Smart Walk: A Culturally Tailored Smartphone-Delivered Physical Activity Intervention for Cardiometabolic Risk Reduction among African American Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Description of the Smart Walk PA Intervention
| SCT Construct | Concepts Leveraged by the Intervention | Description of How the Intervention Targeted Each Theoretical Construct |
|---|---|---|
| Behavioral Capability—PA-related knowledge and skill |
|
|
| Social Support—Extent to which family and friends support, encourage/discourage, and/or influence PA engagement. |
|
|
| Self-efficacy—Confidence in one’s ability to overcome barriers and be physically active |
|
|
| Outcome Expectations—Anticipated outcomes associated with PA engagement |
|
|
| Self-regulation—Management of intrapersonal and interpersonal processes to achieve PA goals |
|
|
2.4. Cultural Tailoring of the Intervention
2.5. Description of the Smart Health Attention-Matched Wellness Comparison Intervention
2.6. Measures
2.6.1. Demographic Characteristics
2.6.2. Feasibility and Acceptability
2.6.3. Physical Activity
2.6.4. Cardiometabolic Risk Markers
2.6.5. Social Cognitive Theory Outcomes
2.7. Protocol
2.8. Statistical Design
2.8.1. Sample Size Considerations
2.8.2. Statistical Analyses
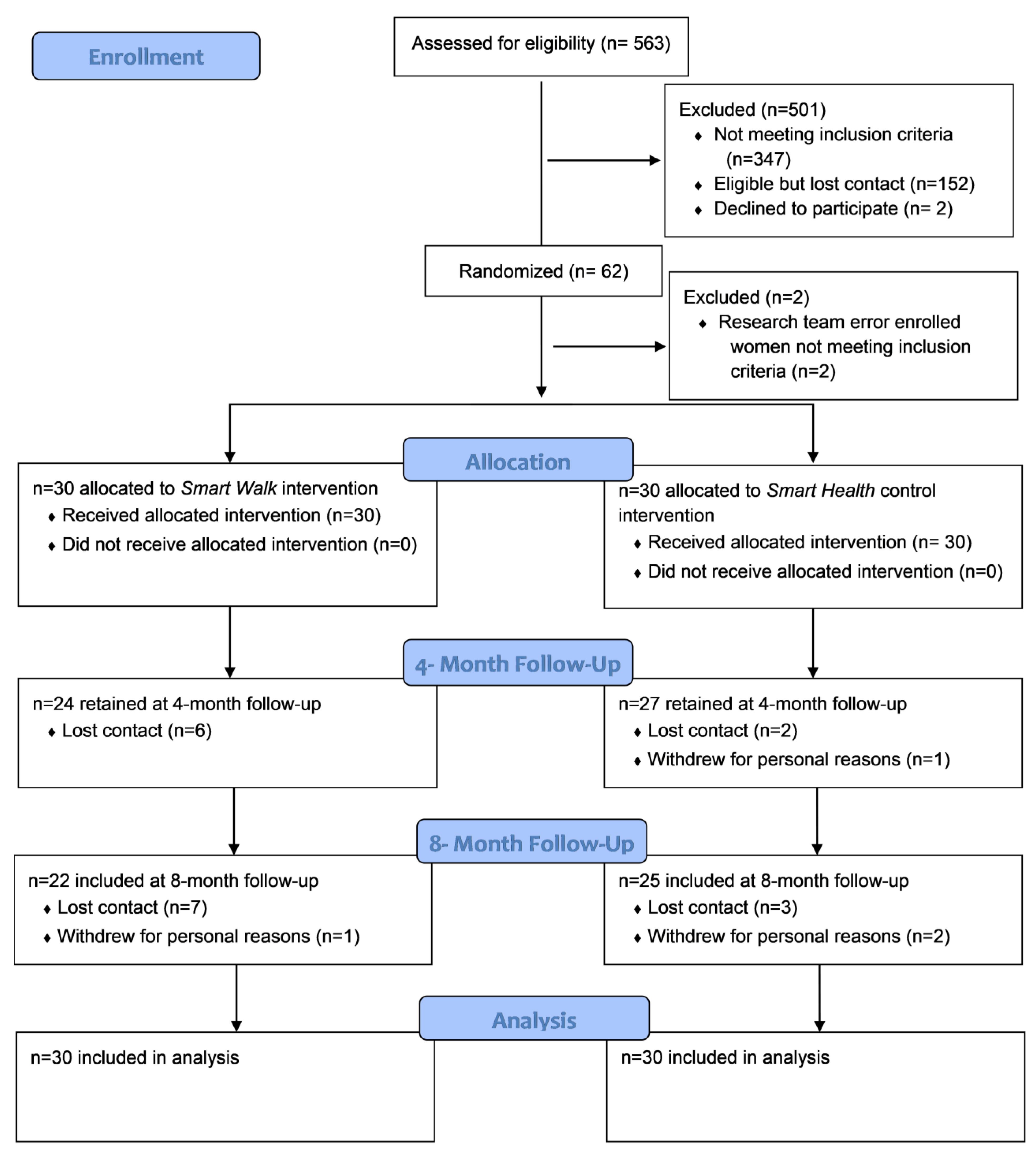
3. Results
3.1. Participant Characteristics
3.2. Feasibility and Acceptability
3.2.1. Recruitment and Retention
3.2.2. Objectively-Measured App Use, Fitbit Wear, and Participant Feedback Regarding the Intervention
3.3. Physical Activity
3.4. Cardiometabolic Risk Markers
3.5. Social Cognitive Theory Outcomes
4. Discussion
4.1. Feasibility and Acceptability
4.2. Physical Activity and Cardiometabolic Disease Risk Outcomes
4.3. Psychosocial Outcomes
4.4. Limitations, Strengths, and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semlitsch, T.; Jeitler, K.; Hemkens, L.G.; Horvath, K.; Nagele, E.; Schuermann, C.; Pignitter, N.; Hermann, K.H.; Waffenschmidt, S.; Siebenhofer, A. Increasing physical activity for the treatment of hypertension: A systematic review and meta-analysis. Sport. Med. 2013, 43, 1009–1023. [Google Scholar] [CrossRef]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H. Effects of exercise, diet and their combination on blood pressure. J. Hum. Hypertens. 2005, 19 (Suppl. S3), S20–S24. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Ascione, A.; Marciano, F.; Volpe, A.; La Sala, G.; Savastano, S.; Colao, A.; Palomba, S. Effects of physical exercise on the female reproductive system. Minerva Endocrinol. 2013, 38, 305–319. [Google Scholar] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Franklin, B. Aerobic exercise and lipids and lipoproteins in patients with cardiovascular disease: A meta-analysis of randomized controlled trials. J. Cardiopulm. Rehabil. 2006, 26, 131–139, quiz 131–140; discussion 134–142. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Roberts, S.; Haskell, W. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. Clin. Nutr. 2012, 31, 156–167. [Google Scholar] [CrossRef]
