Abstract
Hepatitis A is a common form of viral hepatitis. It is usually transmitted through the ingestion of contaminated food and water. This systematic review was carried out to summarise the overall prevalence of Hepatitis A virus (HAV) in different water matrices: untreated and treated wastewater, surface water, groundwater, drinking water, and others (e.g., irrigation water and floodwater). The literature search was performed in four databases: PubMed, Web of Science, Global Index Medicus, and Excerpta Medica Database. Heterogeneity (I2) was assessed using the χ2 test on the Cochran Q statistic and H parameters. A total of 200 prevalence data from 144 articles were included in this meta-analysis. The overall prevalence of HAV in water matrices was 16.7% (95% CI: 13.4–20.3). The prevalence for individual matrix was as follows: 31.4% (95% CI: 23.0–40.4) untreated wastewater, 18.0% (95% CI: 9.5–28.2) treated wastewater, 15.0% (95% CI: 10.1–20.5) surface water, 2.3% (95% CI: 0.1–6.0) in groundwater, 0.3% (95% CI: 0.0–1.7) in drinking water, and 8.5% (95% CI: 3.1–15.6) in other matrices. The prevalence was higher in low-income economies (29.0%). Africa and Eastern Mediterranean were the regions with higher HAV prevalence values. This study showed a high heterogeneity (I2 > 75%) with a significant publication bias (p value Egger test < 0.001). The results of this review suggest that water matrices could be an important route of HAV transmission even in industrialized countries, despite the lower prevalence compared to less industrialized countries, and the availability of advanced water management systems. More effective water/wastewater treatment strategies are needed in developing countries to limit the environmental circulation of HAV.
1. Introduction
Hepatitis A is a highly contagious liver infection [1], first discovered in 1973 [2]. It can cause mild to severe illness and occasionally acute liver failure, which is often fatal. Unlike hepatitis B and C, hepatitis A does not cause chronic liver disorders. In a minority of cases, it can lead to extrahepatic manifestations, including urticarial and maculopapular rash, acute kidney injury, autoimmune hemolytic anemia, aplastic anemia, acute pancreatitis, glomerulonephritis, and thrombocytopenia [3,4]. Approximately 1.5 million people are infected with hepatitis A each year; however, this figure seems to be underestimated, and the infection rate is probably much higher [5], making hepatitis A a global public health concern. According to the World Health Organization (WHO), there were 7134 deaths worldwide caused by this disease in 2016, accounting for 0.5% of the mortality due to viral hepatitis [6]. The disease is caused by hepatitis A virus (HAV), an RNA virus of the Picornaviridae family, genus Hepatovirus. Six genotypes of HAV are currently recognized (genotypes I–VI). Genotypes I, II, and III, each divided into subtypes A and B, infect humans [7,8,9]. HAV is mainly transmitted via the faecal–oral route through contaminated food or water. Drinking water can be a route of major concern for HAV outbreaks; moreover, water-borne outbreaks have resulted from contaminated water supplied in households due to damages or locations of water pipelines close to drain or sewerage systems [10]. Endemicity is high in low-income countries, mainly due to inadequate safe water and poor sanitation and hygiene [11,12]. In these countries, most children (90%) are infected with HAV before the age of 10 years, often without symptoms [6]. Conversely, infection rates are low in high-income countries with good sanitary and hygienic conditions. Limiting environmental contamination is essential to control hepatitis A, hence the importance of understanding environmental sources of HAV. Several studies have reported the detection of HAV in foods, untreated and treated wastewater, and other water environments [13].
Several systematic reviews on HAV in water or food have been published, focusing on certain foods or specific water matrices. Chatziprodromidou et al., showed that HAV was the second most abundant virus in fresh produce [14]. Bellou et al., found that HAV was one of the most common viral pathogens detected in shellfish [15]. Thébault et al. demonstrated the critical role of untreated drinking water, shellfish, and crop products in sporadic hepatitis A infection [16]. A recent review by Gholipour and co-workers highlighted that HAV is frequently found in sewage sludge [17]. Boehm et al. reviewed decay rates of waterborne viruses, including HAV, in surface waters [18]. Finally, Kuodi and coworkers characterised the environmental presence of HAV in low- and middle-income countries, showing that HAV occurrence declined by 10% over the review period (2005–2019) [19]. No systematic review, however, has looked comprehensively at all water matrices thus far, which is important to understand the extent of viral contamination of water environments. Therefore, the objective of this systematic review and meta-analysis was to assess the overall prevalence of HAV in different water matrices, including untreated and treated wastewater, surface water, groundwater, and drinking water.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review and meta-analysis was conducted between March and November 2022. The review protocol was registered on Prospero, number CRD42021289116. This study was designed by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standard guidelines [20].
2.2. Eligibility Criteria
We included all studies published worldwide from inception until March 2022.
2.3. Inclusion and Exclusion Criteria
We included studies that met the following criteria: (a) containing data about the prevalence of HAV in water matrices, (b) original studies, and (c) published in English or French. Letters to editors, comment papers, brief reports, research news, systematic review, meta-analysis, and studies with a number of tested samples (sample size) equal or below 10 were excluded.
2.4. Information about Searches
We searched four databases including PubMed, Excerpta Medica Database (Embase), Web of Science, and Global Index Medicus. The search terms were related to HAV and water matrices (see search strategy in Table S1). A manual search in the list of references of relevant studies was also conducted to identify any additional articles missed by the online search.
2.5. Data Extraction and Management
Firstly, duplicate articles were removed. Two reviewers screened the titles and abstracts of all the articles using Rayyan—Intelligent Systematic Review website (https://www.rayyan.ai/ (accessed on 8 April 2022)). In case of discrepancies, a third reviewer intervened as a referee. After the preliminary screening, the data were extracted from the selected studies using a pre-designed Google data abstraction form. The different data extracted were: name of the first author, year of publication, study period, sampling approach (probabilistic/non-probabilistic), number of sites (monocenter, multicenter and nationally representative), timing of samples collection (prospective, retrospective), country, WHO region, United Nations Statistics Division (UNSD) region [21], country income level [22], type of water matrix (untreated wastewater, treated wastewater, surface water, drinking water, groundwater, and others), sample size, detection method, detected genotypes, and viral loads. In case different prevalence data were obtained for the same samples according to the HAV concentration and/or detection methods, the estimate with the highest prevalence was included. In case water samples were collected at different stages of treatment, we calculated the sum of all the categories of water and considered a single category of treated water. After data extraction, two reviewers screened the data extracted from all included studies.
2.6. Quality Assessment
To assess the quality of the studies, we used the tool developed by Hoy et al. for prevalence studies; this allowed the included studies to be evaluated for risk of bias (Table S2) [23]. Items for risk of bias assessment included external validity controls (e.g., representativity at national level of the prevalence data, sampling size and frame) and internal validity controls (e.g., use of valid and reliable detection assays, acceptable water matrix definition, length of the study period >1 year, etc.).
2.7. Statistical Analysis
Study-specific estimates were pooled using a random-effects model meta-analysis from DerSimonian and Laird [24]. Heterogeneity was assessed by the Cochrane Q statistical test and quantified by I2 values, assuming that the I² values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively [25]. Publication bias was assessed by Egger’s test and the funnel plot [26]. Sensitivity analysis was carried out using studies that had a low risk of bias and studies in which a process control virus (e.g., murine norovirus, mengovirus, etc.) was added to the samples before analysis, in order to monitor all the analytical steps. Subgroup analyses were conducted according to sampling approach, setting, country, WHO and UNSD regions, country income level, and water matrix. A p-value < 0.05 indicated a significant difference. R software version 4.1.0 was used to perform analyses [27,28].
3. Results
3.1. Study Selection
After searching the four databases, we obtained 18,189 articles; an additional 20 papers were included as results of manual searches (Figure 1). A total of 1453 duplicates were removed, as well as 16,162 articles for unfitting titles or abstracts (articles that do not meet inclusion criteria). A total of 594 articles were recorded as eligible, 450 of which were excluded for reasons given in Table S3. Finally, 144 articles were included in this systematic review (Table S5), which resulted in 200 HAV prevalence data from six different water matrices [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173].
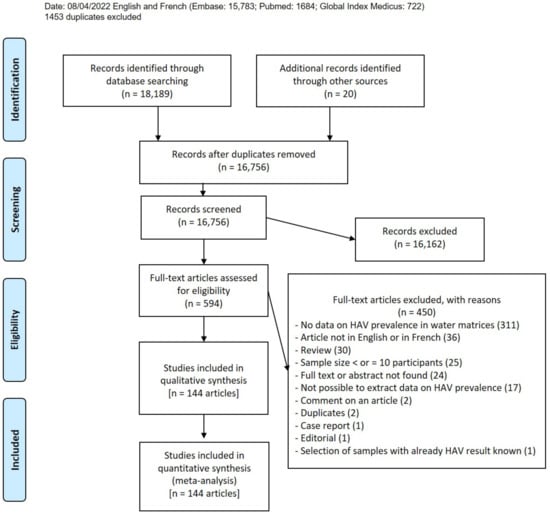
Figure 1.
Study selection.
