Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
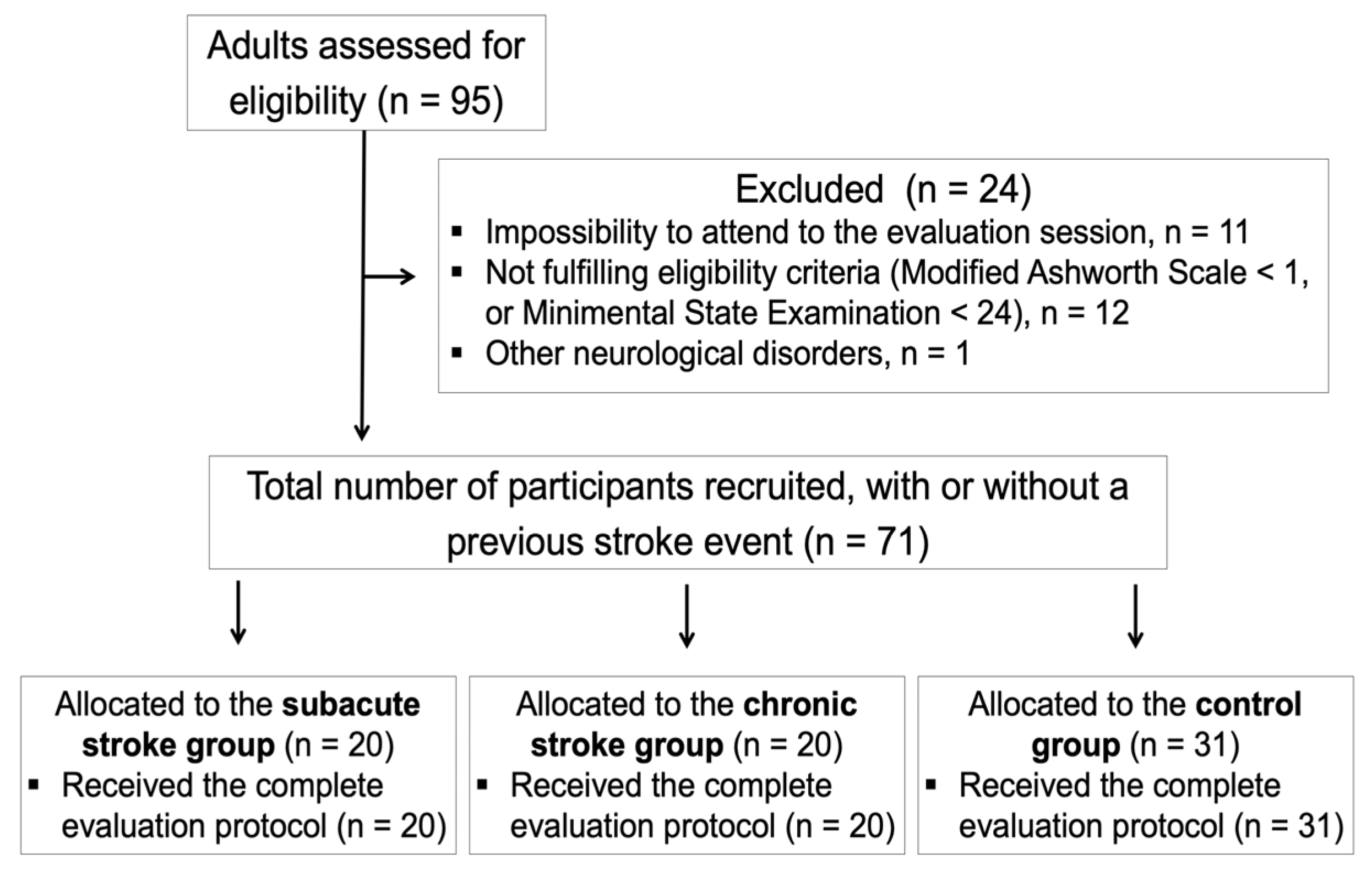
2.2. Participants
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
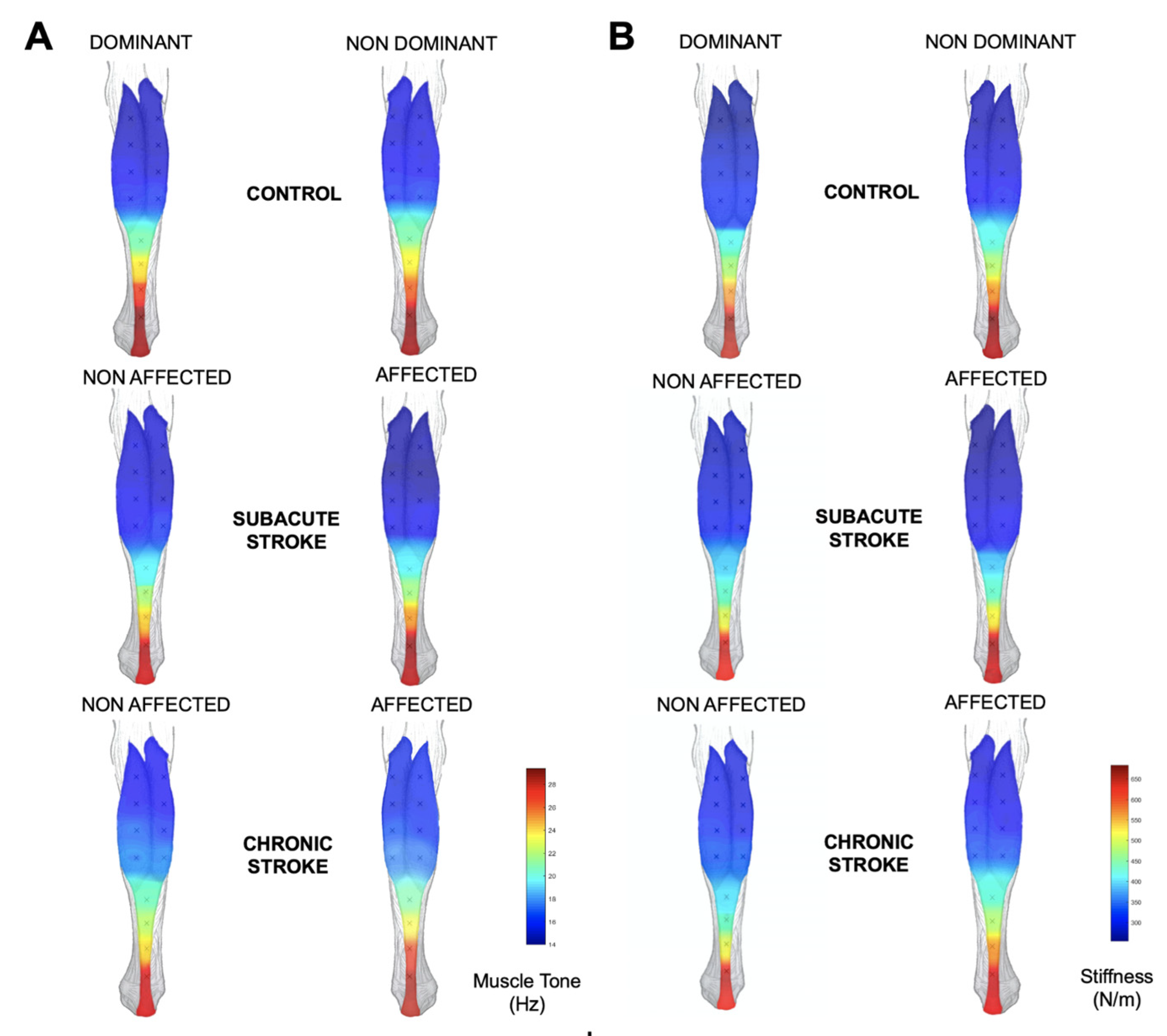
3.1. Mechanical and Structural Muscular Adaptations among Stroke Survivors
3.2. Discriminative Ability between Stroke Survivors and Healthy Controls
4. Discussion
4.1. Mechanical and Structural Muscular Adaptations among Stroke Survivors
4.2. Discriminative Ability between Stroke Survivors and Healthy Controls
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-Stroke Spasticity: Predictors of Early Development and Considerations for Therapeutic Intervention. PM R 2015, 7, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of Spasticity: Implications for Neurorehabilitation. BioMed Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, P. Upper Limb Motor Impairment Post Stroke. Phys. Med. Rehabil. Clin. N. Am. 2016, 26, 599–610. [Google Scholar] [CrossRef]
- Pundik, S.; McCabe, J.; Skelly, M.; Tatsuoka, C.; Daly, J.J. Association of Spasticity and Motor Dysfunction in Chronic Stroke. Ann. Phys. Rehabil. Med. 2019, 62, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal Muscle Changes Following Stroke: A Systematic Review and Comparison to Healthy Individuals. Top. Stroke Rehabil. 2017, 24, 463–471. [Google Scholar] [CrossRef]
- Faturi, F.M.; Lopes Santos, G.; Ocamoto, G.N.; Russo, T.L. Structural Muscular Adaptations in Upper Limb after Stroke: A Systematic Review. Top. Stroke Rehabil. 2019, 26, 73–79. [Google Scholar] [CrossRef]
- Del Prete, C.M.; Viva, M.G.; de Trane, S.; Brindisino, F.; Barassi, G.; Specchia, A.; di Iorio, A.; Pellegrino, R. An Observational Cross-Sectional Study of Gender and Disability as Determinants of Person-Centered Medicine in Botulinum Neurotoxin Treatment of Upper Motoneuron Syndrome. Toxins 2022, 14, 246. [Google Scholar] [CrossRef]
- Lehoux, M.C.; Sobczak, S.; Cloutier, F.; Charest, S.; Bertrand-Grenier, A. Shear Wave Elastography Potential to Characterize Spastic Muscles in Stroke Survivors: Literature Review. Clin. Biomech. 2020, 72, 84–93. [Google Scholar] [CrossRef]
- Luo, Z.; Lo, W.L.A.; Bian, R.; Wong, S.; Li, L. Advanced Quantitative Estimation Methods for Spasticity: A Literature Review. J. Int. Med. Res. 2019, 48, 0300060519888425. [Google Scholar] [CrossRef]