- Eckardt, K.; Taube, A.; Eckel, J. Obesity-associated insulin resistance in skeletal muscle: Role of lipid accumulation and physical inactivity. Rev. Endocr. Metab. Disord. 2011, 12, 163–172. [Google Scholar] [CrossRef]
- Venkatasamy, V.V.; Pericherla, S.; Manthuruthil, S.; Mishra, S.; Hanno, R. Effect of Physical activity on Insulin Resistance, Inflammation and Oxidative Stress in Diabetes Mellitus. J. Clin. Diagn. Res. JCDR 2013, 7, 1764–1766. [Google Scholar] [CrossRef]
- Thomas, G.N.; Jiang, C.Q.; Taheri, S.; Xiao, Z.H.; Tomlinson, B.; Cheung, B.M.; Lam, T.H.; Barnett, A.H.; Cheng, K.K. A systematic review of lifestyle modification and glucose intolerance in the prevention of type 2 diabetes. Curr. Diabetes Rev. 2010, 6, 378–387. [Google Scholar] [CrossRef]
- Jarvie, J.L.; Whooley, M.A.; Regan, M.C.; Sin, N.L.; Cohen, B.E. Effect of Physical Activity Level on Biomarkers of Inflammation and Insulin Resistance Over 5 Years in Outpatients With Coronary Heart Disease (from the Heart and Soul Study). Am. J. Cardiol. 2014, 114, 1192–1197. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2014, 57, 306–314. [Google Scholar] [CrossRef]
- Swift, D.L.; Lavie, C.J.; Johannsen, N.M.; Arena, R.; Earnest, C.P.; O’Keefe, J.H.; Milani, R.V.; Blair, S.N.; Church, T.S. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ. J. 2013, 77, 281–292. [Google Scholar] [CrossRef]
- DeFina, L.F.; Haskell, W.L.; Willis, B.L.; Barlow, C.E.; Finley, C.E.; Levine, B.D.; Cooper, K.H. Physical activity versus cardiorespiratory fitness: Two (partly) distinct components of cardiovascular health? Prog. Cardiovasc. Dis. 2015, 57, 324–329. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Green, D.J.; Eijsvogels, T.; Bouts, Y.M.; Maiorana, A.J.; Naylor, L.H.; Scholten, R.R.; Spaanderman, M.E.; Pugh, C.J.; Sprung, V.S.; Schreuder, T.; et al. Exercise training and artery function in humans: Nonresponse and its relationship to cardiovascular risk factors. J. Appl. Physiol. 2014, 117, 345–352. [Google Scholar] [CrossRef]
- Jennersjo, P.; Ludvigsson, J.; Lanne, T.; Nystrom, F.H.; Ernerudh, J.; Ostgren, C.J. Pedometer-determined physical activity is linked to low systemic inflammation and low arterial stiffness in Type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2012, 29, 1119–1125. [Google Scholar] [CrossRef]
- Graham, M.R.; Evans, P.; Davies, B.; Baker, J.S. Arterial pulse wave velocity, inflammatory markers, pathological GH and IGF states, cardiovascular and cerebrovascular disease. Vasc. Health Risk Manag. 2008, 4, 1361–1371. [Google Scholar] [CrossRef]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W., 3rd; Haskell, W.; Lee, I.M. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef]
- Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Campbell, W.W.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med. Sci. Sport. Exerc. 2019, 51, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: A systematic review and meta-analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef] [PubMed]
- 2018 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report; US Department of Health and Human Services, Ed.; US Department of Health and Human Services: Washington, DC, USA, 2018.
