ijerph20021401
Reina-Ruiz ÁJ, Martínez-Cal J, Molina-Torres G, Romero-Galisteo R-P, Galán-Mercant A, Carrasco-Vega E, González-Sánchez M. Effectiveness of Blood Flow Restriction on Functionality, Quality of Life and Pain in Patients with Neuromusculoskeletal Pathologies: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(2):1401. https://doi.org/10.3390/ijerph20021401
Chicago/Turabian StyleReina-Ruiz, Álvaro Jesús, Jesús Martínez-Cal, Guadalupe Molina-Torres, Rita-Pilar Romero-Galisteo, Alejandro Galán-Mercant, Elio Carrasco-Vega, and Manuel González-Sánchez. 2023. "Effectiveness of Blood Flow Restriction on Functionality, Quality of Life and Pain in Patients with Neuromusculoskeletal Pathologies: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 2: 1401. https://doi.org/10.3390/ijerph20021401
APA StyleReina-Ruiz, Á. J., Martínez-Cal, J., Molina-Torres, G., Romero-Galisteo, R.-P., Galán-Mercant, A., Carrasco-Vega, E., & González-Sánchez, M. (2023). Effectiveness of Blood Flow Restriction on Functionality, Quality of Life and Pain in Patients with Neuromusculoskeletal Pathologies: A Systematic Review. International Journal of Environmental Research and Public Health, 20(2), 1401. https://doi.org/10.3390/ijerph20021401