- Miller, T.; Ying, M.; Sau Lan Tsang, C.; Huang, M.; Pang, M.Y.C. Reliability and Validity of Ultrasound Elastography for Evaluating Muscle Stiffness in Neurological Populations: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzaa188. [Google Scholar] [CrossRef]
- García-Bernal, M.I.; Heredia-Rizo, A.M.; González-García, P.; Cortés-Vega, M.D.; Casuso-Holgado, M.J. Validity and Reliability of Myotonometry for Assessing Muscle Viscoelastic Properties in Patients with Stroke: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 5062. [Google Scholar] [CrossRef]
- Murillo, C.; Falla, D.; Sanderson, A.; Rushton, A.; Heneghan, N.R. Shear Wave Elastography Investigation of Multifidus Stiffness in Individuals with Low Back Pain. J. Electromyogr. Kinesiol. 2019, 47, 19–24. [Google Scholar] [CrossRef]
- García-Bernal, M.; González-García, P.; Casuso-Holgado, M.; Cortés-Vega, M.; Heredia-Rizo, A. Measuring Mechanical Properties of Spastic Muscles after Stroke. Does Muscle Position during Assessment Really Matter? Arch. Phys. Med. Rehabil. 2022, 103, 2368–2374. [Google Scholar] [CrossRef]
- Munneke, M.A.M.; Bakker, C.D.; Goverde, E.A.; Pasman, J.W.; Stegeman, D.F. On the Electrode Positioning for Bipolar EMG Recording of Forearm Extensor and Flexor Muscle Activity after Transcranial Magnetic Stimulation. J. Electromyogr. Kinesiol. 2018, 40, 23–31. [Google Scholar] [CrossRef]
- Alburquerque-Sendín, F.; Madeleine, P.; Fernández-De-Las-Peñas, C.; Camargo, P.R.; Salvini, T.F. Spotlight on Topographical Pressure Pain Sensitivity Maps: A Review. J. Pain Res. 2018, 11, 215–225. [Google Scholar] [CrossRef]
- Heredia-Rizo, A.M.; Petersen, K.K.; Arendt-Nielsen, L.; Madeleine, P. Eccentric Training Changes the Pressure Pain and Stiffness Maps of the Upper Trapezius in Females with Chronic Neck-Shoulder Pain: A Preliminary Study. Pain Med. 2020, 21, 1936–1946. [Google Scholar] [CrossRef]
- Sarasso, S.; Määttä, S.; Ferrarelli, F.; Poryazova, R.; Tononi, G.; Small, S.L. Plastic Changes Following Imitation-Based Speech and Language Therapy for Aphasia: A High-Density Sleep EEG Study. Neurorehabil. Neural Repair 2014, 28, 129–138. [Google Scholar] [CrossRef]
- Kumar, R.T.S.; Pandyan, A.D.; Sharma, A.K. Biomechanical Measurement of Post-Stroke Spasticity. Age Ageing 2006, 35, 371–375. [Google Scholar] [CrossRef]
- Moon, S.H.; Choi, J.H.; Park, S.E. The Effects of Functional Electrical Stimulation on Muscle Tone and Stiffness of Stroke Patients. J. Phys. Ther. Sci. 2017, 29, 238–241. [Google Scholar] [CrossRef]