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Powell, K.E.; Campbell, W.W.; Dipietro, L.; Pate, R.R.; Pescatello, L.S.; Collins, K.A.; Bloodgood, B.; Piercy, K.L. Physical activity and the prevention of weight gain in adults: A systematic review. Med. Sci. Sport. Exerc. 2019, 51, 1262–1269. [Google Scholar] [CrossRef]
- Williams, W.M.; Yore, M.; Whitt-Glover, M. Estimating physical activity trends among blacks in the United States through examination of four national surveys. AIMS Public Health 2018, 5, 144–157. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; US Department of Health and Human Services: Hyattsville, MD, USA, 2018.
- Hales, C.M.; Carrol, M.D.; Fryer, C.D.; Ogden, C.L. Prevalance of Obesity among Adults and Youth: United States, 2015–2016; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017; Centers for Disease Control and Prevention; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2017; p. 20.
- Resnicow, K.; Baranowski, T.; Ahluwalia, J.S.; Braithwaite, R.L. Cultural sensitivity in public health: Defined and demystified. Ethn. Dis. 1999, 9, 10–21. [Google Scholar]
- Whitt-Glover, M.C.; Keith, N.R.; Ceaser, T.G.; Virgil, K.; Ledford, L.; Hasson, R.E. A systematic review of physical activity interventions among African American adults: Evidence from 2009 to 2013. Obes. Rev. 2014, 15 (Suppl. S4), 125–145. [Google Scholar] [CrossRef]
- Jenkins, F.; Jenkins, C.; Gregoski, M.J.; Magwood, G.S. Interventions promoting physical activity in African American women: An integrative review. J. Cardiovasc. Nurs. 2017, 32, 22–29. [Google Scholar] [CrossRef]
- Bland, V.; Sharma, M. Physical activity interventions in African American women: A systematic review. Health Promot. Perspect. 2017, 7, 52–59. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Racial and Ethnic Approaches to Community Health. Available online: https://www.cdc.gov/nccdphp/dnpao/state-local-programs/reach/index.htm (accessed on 15 December 2022).
- Pew Research Center. Mobile Fact Sheet. Available online: https://www.pewresearch.org/internet/fact-sheet/mobile/ (accessed on 15 December 2022).
- Feter, N.; Dos Santos, T.S.; Caputo, E.L.; da Silva, M.C. What is the role of smartphones on physical activity promotion? A systematic review and meta-analysis. Int. J. Public Health 2019, 64, 679–690. [Google Scholar] [CrossRef]
- Mönninghoff, A.; Kramer, J.N.; Hess, A.J.; Ismailova, K.; Teepe, G.W.; Tudor Car, L.; Müller-Riemenschneider, F.; Kowatsch, T. Long-term Effectiveness of mHealth Physical Activity Interventions: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Med. Internet Res. 2021, 23, e26699. [Google Scholar] [CrossRef]
- de Arenas-Arroyo, S.N.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Sequí-Domínguez, I.; Reina-Gutiérrez, S.; Martínez-Vizcaíno, V. Effect of eHealth to increase physical activity in healthy adults over 55 years: A systematic review and meta-analysis. Scand. J. Med. Sci. Sport. 2021, 31, 776–789. [Google Scholar] [CrossRef]
- Joseph, R.P.; Royse, K.E.; Benitez, T.J. A Systematic Review of Electronic and Mobile Health (e- and mHealth) Physical Activity Interventions for African American and Hispanic Women. J. Phys. Act. Health 2019, 16, 230–239. [Google Scholar] [CrossRef]
- Kim, B.H.; Glanz, K. Text messaging to motivate walking in older African Americans: A randomized controlled trial. Am. J. Prev. Med. 2013, 44, 71–75. [Google Scholar] [CrossRef]
- McCoy, P.; Leggett, S.; Bhuiyan, A.; Brown, D.; Frye, P.; Williams, B. Text messaging: An intervention to increase physical activity among African American participants in a faith-based, competitive weight loss program. Int. J. Environ. Res. Public Health 2017, 14, 326. [Google Scholar] [CrossRef]