3.2. Study Characteristics
The characteristics of the included studies are listed in Tables S4–S6. The studies included were published between 1987 and 2022, and the sampling period ranged from 1986 to 2020. Most of the studies were non-probabilistic 195/200 (97.5%), prospective 197/200 (98.5%), and multicenter 130/200 (65.0%). The most represented UNSD Region was Southern Europe (53/200, 26.5%), while the most reported WHO Regions were Europe (74/200, 37.0%) and America (49/200, 24.5%). The most represented countries were Italy (29/200, 14.5%) and Brazil (22/200, 11.0%). High-income countries were prevalent (107/200, 53.5%), followed by upper-middle income countries (54/200, 27.0%). The water matrices were categorized into six groups, and the most represented were surface waters (66/200, 33.0%), followed by untreated wastewater (56/200, 28.0%) (Table S4). As for HAV genotypes, IA was the most frequently detected (749 samples), followed by IB (391 samples), IIIA (17 samples), and V (1 sample) (Table S5). Only nine studies reported information on the presence of infectious HAV by isolation in cell lines [47,66,85,88,132,145,150,158,166] (Table S5). Attempts to isolate HAV was carried out on Buffalo green monkey kidney cells (BGMK), PLC/PRF/5 human liver cells, Colorectal adenocarcinoma cells (Caco-2), Fetal rhesus monkey kidney (FRHK-4), Human lung carcinoma epithelial cells (A549), Verda reno cells (VERO), African rhesus monkey kidney cells (MA104), and Hep-2. Conventional RT-PCR (109/200, 54.5%) and real-time RT-PCR (75/200, 37.5%) were the analytical approaches most frequently used. Of the 75 studies using real-time RT-PCR, 34 obtained quantitative data, viral loads ranging from ˂LOD to 3.70 × 1010 genome copies (gc)/L. The concentration ranges were <1 to 3.7 × 1010 for untreated wastewater, 2.3 × 101 to 3.3 × 107 for treated wastewater, 1.5 × 101 to 107 for surface waters and no data for drinking water and groundwater. Moreover, low concentrations were reported in “other matrices” (6.87 × 10−1 to 7.4 × 10−1). An analytical process control to assess the performance of concentration and extraction of samples was present in 74/200 (37.0%) of the studies; the risk of bias was low in 13/200 studies (6.5%) and moderate in the remaining 187/200 (93.5%) (Table S4).
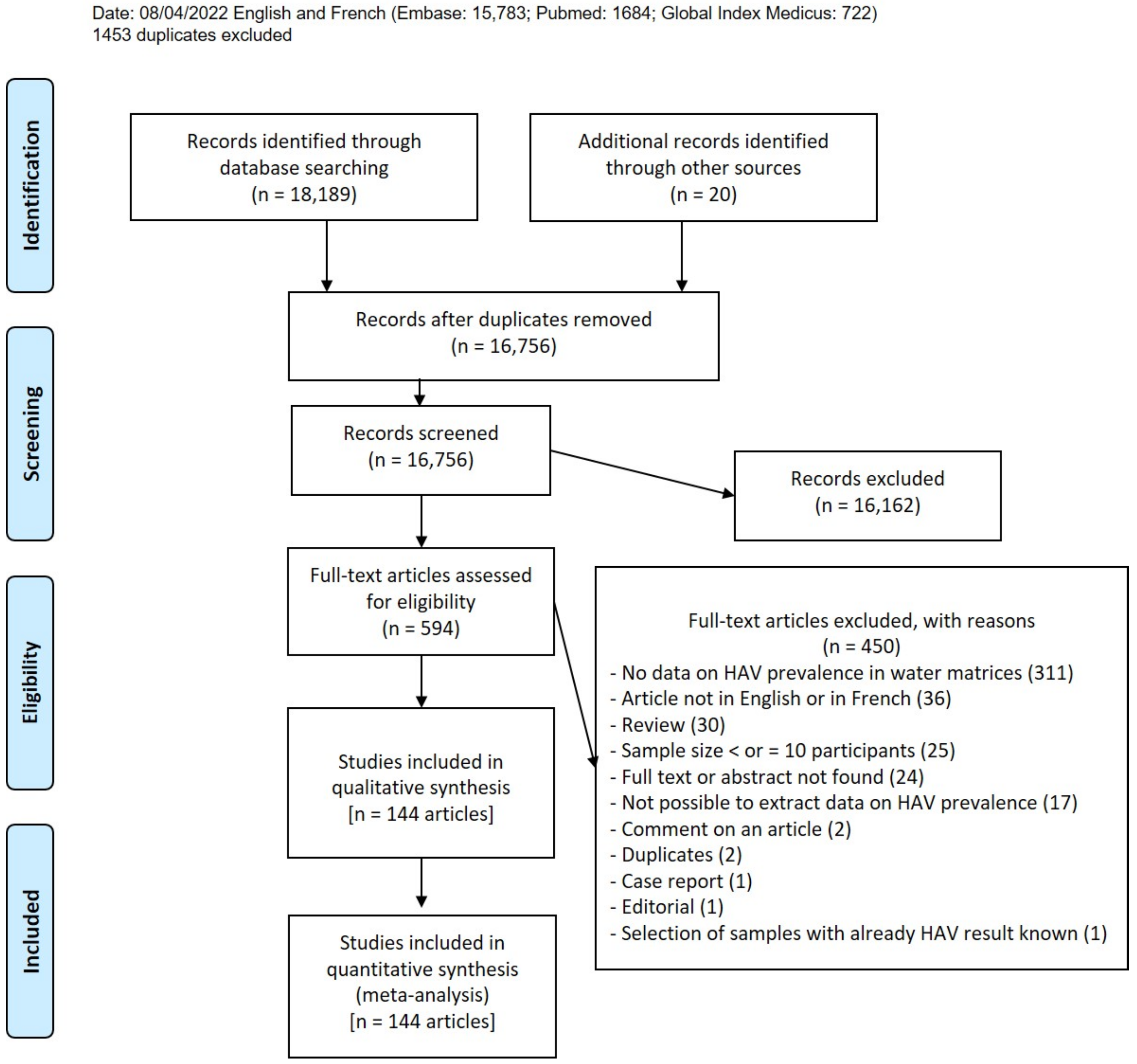
3.3. HAV Prevalence in Water Matrices
Data on HAV prevalence are represented in Figure 2 and Figure S1. The global HAV prevalence in water environments was 16.7% (95% CI: 13.4–20.3). According to the different water matrices, prevalence varied from 0.3% to 31.5%. In detail, the prevalence was as follows: 31.5% (95% CI: 23.1–40.5) in untreated wastewater, 18.0% (95% CI: 9.5–28.3) in treated wastewater, 15.0% (95% CI: 10.2–20.6) in surface water, 2.4% (95% CI: 0.2–6.1) in groundwater, 0.4% (95% CI: 0.0–1.8) in drinking water. Prevalence in other water types was 8.5% (95% CI: 3.2–15.7).
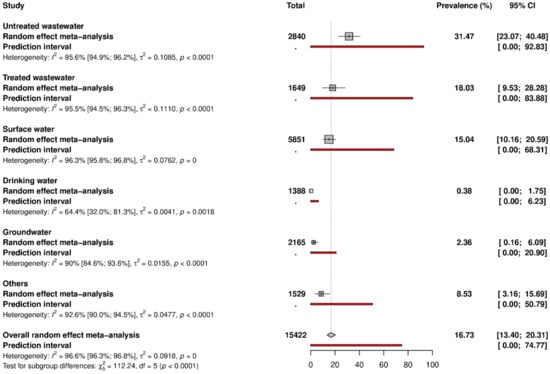
Figure 2.
Data on HAV prevalence.
3.4. Heterogeneity, Publication Bias and Sensitivity Analysis
Table 1 and Figure S2 present the degree of heterogeneity and publication bias. Significant heterogeneity (H ˃ 1 and I2 ˃ 75%) and publication bias (p < 0.05 for Egger’s test) were found to be associated with the estimation of prevalence data in the different water matrices. The publication bias results obtained by Egger’s test were confirmed by the funnel plot (Figure S2). Due to the limited number of studies with low risks of bias, the results of the sensitivity analysis displayed under- (untreated wastewater) or overestimation (surface water) compared to global results (Table 1). In studies applying a process control, on the other hand, the results closely approximated the global results, pointing out the robustness of the analysis.

Table 1.
Summary of global meta-analysis results for the prevalence of hepatitis A virus in different water matrices divided by risk of bias and by use of process control.
3.5. Subgroup Analyses
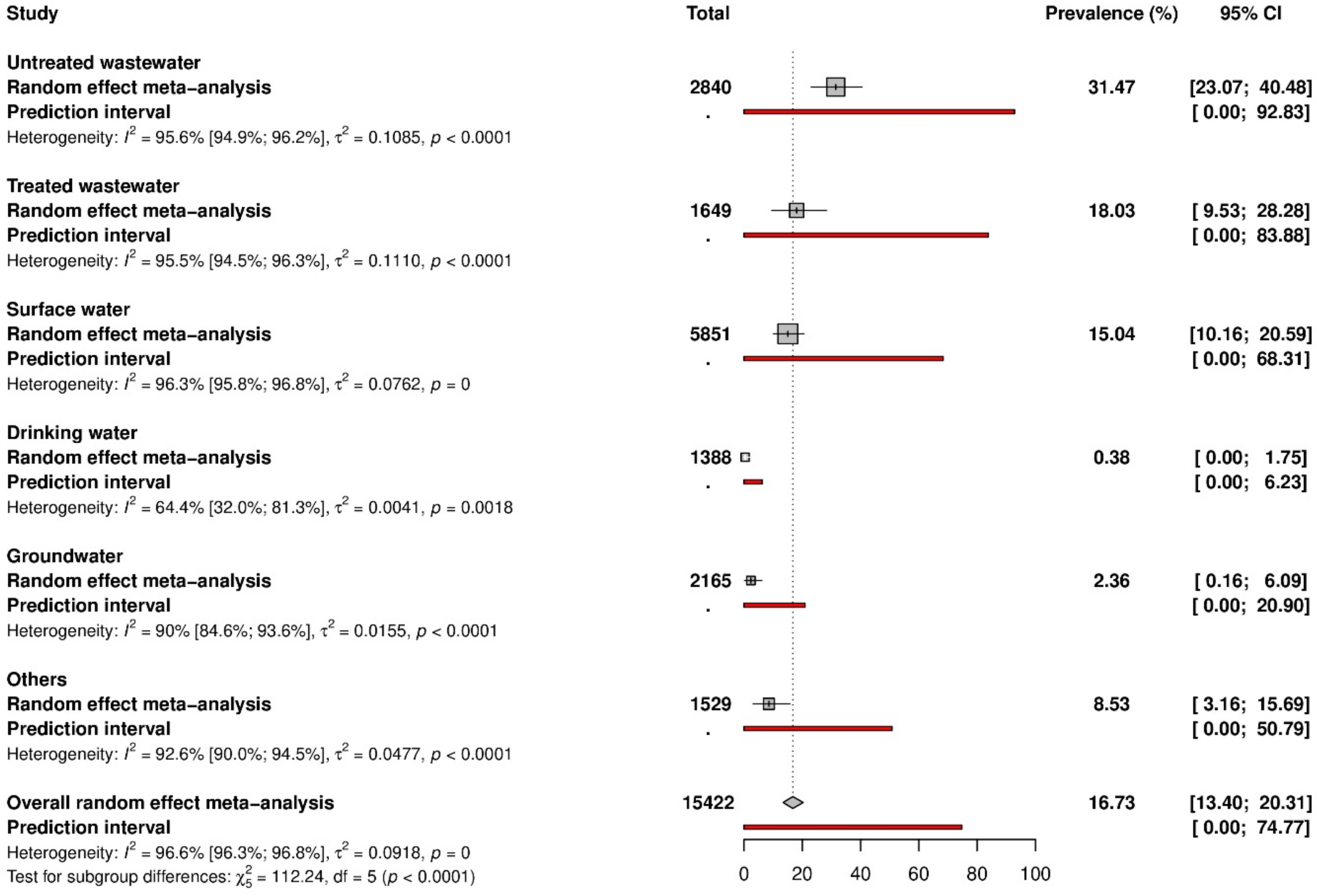
Table S7 presents the subgroup analysis. The global prevalence was significantly different according to countries (p < 0.001) with higher prevalence in Kenya (52.0%, 95% CI: 0.0–100, 3 prevalence data), followed by Tunisia (39.1%, 95% CI: 21.8–57.8, 9 prevalence data), and Uganda (36.8%, 95% CI: 20.7–54.3, 5 prevalence data) (Figure 3). Italy and Brazil, the most represented countries, had a prevalence of 11.5% and 32.5%, respectively. According to the WHO region, a significantly higher prevalence (p < 0.001) was found in Africa (31.8%, 95% CI: 16.9–48.8, 22 prevalence data), followed by Eastern Mediterranean (23.7%, 95% CI: 13.4–35.6, 24 prevalence data) and America (20.0%, 95% CI: 13.2–27.9, 49 prevalence data). For UNSD region, higher prevalence (p < 0. 001) was in Eastern Africa (43.2%, 95% CI: 12.5–77, 8 prevalence data), followed by Northern Africa (34.4%, 95% CI: 20.7–49.4, 14 prevalence data), Southern America (30.0%, 95% CI: 17.5–44.2, 26 prevalence data), and Southern Africa (28.5%, 95% CI: 13.0–47.0, 13 prevalence data). Regarding country income level, the higher prevalence (p < 0.001) was in low-income economies (29.0%, 95% CI: 11.8–49.6, 6 prevalence data), while high-income economies, which are the most represented, had the prevalence of 10.8% (95% CI: 7.8–14.1, 107 prevalence data).