- Ilahi, S.; Masi, A.T.; White, A.; Devos, A.; Henderson, J.; Nair, K. Quantified Biomechanical Properties of Lower Lumbar Myofascia in Younger Adults with Chronic Idiopathic Low Back Pain and Matched Healthy Controls. Clin. Biomech. 2020, 73, 78–85. [Google Scholar] [CrossRef]
- Miller, T.; Ying, M.T.C.; Chung, R.C.K.; Pang, M.Y.C. Convergent Validity and Test-Retest Reliability of Multimodal Ultrasonography and Related Clinical Measures in People With Chronic Stroke. Arch. Phys. Med. Rehabil. 2022, 103, 459–472.e4. [Google Scholar] [CrossRef]
- Barbosa, N.E.; Forero, S.M.; Galeano, C.P.; Hernández, E.D.; Landinez, N.S.; Sunnerhagen, K.S.; Murphy, M.A. Translation and Cultural Validation of Clinical Observational Scales—The Fugl-Meyer Assessment for Post Stroke Sensorimotor Function in Colombian Spanish. Disabil. Rehabil. 2019, 41, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meißner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients After Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Tong, K.Y.; Li, L. The Mechanomyography of Persons after Stroke during Isometric Voluntary Contractions. J. Electromyogr. Kinesiol. 2007, 17, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of Tissue Stiffness of the Infraspinatus, Erector Spinae, and Gastrocnemius Muscle Using Ultrasound Shear Wave Elastography and Superficial Mechanical Deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Liu, C.L.; Feng, Y.N.; Zhang, H.Q.; Li, Y.P.; Zhu, Y.; Zhang, Z.J. Assessing the Viscoelastic Properties of Upper Trapezius Muscle: Intra- and Inter-Tester Reliability and the Effect of Shoulder Elevation. J. Electromyogr. Kinesiol. 2018, 43, 226–229. [Google Scholar] [CrossRef]
- Ge, J.S.; Chang, T.T.; Zhang, Z.J. Reliability of Myotonometric Measurement of Stiffness in Patients with Spinal Cord Injury. Med. Sci. Monit. 2020, 26, e924811-1. [Google Scholar] [CrossRef]
- Marusiak, J.; Jaskólska, A.; Budrewicz, S.; Koszewicz, M.; Jaskólski, A. Increased Muscle Belly and Tendon Stiffness in Patients with Parkinson’s Disease, as Measured by Myotonometry. Mov. Disord. 2011, 26, 2119–2122. [Google Scholar] [CrossRef]
- Son, J.; Rymer, W.Z.; Lee, S.S.M. Limited Fascicle Shortening and Fascicle Rotation May Be Associated with Impaired Voluntary Force-Generating Capacity in Pennate Muscles of Chronic Stroke Survivors. Clin. Biomech. 2020, 75, 105007. [Google Scholar] [CrossRef]