- Joseph, R.P.; Keller, C.; Adams, M.A.; Ainsworth, B.E. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: A randomized pilot trial. BMC Womens Health 2015, 15, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Jemmott Iii, J.B. Mobile app-based small-group physical activity intervention for young African American women: A pilot randomized controlled trial. Prev. Sci. 2019, 20, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.P.; Ainsworth, B.E.; Hollingshead, K.; Todd, M.; Keller, C. Results of a culturally tailored smartphone-delivered physical activity intervention among midlife African American women: Feasibility trial. JMIR Mhealth Uhealth 2021, 9, e27383. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.; Kirk, A.; Macmillan, F.; Mutrie, N. Can physical activity interventions for adults with type 2 diabetes be translated into practice settings? A systematic review using the RE-AIM framework. Transl. Behav. Med. 2014, 4, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Belanger-Gravel, A.; Godin, G.; Vezina-Im, L.A.; Amireault, S.; Poirier, P. The effect of theory-based interventions on physical activity participation among overweight/obese individuals: A systematic review. Obes. Rev. 2011, 12, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef]
- Brook, R.D.; Appel, L.J.; Rubenfire, M.; Ogedegbe, G.; Bisognano, J.D.; Elliott, W.J.; Fuchs, F.D.; Hughes, J.W.; Lackland, D.T.; Staffileno, B.A.; et al. Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the american heart association. Hypertension 2013, 61, 1360–1383. [Google Scholar] [CrossRef]
- Joseph, R.P.; Ainsworth, B.E.; Vega-Lopez, S.; Adams, M.A.; Hollingshead, K.; Hooker, S.P.; Todd, M.; Gaesser, G.A.; Keller, C. Rationale and design of Smart Walk: A randomized controlled pilot trial of a smartphone-delivered physical activity and cardiometabolic risk reduction intervention for African American women. Contemp. Clin. Trials 2019, 77, 46–60. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport Sci. J. Can. Des Sci. Du Sport 1992, 17, 338–345. [Google Scholar]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Framework; Prentice Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Tudor-Locke, C.; Han, H.; Aguiar, E.J.; Barreira, T.V.; Schuna, J.M., Jr.; Kang, M.; Rowe, D.A. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: A narrative review. Br. J. Sport. Med. 2018, 52, 776–788. [Google Scholar] [CrossRef]
- Slaght, J.; Senechal, M.; Hrubeniuk, T.J.; Mayo, A.; Bouchard, D.R. Walking Cadence to Exercise at Moderate Intensity for Adults: A Systematic Review. J. Sport. Med. 2017, 2017, 4641203. [Google Scholar] [CrossRef]
- Joseph, R.P.; Ainsworth, B.E.; Keller, C.; Dodgson, J.E. Barriers to physical activity among African American women: An integrative review of the literature. Women Health 2015, 55, 579–699. [Google Scholar] [CrossRef]
- Joseph, R.P.; Keller, C.; Affuso, O.; Ainsworth, B.E. Designing culturally relevant physical activity programs for African-American women: A framework for intervention development. J. Racial Ethn. Health Disparities 2017, 4, 397–409. [Google Scholar] [CrossRef]
- Joseph, R.P.; Ainsworth, B.E.; Mathis, L.; Hooker, S.P.; Keller, C. Utility of social cognitive theory in intervention design for promoting physical activity among African-American women: A qualitative study. Am. J. Health Behav. 2017, 54, 16. [Google Scholar] [CrossRef]
- Joseph, R.P.; Ainsworth, B.E.; Mathis, L.; Hooker, S.P.; Keller, C. Incorporating religion and spirituality into the design of community-based physical activity programs for African American women: A qualitative inquiry. BMC Res. Notes 2017, 10, 506. [Google Scholar] [CrossRef]
- Joseph, R.P.; Coe, K.; Ainsworth, B.E.; Hooker, S.P.; Mathis, L.; Keller, C. Hair as a barrier to physical activity among African American women: A qualitative exploration. Front. Public Health 2017, 5, 367. [Google Scholar] [CrossRef]
- Hartman, S.J.; Nelson, S.H.; Weiner, L.S. Patterns of Fitbit Use and Activity Levels Throughout a Physical Activity Intervention: Exploratory Analysis from a Randomized Controlled Trial. JMIR Mhealth Uhealth 2018, 6, e29. [Google Scholar] [CrossRef]
- Joseph, R.P.; Pekmezi, D.; Dutton, G.R.; Cherrington, A.L.; Kim, Y.I.; Allison, J.J.; Durant, N.H. Results of a culturally adapted internet-enhanced physical activity pilot intervention for overweight and obese young adult African American women. J. Transcult. Nurs. 2016, 27, 136–146. [Google Scholar] [CrossRef]