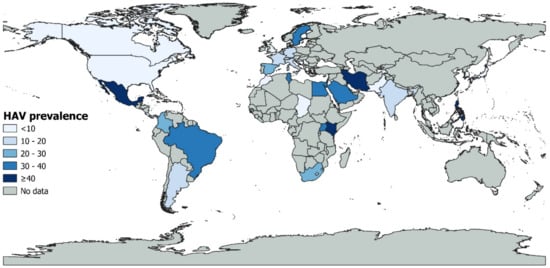
Figure 3.
Global HAV prevalence.
No statistically significant difference in the prevalence values was found according to time periods (Table S7): 14.5% during 1986–2000 (25 studies), 17.3% during 2000–2010 (67 studies), and 15.5 % during 2010–2020 (75 studies).
4. Discussion
Hepatitis A virus is one of the most important causes of acute viral hepatitis worldwide. The virus is present in all regions of the world, both industrialized and non-industrialized countries, making hepatitis A a significant health problem regardless of the country’s economic level. HAV is common in areas with insufficient sanitation and limited access to clean water [174]. In highly endemic areas, epidemics of hepatitis A are uncommon, and a large proportion of adults in the population are immune. Instead, in areas of intermediate endemicity, adolescents and adults are susceptible to infection, and outbreaks are more frequent. In areas of low endemicity, infection is less common, but disease occurs among people in high-risk groups (e.g., people travelling to areas of high endemicity) and as community-wide outbreaks [18]. HAV can be transmitted through ingestion of contaminated water; indeed viral contamination of water environments has been frequently reported as a primary source of hepatitis outbreaks [18]. Thus, understanding the occurrence of HAV in water environments is crucial to better understand the epidemiology of the disease. However, the few reviews published to date only addressed specific water environments [16,17,175] or a specific subset of countries [20].
Here, we aimed at summarizing all the existing literature on the prevalence of HAV in waters, including untreated and treated wastewater, surface water, groundwater, and drinking water. A total of 144 articles and 200 prevalence data were included in this study, spanning a period of 34 years (1986–2020). The overall prevalence of HAV in water was 16.7%, varying significantly according to the degree of water quality. As expected, the prevalence was higher in untreated wastewater (31.4%), with viral concentrations reaching up to 3.7 × 1010 gc/L. Virus occurrence in sewage samples may reflect the epidemiology pattern of virus infections in the population; it is the result of the high viral excretion by infected individuals, ranging from 106 to 1011 viral particles per gram of faeces [176]. Despite the improvement in wastewater treatment technology, water treatments are in some cases unable to provide virus-free wastewater effluents. Indeed, a prevalence of 18.0% and a viral concentration up to 3.3 × 107 cg/L was detected in treated wastewater, though this value reflects the average detection of HAV in wastewater at different levels of treatment and may therefore overestimate its occurrence in the finally treated wastewater. Viruses can be introduced to surface waters through the discharge of untreated or improperly treated sewage. Indeed, a prevalence of 15.0% and maximum concentrations up to 107 cg/L was found in surface waters in this study—including rivers, lakes, marine waters—which are the water environments collecting treated wastewaters. Interestingly, the HAV prevalence in groundwater was 2.3%. Contaminated groundwater has indeed been associated with HAV outbreaks. For example, in USA, the Waterborne Disease and Outbreak Surveillance System (WBDOSS) reported HAV as the most commonly reported etiology for outbreaks associated with untreated groundwater in the period of 1971–2008, suggesting that individual water systems that used wells with untreated groundwater is a common waterborne exposure pathway for HAV [7]. Factors contributing to groundwater contamination include nearby septic systems or sewage, heavy rainfall, improper well construction and maintenance as well as surface water infiltration.
Finally, low HAV prevalence was found in drinking water (0.3%, with only 11 available studies), suggesting the effectiveness of drinking water treatment technologies. These usually include conventional pre-treatments (coagulation, sedimentation, and filtration), and disinfection (e.g., chlorination, UV) to allow pathogens’ inactivation and guarantee the safety of drinking water. However, despite the low prevalence observed in drinking waters, these can be potential sources of HAV contamination. In fact, due to the low infectious dose of HAV, the presence of the virus even at low concentrations can be enough to cause infection.
The regions with the highest HAV prevalence were Africa and Eastern Mediterranean. These regions are also strongly represented by low-income countries, confirming the study results showing that low-income economies had the highest HAV prevalence (29.0%). It has already been demonstrated that the socioeconomic status is strongly associated with HAV seroprevalence [177], and low-income economies represent endemic areas [12,13]. The endemicity of the disease in these regions is thought to be a consequence of water contamination, as these regions are also known for poor access to good quality water, poor sanitation, and lack of financial resources to set up adequate water treatment systems. Most of the European Union (EU) and European Economic Area (EEA) is considered a region of very low HAV endemicity; however, geographical differences exist, supporting the need to reconsider specific prevention and control measures, to further decrease HAV circulation [177].
The differences in prevalence observed in the studies of this review may also depend on the variety of methods used. Indeed, a wide variety of HAV detection methods were used in the collected studies; conventional RT-PCR was the most common (54.5%), followed by real-time PCR (37.5%). Data on HAV genotypes showed that IA was the most frequently detected, followed by IB; genotype IIIA was also detected in some cases. This is in agreement with epidemiological data, on the global distribution of HAV genotypes: genotype I is the most prevalent worldwide, with IA being reported more frequently than IB, and sub-genotype IIIA is prevalent in central Asia (https://www.ecdc.europa.eu/en/hepatitis-A/facts (accessed on 11 November 2022)). In areas of low endemicity, sub-genotype IA dominates, but all genotypes and subtypes have been reported [177].
Prevalence data over time (periods [1986–2000], [2000–2010], and [2010–2020]) showed no statistically significant differences or trends. Different factors may have contributed to such a result in one direction or another, such as the steady improvement of hygiene conditions worldwide, the increased sensitivity of analytical methods, and the progressive increasing number of studies from low and low/middle income economies.
Only 6.5% of the studies included in this review had a low risk of bias, which calls into question the methodological quality of the studies (e.g., errors in design, analysis, or reporting; missing information). A high level of heterogeneity of the data (I2 ˃ 75%) was also observed in this review, with only 3.0% of the studies conducted in low-income economies, in areas of high endemicity and low access to good quality water. This low percentage would considerably reduce the overall prevalence obtained. Publication bias was detected in association with the estimated prevalences in the different water groups (p < 0.05 for Egger’s test). This may point to underreporting of negative results obtained in monitoring studies, or may derive from an a priori selection of areas of investigation, prioritizing environmental studies targeting health-relevant viral pathogens with known high prevalence in the population. This may explain, for example, the unbalance of studies between northern/western and southern Europe in this review (19 studies vs. 53).
5. Conclusions
In conclusion, the results demonstrate that, except for drinking water where low HAV prevalence was observed, the other water matrices, and in particular untreated wastewater, can constitute a fairly important source of HAV contamination. However, based on the data, the water treatments significantly reduce the occurrence of HAV, suggesting that effective wastewater, surface water, and drinking water management systems are the key in the fight against waterborne hepatitis A.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20021054/s1.