- Li, L.; Tong, K.Y.; Hu, X. The Effect of Poststroke Impairments on Brachialis Muscle Architecture as Measured by Ultrasound. Arch. Phys. Med. Rehabil. 2007, 88, 243–250. [Google Scholar] [CrossRef]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W. Rasch Analysis Staging Methodology to Classify Upper Extremity Movement Impairment after Stroke. Arch. Phys. Med. Rehabil. 2013, 94, 1527–1533. [Google Scholar] [CrossRef]
- Narai, E.; Hagino, H.; Komatsu, T.; Togo, F. Accelerometer-Based Monitoring of Upper Limb Movement in Older Adults with Acute and Subacute Stroke. J. Geriatr. Phys. Ther. 2016, 39, 171–177. [Google Scholar] [CrossRef]
- Jones, T.A.; Allred, R.P.; Jefferson, S.C.; Kerr, A.L.; Woodie, D.A.; Cheng, S.Y.; Adkins, D.A.L. Motor System Plasticity in Stroke Models Intrinsically Use-Dependent, Unreliably Useful. Stroke 2013, 44, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Fini, N.A.; Holland, A.E.; Keating, J.; Simek, J.; Bernhardt, J. How Physically Active Are People Following Stroke? Systematic Review and Quantitative Synthesis. Phys. Ther. 2017, 97, 707–717. [Google Scholar] [CrossRef]
- Berenpas, F.; Martens, A.M.; Weerdesteyn, V.; Geurts, A.C.; van Alfen, N. Bilateral Changes in Muscle Architecture of Physically Active People with Chronic Stroke: A Quantitative Muscle Ultrasound Study. Clin. Neurophysiol. 2017, 128, 115–122. [Google Scholar] [CrossRef]
- Wang, J.-S.; Lee, S.-B.; Moon, S.-H. The Immediate Effect of PNF Pattern on Muscle Tone and Muscle Stiffness in Chronic Stroke Patient. J. Phys. Ther. Sci. 2016, 28, 967–970. [Google Scholar] [CrossRef]
- Jakubowski, K.L.; Terman, A.; Santana, R.V.C.; Lee, S.S.M. Passive Material Properties of Stroke-Impaired Plantarflexor and Dorsiflexor Muscles. Clin. Biomech. 2017, 49, 48–55. [Google Scholar] [CrossRef]
- Fröhlich-Zwahlen, A.K.; Casartelli, N.C.; Item-Glatthorn, J.F.; Maffiuletti, N.A. Validity of Resting Myotonometric Assessment of Lower Extremity Muscles in Chronic Stroke Patients with Limited Hypertonia: A Preliminary Study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke Induced Sarcopenia: Muscle Wasting and Disability after Stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef]
- Beckwée, D.; Cuypers, L.; Lefeber, N.; de Keersmaecker, E.; Scheys, E.; van Hees, W.; Perkisas, S.; de Raedt, S.; Kerckhofs, E.; Bautmans, I.; et al. Skeletal Muscle Changes in the First Three Months of Stroke Recovery: A Systematic Review. J. Rehabil. Med. 2022, 54, jrm00308. [Google Scholar] [CrossRef]