- Coleman, K.J.; Ngor, E.; Reynolds, K.; Quinn, V.P.; Koebnick, C.; Young, D.R.; Sternfeld, B.; Sallis, R.E. Initial validation of an exercise "vital sign" in electronic medical records. Med. Sci. Sport. Exerc. 2012, 44, 2071–2076. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Penafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schroder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sport. Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J. Meas. Phys. Behav. 2019, 2, 188. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sport. Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Padrick, K.P. Use of the modified Balke treadmill protocol for determining the aerobic capacity of women with fibromyalgia. Arthritis Care Res. 1989, 2, 165–167. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Foster, C.; Crowe, A.J.; Daines, E.; Dumit, M.; Green, M.A.; Lettau, S.; Thompson, N.N.; Weymier, J. Predicting functional capacity during treadmill testing independent of exercise protocol. Med. Sci. Sport. Exerc. 1996, 28, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Martinez, M.E.; Marshall, J.R.; Graver, E.; Whitacre, R.C.; Woolf, K.; Ritenbaugh, C.; Alberts, D.S. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol. Biomark. Prev. 1999, 8, 941–946. [Google Scholar]
- Thomson, C.A.; Giuliano, A.; Rock, C.L.; Ritenbaugh, C.K.; Flatt, S.W.; Faerber, S.; Newman, V.; Caan, B.; Graver, E.; Hartz, V.; et al. Measuring dietary change in a diet intervention trial: Comparing food frequency questionnaire and dietary recalls. Am. J. Epidemiol. 2003, 157, 754–762. [Google Scholar] [CrossRef]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Sallis, J.F.; Pinski, R.B.; Grossman, R.M.; Patterson, T.L.; Nader, P.R. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ. Res. 1988, 3, 283–292. [Google Scholar] [CrossRef]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef]
- Anderson, E.S.; Winett, R.A.; Wojcik, J.R.; Williams, D.M. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: Social support, self-efficacy, outcome expectations and self-regulation in the guide-to-health trial. J. Health Psychol. 2010, 15, 21–32. [Google Scholar] [CrossRef]
- Anderson, E.S.; Wojcik, J.R.; Winett, R.A.; Williams, D.M. Social-cognitive determinants of physical activity: The influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychol. 2006, 25, 510–520. [Google Scholar] [CrossRef]
- Resnick, B.; Zimmerman, S.I.; Orwig, D.; Furstenberg, A.L.; Magaziner, J. Outcome expectations for exercise scale: Utility and psychometrics. J. Gerontol. B Psychol. Sci. Soc. Sci. 2000, 55, S352–S356. [Google Scholar] [CrossRef]
- Vega, W.A.; Sallis, J.F.; Patterson, T.; Rupp, J.; Atkins, C.; Nader, P.R. Assessing knowledge of cardiovascular health-related diet and exercise behaviors in Anglo- and Mexican-Americans. Prev. Med. 1987, 16, 696–709. [Google Scholar] [CrossRef]
- Kay, M.C.; Carroll, D.D.; Carlson, S.A.; Fulton, J.E. Awareness and knowledge of the 2008 Physical Activity Guidelines for Americans. J. Phys. Act. Health 2014, 11, 693–698. [Google Scholar] [CrossRef]
- Uschner, D.; Schindler, D.; Hilgers, R.-D.; Heussen, N. randomizeR: An R Package for the Assessment and Implementation of Randomization in Clinical Trials. J. Stat. Softw. 2018, 85, 1–22. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Collins, L.; Huang, L.; Dziak, J. MOST: Multiphase Optimization Strategy; R Package Version 0.1.0; The Methodology Center, The Pennsylvania State University: University Park, PA, USA, 2017. [Google Scholar]
- Keller, B.T.; Enders, C.K. Blimp User’s Manual (Version 3). 2021. Available online: http://www.appliedmissingdata.com/multilevel-imputation.html (accessed on 30 December 2022).
- Grund, S.; Robitzsch, A.; Luedtke, O. Tools for Multiple Imputation in Multilevel Modeling; R Package Version 0.4-1. 2021. Available online: https://cran.r-project.org/web/packages/mitml/mitml.pdf (accessed on 30 December 2022).
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Morris, S.B. Distribution of the standardized mean change effect size for meta-analysis on repeated measures. Br. J. Math. Stat. Psychol 2000, 53 (Pt 1), 17–29. [Google Scholar] [CrossRef]