Author Contributions
Conceptualization, G.R.T., S.K., E.S. and G.L.R.; methodology and analysis, J.T.E.-B., C.K.-N., D.S.M., A.B.-N., J.L.N.O., R.K.-M., S.T., J.K.-Z., R.L.F., E.Z.M., G.I.K.-N., J.N.M.-P., C.V., P.M., G.B.F., M.I., L.O. and C.D.G. writing—original draft preparation, G.R.T., S.K., E.S. and G.L.R.; writing—review and editing, G.R.T., S.K., E.S., G.L.R., J.T.E.-B., C.K.-N., D.S.M., A.B.-N., J.L.N.O., R.K.-M., S.T., J.K.-Z., R.L.F., E.Z.M., G.I.K.-N., J.N.M.-P., C.V., P.M., G.B.F., M.I., L.O. and C.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, G.; Jing, W.; Liu, J.; Liu, M. The global trends and regional differences in incidence and mortality of hepatitis A from 1990 to 2019 and implications for its prevention. Hepatol. Int. 2021, 15, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Bharara, T. Epidemiology of Hepatitis A: Past and Current Trends. In Hepatitis A and Other Associated Hepatobiliary Diseases; Costin, T.S., Cristin, C.V., Ion, R., Valeria, T., Silvia, L., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Amarapurkar, D.N.; Amarapurkar, A.D. Extrahepatic manifestations of viral hepatitis. Ann. Hepatol. 2002, 1, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, H.S. Hepatitis A: Clinical manifestations and management. Intervirology 2010, 53, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Andani, A.; van Elten, T.M.; Bunge, E.M.; Marano, C.; Salgado, F.; Jacobsen, K.H. Hepatitis A epidemiology in Latin American countries: A 2020 view from a systematic literature review. Expert Rev. Vaccines 2020, 19, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis, A. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a (accessed on 11 November 2022).
- Barrett, C.E.; Pape, B.J.; Benedict, K.M.; Foster, M.A.; Roberts, V.A.; Rotert, K.; Mattioli, M.C.; Yoder, J.S. Impact of Public Health Interventions on Drinking Water-Associated Outbreaks of Hepatitis A—United States, 1971–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Taffon, S.; Equestre, M.; Cella, E.; Lo Presti, A.; Costantino, A.; Chionne, P.; Madonna, E.; Golkocheva-Markova, E.; Bankova, D.; et al. Hepatitis a virus genotypes and strains from an endemic area of Europe, Bulgaria 2012–2014. BMC Infect. Dis 2017, 17, 497. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Dutta, S. Waterborne & foodborne viral hepatitis: A public health perspective. Indian J. Med. Res. 2019, 150, 432–435. [Google Scholar] [CrossRef]
- Chatziprodromidou, I.P.; Dimitrakopoulou, M.E.; Apostolou, T.; Katopodi, T.; Charalambous, E.; Vantarakis, A. Hepatitis A and E in the Mediterranean: A systematic review. Travel Med. Infect. Dis. 2022, 47, 102283. [Google Scholar] [CrossRef]
- Hu, X.; Collier, M.G.; Xu, F. Hepatitis A Outbreaks in Developed Countries: Detection, Control, and Prevention. Foodborne Pathog. Dis. 2020, 17, 166–171. [Google Scholar] [CrossRef]
- Jacobsen, K.H. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Van der Poel, W.; Rzezutka, A.; Hepatitis, A. In Sanitation and Disease in the 21st Century---Part Three: Specific Excreted Pathogens: Environmental and Epidemiology Aspects (Global Water Pathogen Project), Rose, J.B., Jiménez-Cisneros, B., Eds. Available online: https://www.waterpathogens.org/toc (accessed on 11 November 2022).
- Chatziprodromidou, I.P.; Bellou, M.; Vantarakis, G.; Vantarakis, A. Viral outbreaks linked to fresh produce consumption: A systematic review. J. Appl. Microbiol. 2018, 124, 932–942. [Google Scholar] [CrossRef]
- Bellou, M.; Kokkinos, P.; Vantarakis, A. Shellfish-borne viral outbreaks: A systematic review. Food Environ. Virol. 2013, 5, 13–23. [Google Scholar] [CrossRef]
- Thébault, A.; Roque-Afonso, A.-M.; Kooh, P.; Cadavez, V.; Gonzales-Barron, U.; Pavio, N. Risk factors for sporadic hepatitis A infection: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 100155. [Google Scholar] [CrossRef]
- Gholipour, S.; Ghalhari, M.R.; Nikaeen, M.; Rabbani, D.; Pakzad, P.; Miranzadeh, M.B. Occurrence of viruses in sewage sludge: A systematic review. Sci. Total Environ. 2022, 824, 153886. [Google Scholar] [CrossRef]
- Boehm, A.B.; Silverman, A.I.; Schriewer, A.; Goodwin, K. Systematic review and meta-analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters. Water Res. 2019, 164, 114898. [Google Scholar] [CrossRef]
- Kuodi, P.; Patterson, J.; Silal, S.; Hussey, G.D.; Kagina, B.M. Characterisation of the environmental presence of hepatitis A virus in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2020, 10, e036407. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- UN. UNSD—Methodology. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 8 April 2022).
- World Bank. World Bank Country and Lending Groups—World Bank Data Help Desk. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 8 April 2022).
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. Meta: An R Package for Meta-Analysis. Newsl. R Proj. 2007, 7, 40–45. [Google Scholar]
- Abbaszadegan, M.; Stewart, P.; LeChevallier, M. A strategy for detection of viruses in groundwater by PCR. Appl. Environ. Microbiol. 1999, 65, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Adefisoye, M.A.; Nwodo, U.U.; Green, E.; Okoh, A.I. Quantitative PCR Detection and Characterisation of Human Adenovirus, Rotavirus and Hepatitis A Virus in Discharged Effluents of Two Wastewater Treatment Facilities in the Eastern Cape, South Africa. Food Environ. Virol. 2016, 8, 262–274. [Google Scholar] [CrossRef]
- Ahmad, T.; Adnan, F.; Nadeem, M.; Kakar, S.J.; Anjum, S.; Saad, A.; Waheed, A.; Arshad, N. Assessment of the risk for human health of enterovirus and hepatitis a virus in clinical and water sources from three metropolitan cities of Pakistan. Ann. Agric. Environ. Med. 2018, 25, 708–713. [Google Scholar] [CrossRef]
- Ahmad, T.; Anjum, S.; Zaidi, N.S.S.; Ali, A.; Waqas, M.; Afzal, M.S.; Arshad, N. Frequency of hepatitis E and Hepatitis A virus in water sample collected from Faisalabad, Pakistan. Ann. Agric. Environ. Med. 2015, 22, 661–664. [Google Scholar] [CrossRef]
- Ahmad, T.; Arshad, N.; Adnan, F.; Zaidi, N.U.S.S.; Shahid, M.T.; Zahoor, U.; Afzal, M.S.; Anjum, S. Prevalence of rotavirus, adenovirus, hepatitis a virus and enterovirus in water samples collected from different region of Peshawar, Pakistan. Ann. Agric. Environ. Med. 2016, 23, 576–580. [Google Scholar] [CrossRef]
- Amdiouni, H.; Maunula, L.; Al-Shuwaikh, A.; Nourlil, J. Comparison of two virus concentration methods for enteric viruses detection in Moroccan wastewater and treated effluent. Iraqi JMS 2017, 15, 27–38. [Google Scholar] [CrossRef]
- Anastasi, P.; Bonanni, E.; Cecchini, G.; Divizia, M.; Donia, D.; Di Gianfilippo, F.; Gabrieli, R.; Petrinca, A.R.; Zanobini, A. Virus removal in conventional wastewater treatment process. Ig. Sanità Pubblica 2008, 64, 313–330. [Google Scholar]
- Arankalle, V.A.; Devi, K.L.S.; Lole, K.S.; Shenoy, K.T.; Verma, V.; Haneephabi, M. Molecular characteristics of hepatitis A virus from a large outbreak from Kerala, India. Indian J. Med. Res. 2006, 123, 760–769. [Google Scholar]
- Aw, T.G.; Gin, K.Y.H. Environmental surveillance and molecular characterization of human enteric viruses in tropical urban wastewaters. J. Appl. Microbiol. 2010, 109, 716–730. [Google Scholar] [CrossRef]
- Aw, T.G.; Gin, K.Y.H. Prevalence and genetic diversity of waterborne pathogenic viruses in surface waters of tropical urban catchments. J. Appl. Microbiol. 2011, 110, 903–914. [Google Scholar] [CrossRef]
- Bae, K.S.; Lee, S.; Lee, J.Y.; Kim, J.H.; Joo, Y.L.; Lee, S.H.; Chung, H.M.; You, K.A. Development of diagnostic systems for wide range and highly sensitive detection of two waterborne hepatitis viruses from groundwater using the conventional reverse transcription nested PCR assay. J. Virol. Methods 2022, 299, 114344. [Google Scholar] [CrossRef]
- Bahk, Y.Y.; Kim, M.H.; Kim, T.S.; Park, S.J.; Kim, J.M.; Rhee, O.J.; Lee, S.S. Occurrence of four waterborne viruses at five typical raw water resources in the Republic of Korea during August 2013 to February 2019. J. Microbiol. 2020, 58, 915–925. [Google Scholar] [CrossRef]
- Bai, H.; Shiota, T.; Yoshizaki, S.; Saito-Obata, M.; Malbas, F.F.; Lupisan, S.P.; Oshitani, H.; Takeda, N.; Muramatsu, M.; Wakita, T.; et al. Detection of subgenotype IA and IIIA hepatitis A viruses in rivers flowing through metro Manila, the Philippines. Jpn. J. Infect. Dis. 2019, 72, 53–55. [Google Scholar] [CrossRef]
- Barrella, K.M.; Garrafa, P.; Monezi, T.A.; Hársi, C.M.; Salvi, C.; Violante, P.A.B.C.; Mehnert, D.U. Longitudinal study on occurrence of adenoviruses and hepatitis A virus in raw domestic sewage in the city of Limeira, São Paulo. Braz. J. Microbiol. 2009, 40, 102–107. [Google Scholar] [CrossRef]