- Gao, J.; He, W.; Du, L.J.; Chen, J.; Park, D.; Wells, M.; Fowlkes, B.; O’Dell, M. Quantitative Ultrasound Imaging to Assess the Biceps Brachii Muscle in Chronic Post-Stroke Spasticity: Preliminary Observation. Ultrasound Med. Biol. 2018, 44, 1931–1940. [Google Scholar] [CrossRef]
- Lee, S.S.M.; Jakubowski, K.L.; Spear, S.C.; Rymer, W.Z. Muscle Material Properties in Passive and Active Stroke-Impaired Muscle. J. Biomech. 2019, 83, 197–204. [Google Scholar] [CrossRef]
- Stubbs, P.W.; Nielsen, J.F.; Sinkjær, T.; Mrachacz-Kersting, N. Short-Latency Crossed Spinal Responses Are Impaired Differently in Sub-Acute and Chronic Stroke Patients. Clin. Neurophysiol. 2012, 123, 541–549. [Google Scholar] [CrossRef]
- Mirbagheri, M.M.; Tsao, C.; Rymer, W.Z. Natural History of Neuromuscular Properties after Stroke: A Longitudinal Study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1212–1217. [Google Scholar] [CrossRef]
- Jeon, B.J.; Kim, W.H.; Park, E.Y. Effect of Task-Oriented Training for People with Stroke: A Meta-Analysis Focused on Repetitive or Circuit Training. Top. Stroke Rehabil. 2015, 22, 34–43. [Google Scholar] [CrossRef]
- Tier, L.; Salomoni, S.E.; Hug, F.; Besomi, M.; Hodges, P.W. Shear Modulus of Multifidus and Longissimus Muscles Measured Using Shear Wave Elastography Correlates with Muscle Activity, but Depends on Image Quality. J. Electromyogr. Kinesiol. 2021, 56, 102505. [Google Scholar] [CrossRef]


| Subacute Stroke (n = 20) | Chronic Stroke (n = 20) | Control Group (n = 31) | p Value | |
|---|---|---|---|---|
| Age (years) | 60.2 ± 9.7 | 61.45 ± 9.7 | 60.8 ± 10.6 | 0.926 |
| Sex, female, % (n) | 35% (7) | 35% (7) | 45.2% (14) | 0.689 |
| Time after stroke (weeks) | 17 (6–34) | 242.5 (58–1108) | N/A | <0.001 |
| Affected side, left, % (n) | 55% (11) | 75% (15) | N/A | 0.289 |
| Hand dominance, right, left, ambidextrous, % (n) | 100% (20) | 85% (17); 5% (1); 10% (2) | 80.6% (25); 19.4% (6) | 0.131 |
| Leg dominance, right, left, ambidextrous, % (n) | 95% (19); 5% (1); | 80% (16); 5% (1); 15% (3) | 83.9% (26); 16.1% (5) | 0.319 |
| Control Participants (n = 31) | Subacute Stroke (n = 20) | Chronic Stroke (n = 20) | |||||
|---|---|---|---|---|---|---|---|
| Site | Measure | Dominant Side | Non-Dominant Side | Affected Side | Non-Affected Side | Affected Side | Non-Affected Side |
| Point 1 | Stiffness Tone | 311.1 ± 82.7 17.2 ± 2.7 | 343.9 ± 78.7 18.2 ± 2.5 | 302.9 ± 101.9 16.7 ± 3.1 | 355.5 ± 112.8 18.1 ± 3.4 | 322.4 ± 94.3 17.3 ± 3.0 | 434.1 ± 139.2 20.6 ± 3.9 |