- Fleury, J.; Lee, S.M. The social ecological model and physical activity in African American women. Am. J. Community Psychol. 2006, 37, 129–140. [Google Scholar] [CrossRef]
- Jee, H. Review of researches on smartphone applications for physical activity promotion in healthy adults. J. Exerc. Rehabil. 2017, 13, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Amagai, S.; Pila, S.; Kaat, A.J.; Nowinski, C.J.; Gershon, R.C. Challenges in participant engagement and retention using mobile health apps: Literature review. J. Med. Internet Res. 2022, 24, e35120. [Google Scholar] [CrossRef] [PubMed]
- Göttgens, I.; Oertelt-Prigione, S. The application of human-centered design approaches in health research and innovation: A narrative review of current practices. JMIR Mhealth Uhealth 2021, 9, e28102. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, P.; Deng, H.; Wang, X.; Li, X.; Fu, H. Design features for improving mobile health intervention user engagement: Systematic review and thematic analysis. J. Med. Internet Res. 2020, 22, e21687. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, C.G. What types of exercise are more effective in reducing obesity and blood pressure for middle-aged women? A systematic review with meta-analysis. Biol. Res. Nurs. 2021, 23, 658–675. [Google Scholar] [CrossRef]
- Sundström, J.; Arima, H.; Jackson, R.; Turnbull, F.; Rahimi, K.; Chalmers, J.; Woodward, M.; Neal, B. Effects of blood pressure reduction in mild hypertension: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 184–191. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, M.J.; Cui, Y.J.; Liang, L.; Zhou, M.M.; Yang, Y.W.; Huang, F. Carotid-femoral pulse wave velocity in the prediction of cardiovascular events and mortality: An updated systematic review and meta-analysis. Angiology 2018, 69, 617–629. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitão, C.; Gouveia, M.; Figueiredo, D.; Alves, A.J.; Polonia, J.; Oliveira, J.; et al. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sport. Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The effects of exercise and physical activity on weight loss and maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Rovniak, L.S.; Hovell, M.F.; Wojcik, J.R.; Winett, R.A.; Martinez-Donate, A.P. Enhancing theoretical fidelity: An e-mail-based walking program demonstration. Am. J. Health Promot. 2005, 20, 85–95. [Google Scholar] [CrossRef]
- Young, M.D.; Plotnikoff, R.C.; Collins, C.E.; Callister, R.; Morgan, P.J. Social cognitive theory and physical activity: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 983–995. [Google Scholar] [CrossRef]
- Carey, R.N.; Connell, L.E.; Johnston, M.; Rothman, A.J.; de Bruin, M.; Kelly, M.P.; Michie, S. Behavior Change Techniques and Their Mechanisms of Action: A Synthesis of Links Described in Published Intervention Literature. Ann. Behav. Med. 2019, 53, 693–707. [Google Scholar] [CrossRef]

| Module Topic | |||
|---|---|---|---|
| Week Number | Module Number | Smart Walk Physical Activity Intervention | Smart Health Comparison Intervention |
| 1 | 1 | Overview of the health benefits of PA and the national PA guidelines | Healthy Skin Care Practices |
| 2 | 2 | PA-related health disparities among AA women; Importance being a PA role model in the AA community | Hydration and Water Consumption |
| 3 | 3 | PA time management | Heat Illness: Symptoms and Prevention |
| 4 | 4 | PA goal setting | Communicating with Doctor |
| 5 | 5 | Overcoming general barriers to PA | Breast Cancer: Prevention and Screening |
| 6 | 6 | Tips for increasing daily PA | Cervical Cancer: Prevention and Screening |
| 7 | 7 | Overcoming hair-related barriers to PA | Hair Care |
| 8 | 8 | Social support for PA | Oral Health |
| 9 | 9 | Trying new types of activities | Vision and Eye Health |
| 10 | 10 | Reducing sedentary time | Sickle Cell Disease |
| 11 | 11 | Healthy eating complement PA | Stress Management |
| 12 | 12 | Muscle-strengthening and stretching activities | Cold and Flu Prevention |
| 13 | N/A | ||
| 14 | 13 | Dealing with PA setbacks. | Tips to Stay Healthy when Travelling |