- Béji-Hamza, A.; Khélifi-Gharbi, H.; Hassine-Zaafrane, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M.; Petricca, S.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; et al. Qualitative and Quantitative Assessment of Hepatitis A Virus in Wastewaters in Tunisia. Food Environ. Virol. 2014, 6, 246–252. [Google Scholar] [CrossRef]
- Bisseux, M.; Colombet, J.; Mirand, A.; Roque-Afonso, A.M.; Abravanel, F.; Izopet, J.; Archimbaud, C.; Peigue-Lafeuille, H.; Debroas, D.; Bailly, J.L.; et al. Monitoring human enteric viruses in wastewater and relevance to infections encountered in the clinical setting: A one-year experiment in central France, 2014 to 2015. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Blanco, A.; Abid, I.; Al-Otaibi, N.; Pérez-Rodríguez, F.J.; Fuentes, C.; Guix, S.; Pintó, R.M.; Bosch, A. Glass Wool Concentration Optimization for the Detection of Enveloped and Non-enveloped Waterborne Viruses. Food Environ. Virol. 2019, 11, 184–192. [Google Scholar] [CrossRef]
- Borchardt, M.A.; Bertz, P.D.; Spencer, S.K.; Battigelli, D.A. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl. Environ. Microbiol. 2003, 69, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, M.A.; Haas, N.L.; Hunt, R.J. Vulnerability of drinking-water wells in La Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl. Environ. Microbiol. 2004, 70, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Battistini, R.; Rovini, E.; Verani, M. Viral removal by wastewater treatment: Monitoring of indicators and pathogens. Food Environ. Virol. 2009, 1, 85–91. [Google Scholar] [CrossRef]
- Carducci, A.; Casini, B.; Bani, A.; Rovini, E.; Verani, M.; Mazzoni, F.; Giuntini, A. Virological control of groundwater quality using biomolecular tests. Water Sci. Technol. 2003, 47, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Morici, P.; Pizzi, F.; Battistini, R.; Rovini, E.; Verani, M. Study of the viral removal efficiency in a urban wastewater treatment plant. Water Sci Technol. 2008, 58, 893–897. [Google Scholar] [CrossRef]
- Carducci, A.; Verani, M.; Battistini, R.; Pizzi, F.; Rovini, E.; Andreoli, E.; Casini, B. Epidemiological surveillance of human enteric viruses by monitoring of different environmental matrices. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2006, 54, 239–244. [Google Scholar] [CrossRef]
- Chacón, L.; Barrantes, K.; Santamaría-Ulloa, C.; Solano, M.; Reyes, L.; Taylor, L.; Valiente, C.; Symonds, E.M.; Achí, R. A Somatic Coliphage Threshold Approach to Improve the Management of Activated Sludge Wastewater Treatment Plant Effluents in Resource-Limited Regions. Appl. Environ. Microbiol. 2020, 86, e00616–e00620. [Google Scholar] [CrossRef]
- Chacón, L.; Morales, E.; Valiente, C.; Reyes, L.; Barrantes, K. Wastewater-based epidemiology of enteric viruses and surveillance of acute gastrointestinal illness outbreaks in a resource-limited region. Am. J. Trop. Med. Hyg. 2021, 105, 1004–1012. [Google Scholar] [CrossRef]
- Chigor, V.N.; Okoh, A.I. Quantitative RT-PCR detection of hepatitis A virus, rotaviruses and enteroviruses in the Buffalo River and source water dams in the Eastern Cape Province of South Africa. Int. J. Environ. Res. Public Health 2012, 9, 4017–4032. [Google Scholar] [CrossRef]
- Chitambar, S.; Joshi, M.; Lole, K.; Walimbe, A.; Vaidya, S. Cocirculation of and coinfections with hepatitis A virus subgenotypes IIIA and IB in patients from Pune, western India. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2007, 37, 85–93. [Google Scholar] [CrossRef]
- Cioffi, B.; Ianiro, G.; Iaccarino, D.; D’Apice, F.; Ferraro, A.; Race, M.; Spasiano, D.; Esposito, E.; Monini, M.; Serra, F.; et al. A potential risk assessment tool to monitor pathogens circulation in coastal waters. Environ. Res. 2021, 200, 111748. [Google Scholar] [CrossRef]
- Clemente-Casares, P.; Pina, S.; Buti, M.; Jardi, R.; Martín, M.; Bofill-Mas, S.; Girones, R. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 2003, 9, 448–454. [Google Scholar] [CrossRef]
- Corsi, S.R.; Borchardt, M.A.; Spencer, S.K.; Hughes, P.E.; Baldwin, A.K. Human and bovine viruses in the Milwaukee River watershed: Hydrologically relevant representation and relations with environmental variables. Sci. Total Environ. 2014, 490, 849–860. [Google Scholar] [CrossRef]
- De Giglio, O.; Caggiano, G.; Bagordo, F.; Barbuti, G.; Brigida, S.; Lugoli, F.; Grassi, T.; La Rosa, G.; Lucentini, L.; Uricchio, V.F.; et al. Enteric viruses and fecal bacteria indicators to assess groundwater quality and suitability for irrigation. Int. J. Environ. Res. Public Health 2017, 14, 558. [Google Scholar] [CrossRef]
- De Paula, V.S.; Diniz-Mendes, L.; Villar, L.M.; Luz, S.L.B.; Silva, L.A.; Jesus, M.S.; da Silva, N.M.V.S.; Gaspar, A.M.C. Hepatitis A virus in environmental water samples from the Amazon Basin. Water Res. 2007, 41, 1169–1176. [Google Scholar] [CrossRef]
- De Serres, G.; Cromeans, T.L.; Levesque, B.; Brassard, N.; Barthe, C.; Dionne, M.; Prud’Homme, H.; Paradis, D.; Shapiro, C.N.; Nainan, O.V.; et al. Molecular confirmation of hepatitis A virus from well water: Epidemiology and public health implications. J. Infect. Dis. 1999, 179, 37–43. [Google Scholar] [CrossRef]
- De Souza, F.G.; da Silva, F.P.; Staggemeier, R.; Rigotto, C.; Spilki, F.R. Low occurrence of hepatitis A virus in water samples from an urban area of southern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e69. [Google Scholar] [CrossRef]
- Denis-Mize, K.; Fout, G.S.; Dahling, D.R.; Francy, D.S. Detection of human enteric viruses in stream water with RT-PCR and cell culture. J. Water Health 2004, 2, 37–47. [Google Scholar] [CrossRef]
- Dias, J.; Pinto, R.N.; Vieira, C.B.; de Abreu Corrêa, A. Detection and quantification of human adenovirus (HAdV), JC polyomavirus (JCPyV) and hepatitis A virus (HAV) in recreational waters of Niterói, Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2018, 133, 240–245. [Google Scholar] [CrossRef]
- Divizia, M.; Morace, G.; Gabrieli, R.; Pisani, G.; Pana, A. Application of the PCR technique to the detection of hepatitis A virus in the environment. Water Sci. Technol. 1993, 27, 223–225. [Google Scholar] [CrossRef]
- Divizia, M.; Ruscio, V.; Degener, A.M.; Panà, A. Hepatitis A virus detection in wastewater by PCR and hybridization. New Microbiol. 1998, 21, 161–167. [Google Scholar]
- Dubrou, S.; Kopecka, H.; Lopez Pila, J.M.; Marechal, J.; Prevot, J. Detection of hepatitis A virus and other enteroviruses in wastewater and surface water samples by gene probe assay. Water Sci. Technol. 1991, 24, 267–272. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Fongaro, G.; Schissi, C.D.; Petrucio, M.M.; Barardi, C.R.M. Enteric viruses in surface water and sediment samples from the catchment area of Peri Lagoon, Santa Catarina State, Brazil. J. Water Health 2016, 14, 142–154. [Google Scholar] [CrossRef]
- Elmahdy, M.E.; Fongaro, G.; Magri, M.E.; Petruccio, M.M.; Barardi, C.R. Spatial distribution of enteric viruses and somatic coliphages in a Lagoon used as drinking water source and recreation in Southern Brazil. Int. J. Hyg. Environ. Health 2016, 219, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef]
- Félix, J.L.; Fernandez, Y.C.; Velarde-Félix, J.S.; Torres, B.V.; Cháidez, C. Detection and phylogenetic analysis of hepatitis a virus and norovirus in marine recreational waters of Mexico. J. Water Health 2010, 8, 269–278. [Google Scholar] [CrossRef]
- Fernández-Molina, M.C.; Álvarez, A.; Espigares, M. Presence of hepatitis a virus in water and its relationship with indicators of fecal contamination. Water Air Soil Pollut. 2004, 159, 197–208. [Google Scholar] [CrossRef]
- Fongaro, G.; Nascimento, M.A.; Viancelli, A.; Tonetta, D.; Petrucio, M.M.; Barardi, C.R.M. Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Sci. Technol. 2012, 66, 2682–2687. [Google Scholar] [CrossRef]
- Fongaro, G.; Viancelli, A.; dos Reis, D.A.; Santiago, A.F.; Hernández, M.; Michellon, W.; da Silva Lanna, M.C.; Treichel, H.; Rodríguez-Lázaro, D. Mineral Waste Containing High Levels of Iron from an Environmental Disaster (Bento Rodrigues, Mariana, Brazil) is Associated with Higher Titers of Enteric Viruses. Food Environ. Virol. 2019, 11, 178–183. [Google Scholar] [CrossRef]
- Formiga-Cruz, M.; Hundesa, A.; Clemente-Casares, P.; Albiñana-Gimenez, N.; Allard, A.; Girones, R. Nested multiplex PCR assay for detection of human enteric viruses in shellfish and sewage. J. Virol. Methods 2005, 125, 111–118. [Google Scholar] [CrossRef]