| Point 2 | Stiffness Tone | 359.5 ± 55.9 19.0 ± 2.4 | 372.9 ± 58.1 19.3 ± 2.3 | 357.7 ± 59.8 18.5 ± 2.2 | 400.5 ± 97.1 19.5 ± 4.1 | 388.9 ± 85.6 19.5 ± 3.5 | 478.2 ± 111.0 22.9 ± 4.2 |
| Point 3 | Stiffness Tone | 264.9 ± 49.8 15.1 ± 2.0 | 283.4 ± 49.1 15.6 ± 1.9 | 260.9 ± 48.0 15 ± 2.0 | 293.4 ± 71.9 15.7 ± 2.5 | 254.6 ± 46.4 14.5 ± 1.9 | 315.7 ± 61.1 16.7 ± 2.2 |
| Point 4 | Stiffness Tone | 335.7 ± 52.7 17.6 ± 2.2 | 346.5 ± 43.2 18.0 ± 1.7 | 335.7 ± 55.3 17.3 ± 2.6 | 370.8 ± 69.8 17.9 ± 3.2 | 332.5 ± 51.5 17.1 ± 2.6 | 407.4 ± 85.4 19.8 ± 3.2 |
| Point 5 | Stiffness Tone | 250.5 ± 47.7 14.1 ± 2.0 | 256.9 ± 52.2 14.2 ± 1.9 | 227.4 ± 41.3 13.5 ± 1.6 | 267.3 ± 63.3 15.0 ± 2.5 | 223.0 ± 47.2 13.2 ± 2.0 | 265.6 ± 67.1 14.5 ± 2.6 |
| Point 6 | Stiffness Tone | 319.1 ± 49.7 16.6 ± 2.2 | 340.6 ± 57.6 17.4 ± 2.3 | 339.7 ± 82.9 17.1 ± 3.2 | 369.7 ± 89.3 17.8 ± 3.4 | 318.5 ± 67.6 16.6 ± 2.9 | 380.1 ± 96.0 18.6 ± 3.5 |
| Point 7 | Stiffness Tone | 235.2 ± 26.4 13.4 ± 1.6 | 240.2 ± 29.8 13.2 ± 1.4 | 220.4 ± 35.6 13.0 ± 1.5 | 242.9 ± 30.7 13.7 ± 1.5 | 228.0 ± 29.7 13.5 ± 1.3 | 238.9 ± 31.8 13.4 ± 1.3 |
| Point 8 | Stiffness Tone | 307.2 ± 78.7 15.9 ± 2.9 | 312.4 ± 67.5 16.1 ± 2.5 | 297.8 ± 75.5 15.5 ± 2.5 | 381.6 ± 131.3 17.8 ± 4.0 | 315.3 ± 70.4 16.1 ± 2.2 | 364.1 ± 101.2 17.7 ± 3.4 |
| Point 9 | Stiffness Tone | 237.3 ± 23.9 13.3 ± 1.3 | 235.3 ± 19.8 13.1 ± 1.0 | 223.9 ± 31.4 12.9 ± 1.6 | 245.4 ± 29.1 14.1 ± 1.9 | 242.6 ± 31.9 13.7 ± 1.5 | 245.3 ± 23.7 13.7 ± 1.2 |
| Point 10 | Stiffness Tone | 262.1 ± 38.9 14.4 ± 1.8 | 264.9 ± 37.1 14.4 ± 1.9 | 260.1 ± 41.5 14.2 ± 1.6 | 294.1 ± 47.4 15.2 ± 1.9 | 267.9 ± 30.7 14.9 ± 1.5 | 302.2 ± 44.1 15.9 ± 2.1 |
| Point 11 | Stiffness Tone | 242.5 ± 20.8 13.7 ± 1.0 | 246.9 ± 26.7 13.9 ± 1.2 | 233.9 ± 26.7 13.6 ± 1.5 | 251.8 ± 37.8 14.5 ± 1.8 | 257.2 ± 32.6 14.6 ± 1.9 | 263.3 ± 25.7 14.2 ± 1.6 |
| Point 12 | Stiffness Tone | 247.9 ± 44.9 14.2 ± 1.8 | 252.9 ± 37.8 14.0 ± 1.6 | 243.3 ± 32.5 13.5 ± 1.2 | 276.1 ± 43.1 14.8 ± 1.6 | 248.2 ± 23.2 14.1 ± 1.4 | 280.3 ± 37.3 14.9 ± 1.9 |
| Point 13 | Stiffness Tone | 253.4 ± 39.7 14.3 ± 1.4 | 258.8 ± 48.4 14.5 ± 1.9 | 245.6 ± 33.2 14.3 ± 1.3 | 251.9 ± 54.2 14.4 ± 2.5 | 275.7 ± 75.2 15.1 ± 2.9 | 259.9 ± 39.1 14.2 ± 1.7 |
| Muscle belly (MB) sites | Stiffness Tone | 256.8 ± 27.1 14.3 ± 1.4 | 262.2 ± 28.1 14.3 ± 1.3 | 248.1 ± 36.3 13.9 ± 1.3 | 277.7 ± 35.8 15.1 ± 1.5 | 254.8 ± 27.6 14.4 ± 1.2 | 280.9 ± 35.4 15.0 ± 1.5 |