| 15 | N/A | ||
| 16 | 14 | Review of previous modules and PA maintenance | Review of Previous Modules |
| Cultural Consideration | Description | How the Cultural Consideration Was Incorporated into the Intervention |
|---|---|---|
| Collectivism | Attending to the needs and well-being of others over the needs and well-being of one’s self. This can contribute to AA women reporting lack of time, energy, or resources for PA. Notably, this consideration is reported among women regardless of races/ethnicities, but previous research suggests this concept may be more accentuated in the AA community. | PA promotion materials:
|
| Racial pride/Role modelling | Awareness and interest in how one’s behavior can contribute the collective health and well-being of the AA community. | Intervention materials underscored the notion that physically active AA women are positive role models, which can encourage other members of the AA community to be physically active |
| Physical appearance preferences | Some AA women are hesitant to engage in PA because:
| Intervention materials:
|
| Intervention Component | Smart Walk Intervention | Smart Health Intervention |
|---|---|---|
| Features available on the smartphone application | ||
| Personal profiles | X | X |
| Weekly multi-media modules | X | X |
| Discussion boards | X | X |
| Activity tracker that integrates with the Fibit device | X | |
| Text messages delivered 3 times per week | X | X |
| Characteristic | Smart Walk | Comparison |
|---|---|---|
| Age (years; M and SD) | 37.2 (7.0) | 39.5 (6.8) |
| Relationship status (n and %) | ||
| Married | 10 (33%) | 12 (40%) |
| Committed partnership | 6 (20%) | 2 (7%) |
| Divorced | 6 (20%) | 3 (10%) |
| Separated | 0 (0%) | 1 (3%) |
| Never married | 8 (27%) | 12 (40%) |
| Number of children < 18 years (Median and range) | 1 (0–5) | 1 (0–4) |
| Education (n and %) | ||
| Some high school | 1 (3%) | 0 (0%) |
| High school diploma or GED | 5 (17%) | 2 (7%) |
| Some college or technical school | 13 (43%) | 11 (37%) |
| Bachelor’s degree | 5 (17%) | 7 (23%) |
| Master’s degree | 5 (17%) | 10 (33%) |
| Doctoral degree | 1 (3%) | 0 (0%) |
| Annual income (n and %) | ||
| <USD 25 K | 6 (20%) | 3 (10%) |
| USD 25,001–USD 50 K | 12 (40%) | 11 (37%) |
| USD 50,001–USD 75 K | 10 (33%) | 12 (40%) |
| USD 75,001–USD 100 K | 2 (7%) | 3 (10%) |
| >USD 100 K | 0 (0%) | 1 (3%) |
| Employment status (n and %) | ||
| Employed, Part-time | 6 (20%) | 4 (14%) |
| Employed, Full-time | 19 (63%) | 24 (83%) |
| Currently unemployed | 4 (13%) | 2 (7%) |
| Module/Intervention Week a | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Mean # of module views/participant | 2.5 | 2.3 | 1.8 | 1.2 | 1.2 | 1.0 | 1.1 | 0.9 | 0.8 | 1.0 | 0.6 | 0.7 | n/a | 0.9 | n/a | 0.3 |
| Mean # of discussion posts/participant | 2.1 | 1.1 | 1.1 | 0.8 | 0.3 | 0.6 | 0.4 | 0.4 | 0.9 | 0.4 | 0.4 | 0.4 | n/a | 0.4 | n/a | 0.3 |
| Mean # Fitbit wear days/week/participant b | 5.7 | 5.7 | 5.9 | 5.5 | 5.3 | 4.7 | 4.9 | 4.7 | 4.7 | 4.4 | 4.1 | 3.7 | 3.5 | 3.7 | 4.0 | 3.9 |
| Baseline | 4-Month | 8-Month | Baseline to 4-Month Change | Baseline to 8-Month Change | ||||
|---|---|---|---|---|---|---|---|---|
| PA Outcome | Study Arm | M (SD) | M (SD) | M (SD) | dc (95% CI) | pe | dc (95% CI) | pe |
| Self-reported MVPA (min/week) a | Smart Walk | 30.7 (37.3) | 102.2 (73.5) | 114.1 (116.1) | 0.69 | 0.018 | 0.63 | 0.046 |
| Comparison | 30.0 (43.8) | 58.5 (56.6) | 56.9 (74.8) | (0.16, 1.22) | (0.10, 1.16) | |||
| Self-reported Estimated Energy Expenditure (MET-min/week) b | ||||||||
| Light PA | Smart Walk | 79.1 (187.6) | 266.2 (436.2) | 171.1 (218.1) | 0.41 | 0.159 | 0.72 | 0.030 |
| Comparison | 77.0 (109.6) | 131.4 (199.5) | 53.6 (89.1) | (−0.11, 0.93) | (0.19, 1.25) | |||
| Moderate PA | Smart Walk | 81.7 (234.9) | 222.5 (363.6) | 95.2 (131.0) | 0.50 | 0.090 | 0.74 | 0.031 |
| Comparison | 17.7 (35.0) | 80.0 (168.2) | 17.6 (88.6) | (−0.02, 1.02) | (0.21, 1.27) | |||
| Vigorous PA | Smart Walk | 80.5 (128.2) | 324.2 (514.1) | 247.8 (228.5) | 0.38 | 0.196 | 0.60 | 0.052 |