- Fout, G.S.; Martinson, B.C.; Moyer, M.W.N.; Dahling, D.R. A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater. Appl. Environ. Microbiol. 2003, 69, 3158–3164. [Google Scholar] [CrossRef]
- Fumian, T.M.; Victoria, M.; Vieira, C.B.; Fioretti, J.M.; Rocha, M.S.; Prado, T.; Guimarães, F.R.; da Gama, N.P.; de Oliveira, J.M.; Mendes, A.C.O.; et al. Enteric viruses’ dissemination in a private reserve of natural heritage. Lett. Appl. Microbiol. 2018, 66, 313–320. [Google Scholar] [CrossRef]
- Gcilitshana, O.; Sibanda, T.; Zhou, L.; Okoh, A.I. Assessment of the prevalence of enteric viruses in the final effluents of two peri-urban wastewater treatment plants. Asian Pac. J. Trop. Dis. 2017, 7, 121–126. [Google Scholar] [CrossRef]
- Gersberg, R.M.; Rose, M.A.; Robles-Sikisaka, R.; Dhar, A.K. Quantitative detection of hepatitis A virus and enteroviruses near the United States-Mexico border and correlation with levels of fecal indicator bacteria. Appl. Environ. Microbiol. 2006, 72, 7438–7444. [Google Scholar] [CrossRef]
- Gharbi-Khelifi, H.; Abid, N.B.S.; Sdiri, K.; Harrath, R.; Beji, A.; Bhiri, L.; Billaudel, S.; Ferre, V.; Aouni, M. Characterization of outbreak hepatitis a isolates in five Tunisian childcare centers. Braz. J. Microbiol. 2011, 42, 1204–1212. [Google Scholar] [CrossRef]
- Girones, R.; Puig, M.; Allard, A.; Lucena, F.; Wadell, G.; Jofre, J. Detection of adenovirus and enterovirus by PCR amplification in polluted waters. Water Sci. Technol. 1995, 31, 351–357. [Google Scholar] [CrossRef]
- Gozlan, Y.; Bar-Or, I.; Volnowitz, H.; Asulin, E.; Rich, R.; Anis, E.; Shemer, Y.; Cohen, M.S.; Dahary, E.L.; Schreiber, L.; et al. Lessons from intensified surveillance of viral hepatitis a, Israel, 2017 and 2018. Eurosurveillance 2021, 26, 2000001. [Google Scholar] [CrossRef]
- Gozlan, Y.; Volnowitz, H.; Bar-or, I.; Rich, R.; Mendelson, E.; Ari, Z.B.; Mor, O. Circulation of hepatitis a genotypes in Israel 2017-2018: Environmental surveillance supports clinical findings. J. Hepatol. 2019, 70, e722. [Google Scholar] [CrossRef]
- Grabow, W.O.K.; Taylor, M.B.; De Villiers, J.C. New methods for the detection of viruses: Call for review of drinking water quality guidelines. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Guerrero-Latorre, L.; Carratala, A.; Rodriguez-Manzano, J.; Calgua, B.; Hundesa, A.; Girones, R. Occurrence of water-borne enteric viruses in two settlements based in Eastern Chad: Analysis of hepatitis E virus, hepatitis A virus and human adenovirus in water sources. J. Water Health 2011, 9, 515–524. [Google Scholar] [CrossRef]
- Hassine, M.; Sdiri, K.; Riabi, S.; Beji, A.; Aouni, Z.; Aouni, M. Detection of enteric viruses in wastewater of Monastir region by RT-PCR method. Tunis. Med. 2010, 88, 57–62. [Google Scholar]
- Hee, J.K.; Young, O.S.; Sang, H.K. Detection of enteroviruses and mammalian reoviruses in Korean environmental waters. Microbiol. Immunol. 2006, 50, 781–786. [Google Scholar] [CrossRef]
- Hernandez-Morga, J.; Leon-Felix, J.; Peraza-Garay, F.; Gil-Salas, B.G.; Chaidez, C. Detection and characterization of hepatitis a virus and norovirus in estuarine water samples using ultrafiltration—RT-PCR integrated methods. J. Appl. Microbiol. 2009, 106, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Hot, D.; Legeay, O.; Jacques, J.; Gantzer, C.; Caudrelier, Y.; Guyard, K.; Lange, M.; Andréoletti, L. Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Res. 2003, 37, 4703–4710. [Google Scholar] [CrossRef] [PubMed]
- Iaconelli, M.; Muscillo, M.; Libera, S.D.; Fratini, M.; Meucci, L.; De Ceglia, M.; Giacosa, D.; La Rosa, G. One-year Surveillance of Human Enteric Viruses in Raw and Treated Wastewaters, Downstream River Waters, and Drinking Waters. Food Environ. Virol. 2017, 9, 79–88. [Google Scholar] [CrossRef]
- Iaconelli, M.; Purpari, G.; Libera, S.D.; Petricca, S.; Guercio, A.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Fratini, M.; et al. Hepatitis A and E Viruses in Wastewaters, in River Waters, and in Bivalve Molluscs in Italy. Food Environ. Virol. 2015, 7, 316–324. [Google Scholar] [CrossRef]
- Janahi, E.M.; Mustafa, S.; Parkar, S.F.D.; Naser, H.A.; Eisa, Z.M. Detection of enteric viruses and bacterial indicators in a sewage treatment center and shallow water bay. Int. J. Environ. Res. Public Health 2020, 17, 6483. [Google Scholar] [CrossRef]
- Jebri, S.; Jofre, J.; Barkallah, I.; Saidi, M.; Hmaied, F. Presence and fate of coliphages and enteric viruses in three wastewater treatment plants effluents and activated sludge from Tunisia. Environ. Sci. Pollut. Res. 2012, 19, 2195–2201. [Google Scholar] [CrossRef]
- Jehl-Pietri, C.; Hugues, B.; Andre, M.; Diez, J.M.; Bosch, A. Comparison of immunological and molecular hybridization detection methods for the detection of hepatitis A virus in sewage. Lett. Appl. Microbiol. 1993, 17, 162–166. [Google Scholar] [CrossRef]
- Jiang, S.C.; Chu, W. PCR detection of pathogenic viruses in southern California urban rivers. J. Appl. Microbiol. 2004, 97, 17–28. [Google Scholar] [CrossRef]
- Jones, T.H.; Brassard, J.; Topp, E.; Wilkes, G.; Lapen, D.R. Waterborne Viruses and F-Specific Coliphages in Mixed-Use Watersheds: Microbial Associations, Host Specificities, and Affinities with Environmental/Land Use Factors. Appl. Environ. Microbiol. 2017, 83, e02763-16. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cliver, D.O.; Mariam, T.W. Immunomagnetic capture PCR for rapid concentration and detection of hepatitis A virus from environmental samples. Appl. Environ. Microbiol. 1998, 64, 504–508. [Google Scholar] [CrossRef]
- Jothikumar, N.; Paulmurugan, R.; Padmanabhan, P.; Balathiripura Sundari, R.; Kamatchiammal, S.; Subba Rao, K. Duplex RT-PCR for simultaneous detection of hepatitis A and hepatitis E virus isolated from drinking water samples. J. Environ. Monit. 2000, 2, 587–590. [Google Scholar] [CrossRef]
- Jung, J.H.; Yoo, C.H.; Koo, E.S.; Kim, H.M.; Na, Y.; Jheong, W.H.; Jeong, Y.S. Occurrence of norovirus and other enteric viruses in untreated groundwaters of Korea. J. Water Health 2011, 9, 544–555. [Google Scholar] [CrossRef]
- Kaas, L.; Gourinat, A.C.; Urbès, F.; Langlet, J. A 1-Year Study on the Detection of Human Enteric Viruses in New Caledonia. Food Environ. Virol. 2016, 8, 46–56. [Google Scholar] [CrossRef]
- Kamel, A.H.; Ali, M.A.; El-Nady, H.G.; Deraz, A.; Aho, S.; Pothier, P.; Belliot, G. Presence of enteric hepatitis viruses in the sewage and population of Greater Cairo. Clin. Microbiol. Infect. 2011, 17, 1182–1185. [Google Scholar] [CrossRef]
- Katukiza, A.Y.; Temanu, H.; Chung, J.W.; Foppen, J.W.A.; Lens, P.N.L. Genomic copy concentrations of selected waterborne viruses in a slum environment in Kampala, Uganda. J. Water Health 2013, 11, 358–370. [Google Scholar] [CrossRef]
- Kittigul, L.; Raengsakulrach, B.; Siritantikorn, S.; Kanyok, R.; Utrarachkij, F.; Diraphat, P.; Thirawuth, V.; Siripanichgon, K.; Pungchitton, S.; Chitpirom, K.; et al. Detection of poliovirus, hepatitis A virus and rotavirus from sewage and water samples. Southeast. Asian J. Trop. Med. Public Health 2000, 31, 41–46. [Google Scholar]
- Kittigul, L.; Uthaisin, A.; Ekchaloemkiet, S.; Utrarachkij, F.; Luksamijarulkul, P. Detection and characterization of hepatitis A virus in water samples in Thailand. J. Appl. Microbiol. 2006, 100, 1318–1323. [Google Scholar] [CrossRef]
- Kiulia, N.M.; Netshikweta, R.; Page, N.A.; Van Zyl, W.B.; Kiraithe, M.M.; Nyachieo, A.; Mwenda, J.M.; Taylor, M.B. The detection of enteric viruses in selected urban and rural river water and sewage in Kenya, with special reference to rotaviruses. J. Appl. Microbiol. 2010, 109, 818–828. [Google Scholar] [CrossRef]
- Kokkinos, P.; Filippidou, S.; Karlou, K.; Vantarakis, A. Molecular typing of enteroviruses, adenoviruses, and hepatitis a viruses in untreated and treated sewage of a biological treatment plant in Greece. Food Environ. Virol. 2010, 2, 89–96. [Google Scholar] [CrossRef]
- Kokkinos, P.; Karayanni, H.; Meziti, A.; Feidaki, R.; Paparrodopoulos, S.; Vantarakis, A. Assessment of the Virological Quality of Marine and Running Surface Waters in NW Greece: A Case Study. Food Environ. Virol. 2018, 10, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Ziros, P.; Meri, D.; Filippidou, S.; Kolla, S.; Galanis, A.; Vantarakis, A. Environmental surveillance. An additional/alternative approach for virological surveillance in Greece? Int. J. Environ. Res. Public Health 2011, 8, 1914–1922. [Google Scholar] [CrossRef]