| Musculotendinous (MT) sites | Stiffness Tone | 295.3 ± 41.2 16.4 ± 1.7 | 310.2 ± 36.0 16.8 ± 1.5 | 288.7 ± 37.8 16.1 ± 1.5 | 318.1 ± 58.1 16.7 ± 2.3 | 302.4 ± 49.9 16.4 ± 2.1 | 351.9 ± 50.7 18.1 ± 2.1 |
| Control Participants (n = 31) | Subacute Stroke (n = 20) | Chronic Stroke (n = 20) | |||||
|---|---|---|---|---|---|---|---|
| Site | Measure | Dominant Side | Non-Dominant Side | Affected Side | Non- Affected Side | Affected Side | Non-Affected Side |
| Point 1 | Stiffness Tone | 255.4 ± 22.1 14.6 ± 1.1 | 271.5 ± 37.6 15.3 ± 1.8 | 253.9 ± 29.6 14.4 ± 1.5 | 273.5 ± 47.4 15.2 ± 2.3 | 283.4 ± 52.5 15.4 ± 2.2 | 295.1 ± 65.8 15.8 ± 2.5 |
| Point 2 | Stiffness Tone | 259.3 ± 28.1 14.9 ± 1.2 | 267.2 ± 33.1 15.2 ± 1.4 | 263.1 ± 39.2 14.5 ± 1.8 | 273.3 ± 51.6 14.9 ± 2.4 | 292.8 ± 43.3 15.8 ± 1.8 | 291.5 ± 73.4 15.7 ± 2.8 |
| Point 3 | Stiffness Tone | 277.5 ± 24.8 14.9 ± 1.4 | 280.7 ± 23.2 15.0 ± 1.3 | 265.0 ± 25.2 14.2 ± 1.4 | 282.8 ± 40.5 15.0 ± 2.2 | 298.1 ± 43.3 15.4 ± 2.5 | 300.8 ± 59.4 15.9 ± 2.6 |
| Point 4 | Stiffness Tone | 279.1 ± 19.0 14.7 ± 1.4 | 283.4 ± 26.4 15.3 ± 1.6 | 269.9 ± 19.6 14.0 ± 1.3 | 281.4 ± 30.8 14.8 ± 1.8 | 289.9 ± 40.7 15.6 ± 2.3 | 290.7 ± 66.2 15.6 ± 2.9 |
| Point 5 | Stiffness Tone | 288.8 ± 26.6 15.7 ± 1.4 | 290.7 ± 28.1 15.9 ± 1.5 | 278.7 ± 31.3 14.9 ± 1.8 | 289.3 ± 29.7 15.7 ± 2.0 | 317.4 ± 51.7 16.8 ± 2.8 | 312.9 ± 58.4 17.1 ± 3.0 |
| Point 6 | Stiffness Tone | 282.3 ± 18.7 14.9 ± 1.5 | 281.6 ± 26.2 15.3 ± 1.7 | 278.0 ± 26.7 14.4 ± 1.7 | 284.3 ± 30.2 14.8 ± 2.0 | 293.5 ± 40.7 15.8 ± 2.3 | 295.7 ± 66.5 16.2 ± 2.6 |
| Point 7 | Stiffness Tone | 306.8 ± 26.3 16.0 ± 1.4 | 308.1 ± 25.4 16.2 ± 1.5 | 290.4 ± 25.1 15.3 ± 1.5 | 299.2 ± 31.7 15.7 ± 2.2 | 334.0 ± 58.3 17.9 ± 3.1 | 327.0 ± 66.2 17.7 ± 2.8 |
| Point 8 | Stiffness Tone | 314.7 ± 29.2 16.2 ± 1.7 | 310.7 ± 27.9 16.5 ± 1.6 | 305.4 ± 26.8 15.7 ± 1.8 | 308.8 ± 37.3 16.4 ± 2.5 | 332.3 ± 47.1 17.5 ± 2.7 | 329.4 ± 57.2 17.6 ± 3.2 |
| Point 9 | Stiffness Tone | 416.4 ± 40.4 21.1 ± 2.2 | 421.6 ± 40.9 21.1 ± 2.2 | 380.6 ± 49.1 19.3 ± 2.3 | 386.9 ± 44.5 19.7 ± 2.4 | 428.9 ± 81.9 20.9 ± 3.2 | 400.8 ± 71.4 20.8 ± 3.1 |
| Point 10 | Stiffness Tone | 483.2 ± 58.6 23.9 ± 3.3 | 482.5 ± 56.5 23.5 ± 2.6 | 435.3 ± 59.8 21.8 ± 2.7 | 440.2 ± 60.8 22.0 ± 2.5 | 482.3 ± 89.5 23.4 ± 3.6 | 445.7 ± 77.6 22.4 ± 3.5 |
| Point 11 | Stiffness Tone | 562.1 ± 90.1 26.9 ± 3.9 | 571.3 ± 98.3 26.3 ± 3.6 | 521.7 ± 98.9 25.2 ± 3.9 | 509.5 ± 66.6 24.4 ± 3.1 | 572.1 ± 117.5 26.8 ± 4.5 | 510.4 ± 98.2 24.0 ± 4.0 |