- La Rosa, G.; Della Libera, S.; Iaconelli, M.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Alfonsi, V.; Rizzo, C.; Tosti, M.E.; et al. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect. Dis. 2014, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Mancini, P.; Ferraro, G.B.; Iaconelli, M.; Veneri, C.; Paradiso, R.; De Medici, D.; Vicenza, T.; Proroga, Y.T.R.; Di Maro, O.; et al. Hepatitis A Virus Strains Circulating in the Campania Region (2015–2018) Assessed through Bivalve Biomonitoring and Environmental Surveillance. Viruses 2021, 13, 16. [Google Scholar] [CrossRef]
- La Rosa, G.; Sanseverino, I.; Della Libera, S.; Iaconelli, M.; Ferrero, V.E.V.; Barra Caracciolo, A.; Lettieri, T. The impact of anthropogenic pressure on the virological quality of water from the Tiber River, Italy. Lett. Appl. Microbiol. 2017, 65, 298–305. [Google Scholar] [CrossRef]
- Lazić, G.; Grubač, S.; Lupulović, D.; Bugarski, D.; Lazić, S.; Knežević, P.; Petrović, T. Presence of Human and Animal Viruses in Surface Waters in Vojvodina Province of Serbia. Food Environ. Virol. 2015, 7, 149–158. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.J. Molecular detection of human enteric viruses in urban rivers in Korea. J. Microbiol. Biotechnol. 2008, 18, 1156–1163. [Google Scholar]
- Lee, G.C.; Kim, M.J.; Nam, S.; Lee, C.H. Incidence and molecular characterization of hepatitis A viruses in Korean surface water between 2007 and 2010. Microbiol. Immunol. 2014, 58, 342–351. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Corsi, S.R.; Borchardt, M.A.; Spencer, S.K.; Baldwin, A.K.; Lutz, M.A. Hydrologic, land cover, and seasonal patterns of waterborne pathogens in Great Lakes tributaries. Water Res. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Masachessi, G.; Pisano, M.B.; Prez, V.E.; Martínez, L.C.; Michelena, J.F.; Martínez-Wassaf, M.; Giordano, M.O.; Isa, M.B.; Pavan, J.V.; Welter, A.; et al. Enteric Viruses in Surface Waters from Argentina: Molecular and Viable-Virus Detection. Appl. Environ. Microbiol. 2018, 84, e02327-17. [Google Scholar] [CrossRef]
- Miagostovich, M.P.; Guimarães, F.R.; Vieira, C.B.; Fumian, T.M.; da Gama, N.P.; Victoria, M.; de Oliveira, J.M.; de Oliveira Mendes, A.C.; Gaspar, A.M.C.; Leite, J.P.G. Assessment of Water Quality in a Border Region between the Atlantic Forest and an Urbanised Area in Rio de Janeiro, Brazil. Food Environ. Virol. 2014, 6, 110–115. [Google Scholar] [CrossRef]
- Morace, G.; Pisani, G.; Divizia, M.; Panà, A. Detection of hepatitis A virus in concentrated river water by polymerase chain reaction. Zent. Für Hyg. Und Umweltmed. Int. J. Hyg. Environ. Med. 1993, 193, 521–527. [Google Scholar]
- Moresco, V.; Viancelli, A.; Nascimento, M.A.; Souza, D.S.M.; Ramos, A.P.D.; Garcia, L.A.T.; Simões, C.M.O.; Barardi, C.R.M. Microbiological and physicochemical analysis of the coastal waters of southern Brazil. Mar. Pollut. Bull. 2012, 64, 40–48. [Google Scholar] [CrossRef]
- Nasiri, M.; Ghalejoogh, Z.Y.; Ataei-Pirkooh, A.; Bokharaei-Salim, F.; Monavari, S.H.; Tavakoli, A.; Asadifar, B.; Esghaei, M.; Pasalari, H.; Samimi-Rad, K.; et al. Detection and Phylogenetic Analysis of Hepatitis A Virus in the Wastewater Treatment Plant of Ekbatan Town in Tehran, Iran. Hepat. Mon. 2021, 21, e121270. [Google Scholar] [CrossRef]
- Nasser, A.M.; Metcalf, T.G. An A-ELISA to detect hepatitis A virus in estuarine samples. Appl. Environ. Microbiol. 1987, 53, 1192–1195. [Google Scholar] [CrossRef]
- Ngaosuwankul, N.; Thippornchai, N.; Yamashita, A.; Vargas, R.E.M.; Tunyong, W.; Mahakunkijchareon, Y.; Ikuta, K.; Singhasivanon, P.; Okabayashi, T.; Leaungwutiwong, P. Detection and characterization of enteric viruses in flood water from the 2011 Thai flood. Jpn. J. Infect. Dis. 2013, 66, 398–403. [Google Scholar] [CrossRef]
- Novaković, T.; Agolli, B.; Maretić, Z.; Mijajlović, Z.; Cobeljić, M.; Zivanović-Marinković, V.; Krstić, L.; Birtasević, B. An epidemic of viral hepatitis A after consumption of mussels from polluted seawater. Vojnosanit. Pregl. Mil. Med. Pharm. Rev. 1983, 40, 163–167. [Google Scholar]
- O’Brien, E.; Nakyazze, J.; Wu, H.; Kiwanuka, N.; Cunningham, W.; Kaneene, J.B.; Xagoraraki, I. Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res. 2017, 127, 41–49. [Google Scholar] [CrossRef]
- Osuolale, O.; Okoh, A. Incidence of human adenoviruses and Hepatitis A virus in the final effluent of selected wastewater treatment plants in Eastern Cape Province, South Africa. Virol. J. 2015, 12, 98. [Google Scholar] [CrossRef]
- Ouardani, I.; Manso, C.F.; Aouni, M.; Romalde, J.L. Efficiency of hepatitis A virus removal in six sewage treatment plants from central Tunisia. Appl. Microbiol. Biotechnol. 2015, 99, 10759–10769. [Google Scholar] [CrossRef] [PubMed]
- Ouardani, I.; Turki, S.; Aouni, M.; Romalde, J.L. Detection and Molecular Characterization of Hepatitis A Virus from Tunisian Wastewater Treatment Plants with Different Secondary Treatments. Appl. Environ. Microbiol. 2016, 82, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Peláez, D.; Guzmán, B.L.; Rodríguez, J.; Acero, F.; Nava, G. Presence of enteric viruses in water samples for consumption in Colombia: Challenges for supply systems. Biomed. Rev. Inst. Nac. Salud 2016, 36, 169–178. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Galli, C.; Binda, S.; Primache, V.; Tagliacarne, C.; Pizza, F.; Mazzini, R.; Pariani, E.; Romanò, L. Molecular Characterization and Phylogenetic Analysis of Enteroviruses and Hepatitis A Viruses in Sewage Samples, Northern Italy, 2016. Food Environ. Virol. 2019, 11, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Petrinca, A.R.; Donia, D.; Pierangeli, A.; Gabrieli, R.; Degener, A.M.; Bonanni, E.; Diaco, L.; Cecchini, G.; Anastasi, P.; Divizia, M. Presence and environmental circulation of enteric viruses in three different wastewater treatment plants. J. Appl. Microbiol. 2009, 106, 1608–1617. [Google Scholar] [CrossRef]
- Pietri, C.; Hugues, B.; Puel, D. Immune electron microscopy in the detection of viruses other than enteroviruses on cell culture in untreated sewage. Zent. Fur Bakteriol. Mikrobiol. Hygiene Ser. B Umwelthyg. Krankenh. Arb. Prav. Med. 1988, 186, 67–72. [Google Scholar]
- Pina, S.; Buti, M.; Jardí, R.; Clemente-Casares, P.; Jofre, J.; Girones, R. Genetic analysis of hepatitis A virus strains recovered from the environment and from patients with acute hepatitis. J. Gen. Virol. 2001, 82, 2955–2963. [Google Scholar] [CrossRef]
- Pina, S.; Puig, M.; Lucena, F.; Jofre, J.; Girones, R. Viral pollution in the environment and in shellfish: Human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 1998, 64, 3376–3382. [Google Scholar] [CrossRef]
- Pintó, R.M.; Alegre, D.; Domíngueza, A.; El-Senousy, W.M.; Sánchez, G.; Villena, C.; Costafreda, M.I.; Aragonès, L.; Bosch, A. Hepatitis A virus in urban sewage from two Mediterranean countries. Epidemiol. Infect. 2007, 135, 270–273. [Google Scholar] [CrossRef]
- Prado, T.; Barbosa, M.R.F.; Araújo, R.S.; Garcia, S.C.; Melo, A.J.; Galvani, A.T.; Brandão, C.J.; Silva, R.L.O.; Sato, M.I.Z. Hepatitis A Outbreaks and Environmental Circulation of Genotype IA Strains in the São Paulo City, 2017–2018. Food Environ. Virol. 2021, 13, 520–527. [Google Scholar] [CrossRef]
- Prado, T.; Fumian, T.M.; Miagostovich, M.P.; Gaspar, A.M.C. Monitoring the hepatitis A virus in urban wastewater from Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 104–109. [Google Scholar] [CrossRef]
- Prado, T.; Silva, D.M.; Guilayn, W.C.; Rose, T.L.; Gaspar, A.M.C.; Miagostovich, M.P. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011, 45, 1287–1297. [Google Scholar] [CrossRef]
- Prevost, B.; Lucas, F.S.; Goncalves, A.; Richard, F.; Moulin, L.; Wurtzer, S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ. Int. 2015, 79, 42–50. [Google Scholar] [CrossRef]
- Puntaric, D.; Cecuk, D.; Grce, M.; Vodopija, I.; Ljubicic, M.; Baklaic, Z. Human virus detection in drinking water in Zagreb from 1991 to 1994. Period. Biol. 1995, 97, 347–349. [Google Scholar]
- Purpari, G.; Macaluso, G.; Di Bella, S.; Gucciardi, F.; Mira, F.; Di Marco, P.; Lastra, A.; Petersen, E.; La Rosa, G.; Guercio, A. Molecular characterization of human enteric viruses in food, water samples, and surface swabs in Sicily. Int. J. Infect. Dis. 2019, 80, 66–72. [Google Scholar] [CrossRef]
- Pusch, D.; Oh, D.Y.; Wolf, S.; Dumke, R.; Schröter-Bobsin, U.; Höhne, M.; Röske, I.; Schreier, E. Detection of enteric viruses and bacterial indicators in German environmental waters. Arch. Virol. 2005, 150, 929–947. [Google Scholar] [CrossRef]
- Rachida, S.; Matsapola, P.N.; Wolfaardt, M.; Taylor, M.B. Genetic characterization of a novel hepatitis a virus strain in irrigation water in South Africa. J. Med. Virol. 2016, 88, 734–737. [Google Scholar] [CrossRef]
- Rachida, S.; Taylor, M.B. Potentially infectious novel hepatitis a virus strains detected in selected treated wastewater discharge sources, South Africa. Viruses 2020, 12, 1468. [Google Scholar] [CrossRef]
- Rigotto, C.; Victoria, M.; Moresco, V.; Kolesnikovas, C.K.; Corrêa, A.; Souza, D.S.M.; Miagostovich, M.P.; Simões, C.M.O.; Barardi, C.R.M. Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis, South Brazil. J. Appl. Microbiol. 2010, 109, 1979–1987. [Google Scholar] [CrossRef]
- Rodriguez-Manzano, J.; Alonso, J.L.; Ferrús, M.A.; Moreno, Y.; Amorós, I.; Calgua, B.; Hundesa, A.; Guerrero-Latorre, L.; Carratala, A.; Rusiñol, M.; et al. Standard and new faecal indicators and pathogens in sewage treatment plants, microbiological parameters for improving the control of reclaimed water. Water Sci. Technol. 2012, 66, 2517–2523. [Google Scholar] [CrossRef]
- Rodriguez-Manzano, J.; Miagostovich, M.; Hundesa, A.; Clemente-Casares, P.; Carratala, A.; Buti, M.; Jardi, R.; Girones, R. Analysis of the evolution in the circulation of HAV and HEV in Eastern Spain by testing urban sewage samples. J. Water Health 2010, 8, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, I.; Ababneh, Q.; Jaradat, Z. Genomic detection of waterborne enteric viruses as water quality indicators in Al-Zarqa River, Jordan. J. Water Health 2021, 19, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Saïd, R.; Wolfaardt, M.; Taylor, M.B. Molecular characterisation of hepatitis A virus strains from water sources in South Africa. Water Sci. Technol. 2014, 69, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Schlindwein, A.D.; Rigotto, C.; Simões, C.M.; Barardi, C.R. Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2010, 61, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Schvoerer, E.; Bonnet, F.; Dubois, V.; Cazaux, G.; Serceau, R.; Fleury, H.J.A.; Lafon, M.E. PCR detection of human enteric viruses in bathing areas, waste waters and human stools in southwestern France. Res. Microbiol. 2000, 151, 693–701. [Google Scholar] [CrossRef]
- Shaheen, M.N.F.; Elmahdy, E.M.; Chawla-Sarkar, M. Quantitative PCR-based identification of enteric viruses contaminating fresh produce and surface water used for irrigation in Egypt. Environ. Sci. Pollut. Res. Int. 2019, 26, 21619–21628. [Google Scholar] [CrossRef]
- Shin, H.; Park, H.; Seo, D.J.; Jung, S.; Yeo, D.; Wang, Z.; Park, K.H.; Choi, C. Foodborne Viruses Detected Sporadically in the Fresh Produce and Its Production Environment in South Korea. Foodborne Pathog. Dis. 2019, 16, 411–420. [Google Scholar] [CrossRef]
- Simmons, F.J.; Xagoraraki, I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011, 45, 3590–3598. [Google Scholar] [CrossRef]
- Staggemeier, R.; Heck, T.M.S.; Demoliner, M.; Ritzel, R.G.F.; Röhnelt, N.M.S.; Girardi, V.; Venker, C.A.; Spilki, F.R. Enteric viruses and adenovirus diversity in waters from 2016 Olympic venues. Sci. Total Environ. 2017, 586, 304–312. [Google Scholar] [CrossRef]
- Steyer, A.; Gutiérrez-Aguirre, I.; Rački, N.; Glaser, S.B.; Humar, B.B.; Stražar, M.; Škrjanc, I.; Poljšak-Prijatelj, M.; Ravnikar, M.; Rupnik, M. The Detection Rate of Enteric Viruses and Clostridium difficile in a Waste Water Treatment Plant Effluent. Food Environ. Virol. 2015, 7, 164–172. [Google Scholar] [CrossRef]
- Stokdyk, J.P.; Firnstahl, A.D.; Walsh, J.F.; Spencer, S.K.; de Lambert, J.R.; Anderson, A.C.; Rezania, L.W.; Kieke, B.A., Jr.; Borchardt, M.A. Viral, bacterial, and protozoan pathogens and fecal markers in wells supplying groundwater to public water systems in Minnesota, USA. Water Res. 2020, 178, 115814. [Google Scholar] [CrossRef]
- Taylor, M.B.; Cox, N.; Vrey, M.A.; Grabow, W.O.K. The occurrence of hepatitis A and astroviruses in selected river and dam waters in South Africa. Water Res. 2001, 35, 2653–2660. [Google Scholar] [CrossRef]
- Thongprachum, A.; Fujimoto, T.; Takanashi, S.; Saito, H.; Okitsu, S.; Shimizu, H.; Khamrin, P.; Maneekarn, N.; Hayakawa, S.; Ushijima, H. Detection of nineteen enteric viruses in raw sewage in Japan. Infect. Genet. Evol. 2018, 63, 17–23. [Google Scholar] [CrossRef]
- Truchado, P.; Garre, A.; Gil, M.I.; Simón-Andreu, P.J.; Sánchez, G.; Allende, A. Monitoring of human enteric virus and coliphages throughout water reuse system of wastewater treatment plants to irrigation endpoint of leafy greens. Sci. Total Environ. 2021, 782, 146837. [Google Scholar] [CrossRef]
- Tucker, J.D.; Grasso-Knight, G. Environmental hepatitis A detection and awareness on a Native American reservation. Bull. World Health Organ. 2000, 78, 948. [Google Scholar]
- Vaidya, S.R.; Chitambar, S.D.; Arankalle, V.A. Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J. Hepatol. 2002, 37, 131–136. [Google Scholar] [CrossRef]
- Van Zyl, W.B.; Zhou, N.A.; Wolfaardt, M.; Matsapola, P.N.; Ngwana, F.B.; Symonds, E.M.; Fagnant-Sperati, C.S.; Shirai, J.H.; Kossik, A.L.; Beck, N.K.; et al. Detection of potentially pathogenic enteric viruses in environmental samples from Kenya using the bag-mediated filtration system. Water Sci. Technol. Water Supply 2019, 19, 1668–1676. [Google Scholar] [CrossRef]
- Vantarakis, A.; Papapetropoulou, M. Detection of enteroviruses, adenoviruses and Hepatitis A viruses in raw sewage and treated effluents by nested-PCR. Water Air Soil Pollut. 1999, 114, 85–93. [Google Scholar] [CrossRef]
- Vantarakis, A.C.; Tsibouxi, A.; Venieri, D.; Komninou, G.; Athanassiadou, A.; Papapetropoulou, M. Evaluation of microbiological quality of coastal waters in Greece. J. Water Health 2005, 3, 371–380. [Google Scholar] [CrossRef]
- Venter, J.M.; van Heerden, J.; Vivier, J.C.; Grabow, W.O.; Taylor, M.B. Hepatitis A virus in surface water in South Africa: What are the risks? J. Water Health 2007, 5, 229–240. [Google Scholar] [CrossRef]
- Verma, V.; Arankalle, V.A. Hepatitis e virus-based evaluation of a virion concentration method and detection of enteric viruses in environmental samples by multiplex nested RT-PCR. J. Appl. Microbiol. 2010, 108, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; De Paula, V.S.; Diniz-Mendes, L.; Guimarães, F.R.; Ferreira, F.F.M.; Shubo, T.C.; Miagostovich, M.P.; Lampe, E.; Gaspar, A.M.C. Molecular detection of hepatitis A virus in urban sewage in Rio de Janeiro, Brazil. Lett. Appl. Microbiol. 2007, 45, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Neyvaldt, J.; Enache, L.; Sikora, P.; Mattsson, A.; Johansson, A.; Lindh, M.; Bergstedt, O.; Norder, H. Variations among Viruses in Influent Water and Effluent Water at a Wastewater Plant over One Year as Assessed by Quantitative PCR and Metagenomics. Appl. Environ. Microbiol. 2020, 86, e02073-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sikora, P.; Rutgersson, C.; Lindh, M.; Brodin, T.; Björlenius, B.; Larsson, D.G.J.; Norder, H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Environ. Health 2018, 221, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shin, H.; Jung, S.; Yeo, D.; Park, H.; Shin, S.; Seo, D.J.; Park, K.H.; Choi, C. Effects of Weather and Environmental Factors on the Seasonal Prevalence of Foodborne Viruses in Irrigation Waters in Gyeonggi Province, Korea. Microorganisms 2020, 8, 1224. [Google Scholar] [CrossRef]
- Wong, M.V.; Hashsham, S.A.; Gulari, E.; Rouillard, J.M.; Aw, T.G.; Rose, J.B. Detection and characterization of human pathogenic viruses circulating in community wastewater using multi target microarrays and polymerase chain reaction. J. Water Health 2013, 11, 659–670. [Google Scholar] [CrossRef]
- Yanez, L.A.; Lucero, N.S.; Barril, P.A.; Díaz, M.D.P.; Tenaglia, M.M.; Spinsanti, L.I.; Nates, S.V.; Isa, M.B.; Ré, V.E. Evidence of Hepatitis A virus circulation in central Argentina: Seroprevalence and environmental surveillance. J. Clin. Virol. 2014, 59, 38–43. [Google Scholar] [CrossRef]
- Jacobsen, K.H.; Koopman, J.S. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int. J. Epidemiol. 2005, 34, 600–609. [Google Scholar] [CrossRef]
- Nelson, N.P. Chapter 4: Travel-Related Infectious Diseases, Hepatitis A. In CDC Yellow Book; Oxford Universitiy Press: Oxford, UK, 2018; Volume 8, p. a031716. [Google Scholar]
- Costafreda, M.I.; Bosch, A.; Pintó, R.M. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Carrillo-Santisteve, P.; Tavoschi, L.; Severi, E.; Bonfigli, S.; Edelstein, M.; Byström, E.; Lopalco, P. Seroprevalence and susceptibility to hepatitis A in the European Union and European Economic Area: A systematic review. Lancet. Infect. Dis. 2017, 17, e306–e319. [Google Scholar] [CrossRef]
- Desbois, D.; Couturier, E.; Mackiewicz, V.; Graube, A.; Letort, M.J.; Dussaix, E.; Roque-Afonso, A.M. Epidemiology and genetic characterization of hepatitis A virus genotype IIA. J. Clin. Microbiol. 2010, 48, 3306–3315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).