| Point 12 | Stiffness Tone | 679.4 ± 91.1 29.3 ± 2.8 | 683.8 ± 119.5 29.4 ± 3.8 | 670.1 ± 164.1 29.2 ± 4.6 | 663.8 ± 109.8 28.6 ± 3.5 | 651.4 ± 121.5 28.4 ± 3.5 | 663.6 ± 164.9 28.2 ± 5.0 |
| Muscle belly (MB) sites | Stiffness Tone | 280.3 ± 13.7 15.1 ± 0.9 | 284.2 ± 17.4 15.5 ± 1.1 | 273.3 ± 19.8 14.6 ± 1.0 | 284.0 ± 29.4 15.2 ± 1.7 | 301.3 ± 35.5 16.0 ± 1.9 | 302.3 ± 61.1 16.3 ± 2.5 |
| Musculotendinous (MT) sites | Stiffness Tone | 515.9 ± 56.1 24.8 ± 2.4 | 475.2 ± 55.3 23.1 ± 2.4 | 476.4 ± 70.5 23.2 ± 2.8 | 478.2 ± 55.4 23.1 ± 2.3 | 518.1 ± 98.4 24.4 ± 3.4 | 484.2 ± 84.2 23.4 ± 3.3 |
| Subacute Stroke (n = 20) | Chronic Stroke (n = 20) | Control Group (n = 31) | |||||
|---|---|---|---|---|---|---|---|
| Muscle | Site | Affected Side | Non-Affected Side | Affected Side | Non-Affected Side | Dominant Side | Non-Dominant Side |
| Biceps brachii | Point 7 Point 8 | 1.97 ± 0.52 1.41 ± 0.36 | 2.27 ± 0.66 1.78 ± 0.59 | 1.83 ± 0.39 1.24 ± 0.28 | 2.33 ± 0.45 1.66 ± 0.51 | 2.41 ± 0.51 1.75 ± 0.72 | 2.37 ± 0.59 1.57 ± 0.48 |
| Gastroc-nemius | Point 5 Point 6 | 1.47 ± 0.42 1.34 ± 0.45 | 1.60 ± 0.26 1.29 ± 0.46 | 1.25 ± 0.51 1.12 ± 0.41 | 1.45 ± 0.47 1.35 ± 0.44 | 1.70 ± 0.41 1.41 ± 0.29 | 1.75 ± 0.40 1.41 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Bernal, M.I.; González-García, P.; Madeleine, P.; Casuso-Holgado, M.J.; Heredia-Rizo, A.M. Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke. Int. J. Environ. Res. Public Health 2023, 20, 1405. https://doi.org/10.3390/ijerph20021405
García-Bernal MI, González-García P, Madeleine P, Casuso-Holgado MJ, Heredia-Rizo AM. Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke. International Journal of Environmental Research and Public Health. 2023; 20(2):1405. https://doi.org/10.3390/ijerph20021405
Chicago/Turabian StyleGarcía-Bernal, María Isabel, Paula González-García, Pascal Madeleine, María Jesús Casuso-Holgado, and Alberto Marcos Heredia-Rizo. 2023. "Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke" International Journal of Environmental Research and Public Health 20, no. 2: 1405. https://doi.org/10.3390/ijerph20021405
APA StyleGarcía-Bernal, M. I., González-García, P., Madeleine, P., Casuso-Holgado, M. J., & Heredia-Rizo, A. M. (2023). Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke. International Journal of Environmental Research and Public Health, 20(2), 1405. https://doi.org/10.3390/ijerph20021405