| Comparison | 100.8 (139.8) | 183.2 (309.6) | 128.4 (181.3) | (−0.14, 0.90) | (0.07, 1.13) | |||
| Total PA | Smart Walk | 241.3 (500.4) | 871.9 (1065.9) | 524.9 (437.5) | 0.53 | 0.074 | 0.87 | 0.007 |
| Comparison | 410.2 (1169.6) | 396.1 (554.9) | 212.2 (275.9) | (0.01, 1.05) | (0.33, 1.41) | |||
| Accelerometer-measured MVPA (min/day) | ||||||||
| 1 min bouts | Smart Walk | 16.9 (13.1) | 13.8 (21.4) | 15.5 (16.0) | −0.39 | 0.202 | −0.41 | 0.078 |
| Comparison | 13.4 (8.1) | 20.0 (15.9) | 14.6 (8.1) | (−0.91, 0.13) | (−0.93, 0.11) | |||
| 10 min bouts | Smart Walk | 3.4 (7.1) | 1.3 (4.9) | 1.4 (4.3) | −0.25 | 0.536 | −0.25 | 0.430 |
| Comparison | 1.0 (2.9) | 1.4 (5.0) | 1.3 (2.2) | (−0.77, 0.27) | (−0.77, 0.27) | |||
| Baseline | 4-Month | 8-Month | Baseline to 4-Month Change | Baseline to 8-Month Change | ||||
|---|---|---|---|---|---|---|---|---|
| PA Outcome | Study Arm | M (SD) | M (SD) | M (SD) | de (95% CI) | pf | de (95% CI) | pf |
| BMI (kg/m2) a | Smart Walk | 40.0 (6.7) | 39.2 (6.6) | 36.3 (9.9) | −0.14 | 0.147 | 0.27 | 0.317 |
| Comparison | 41.2 (7.3) | 41.3 (7.5) | 41.2 (7.0) | (−0.66, 0.38) | (−0.49, 1.03) | |||
| Systolic Blood Pressure (mmHG) | Smart Walk | 134.6 (14.6) | 130.8 (17.2) | 130.2 (20.3) | −0.22 | 0.391 | −0.01 | 0.977 |
| Comparison | 136.2 (15.8) | 135.2 (16.1) | 134.1 (15.6) | (−0.74, 0.30) | (−0.78, 0.76) | |||
| Diastolic Blood Pressure (mmHG) | Smart Walk | 83.4 (11.8) | 79.1 (13.5) | 81.8 (9.6) | −0.37 | 0.179 | −0.16 | 0.602 |
| Comparison | 84.6 (10.9) | 84.0 (11.3) | 85.2 (9.0) | (−0.89, 0.15) | (−0.93, 0.61) | |||
| Cardiorespiratory Fitness (mL/kg/min) | Smart Walk | 18.8 (2.9) | 19.8 (4.7) | 18.3 (2.9) | 0.40 | 0.090 | −0.17 | 0.518 |
| Comparison | 18.5 (3.7) | 17.8 (4.2) | 17.4 (2.9) | (−0.12, 0.92) | (−0.98, 0.64) | |||
| Pulse wave velocity (m/s) | Smart Walk | 7.7 (1.4) | 7.4 (1.6) | 7.3 (1.1) | −0.33 | 0.131 | −0.01 | 0.976 |
| Comparison | 7.2 (1.0) | 7.3 (1.3) | 7.1 (1.0) | (−0.85, 0.19) | (−0.78, 0.76) | |||
| Total cholesterol (mg/dL) b | Smart Walk | 184.4 (39.2) | 193.2 (44.9) | 186.8 (50.4) | 0.13 | 0.986 | 0.18 | 0.364 |
| Comparison | 188.6 (29.4) | 197.8 (40.4) | 192.2 (35.2) | (−0.39, 0.65) | (−0.63, 0.99) | |||
| LDL cholesterol (mg/dL) c | Smart Walk | 119.9 (33.7) | 124.5 (36.9) | 117.2 (38.6) | −0.11 | 0.878 | 0.27 | 0.253 |
| Comparison | 122.2 (27.0) | 128.6 (31.5) | 127.7 (26.8) | (−0.63, 0.41) | (−0.54, 1.08) | |||
| HDL cholesterol (mg/dL) c | Smart Walk | 46.6 (8.4) | 50.2 (12.6) | 50.3 (12.1) | 0.25 | 0.336 | 0.00 | 0.982 |
| Comparison | 48.5 (11.3) | 48.9 (14.0) | 48.7 (11.1) | (−0.27, 0.77) | (−0.80, 0.80) | |||
| Triglycerides (mg/dL) c | Smart Walk | 88.6 (37.5) | 91.9 (35.4) | 96.3 (40.2) | −0.12 | 0.873 | 0.06 | 0.213 |
| Comparison | 89.2 (51.4) | 90.6 (42.7) | 78.9 (39.4) | (−0.64, 0.40) | (−0.74, 0.86) | |||
| Fasting glucose (mg/dl) d | Smart Walk | 107.5 (54.6) | 112.0 (46.4) | 100.0 (8.9) | 0.14 | 0.402 | 0.06 | 0.858 |
| Comparison | 107.4 (29.4) | 106.9 (29.2) | 108.2 (51.0) | (−0.38, 0.66) | (−0.74, 0.86) | |||
| Insulin (µIU/mL) d | Smart Walk | 20.9 (18.8) | 21.3 (28.0) | 27.4 (18.7) | 0.20 | 0.916 | 0.40 | 0.324 |
| Comparison | 22.8 (17.2) | 20.9 (15.6) | 23.8 (16.4) | (−0.32, 0.72) | (−0.41, 1.21) | |||
| TNF-α (pg/mL) | Smart Walk | 1.5 (1.5) | 1.6 (1.8) | 1.1 (0.5) | 0.08 | 0.361 | −0.16 | 0.439 |
| Comparison | 1.3 (0.5) | 1.3 (0.5) | 1.4 (0.6) | (−0.44, 0.60) | (−0.97, 0.65) | |||
| Baseline | 4-Month | 8-Month | Baseline to 4-Month Change | Baseline to 8-Month Change | |||||
|---|---|---|---|---|---|---|---|---|---|
| SCT Construct | Range a | Study Arm | M (SD) | M (SD) | M (SD) | db (95% CI) | pc | db (95% CI) | pc |
| PA Behavioral Capability | 1–5 | Smart Walk | 1.5 (1.0) | 3.1 (1.5) | 2.9 (1.7) | 1.25 | <0.001 | 0.92 | 0.009 |
| Comparison | 1.7 (0.8) | 1.8 (0.9) | 1.6 (1.4) | (0.68, 1.82) | (0.38, 1.46) | ||||
| Exercise Self-Efficacy | 1–5 | Smart Walk | 4.0 (0.7) | 4.1 (0.8) | 3.6 (1.0) | 0.39 | 0.130 | −0.17 | 0.723 |
| Comparison | 3.9 (0.6) | 3.8 (0.7) | 3.6 (0.8) | (−0.13, 0.91) | (−0.69, 0.35) | ||||
| PA Self-regulation | 1–5 | Smart Walk | 2.2 (0.9) | 2.7 (0.8) | 2.5 (1.1) | 0.29 | 0.319 | 0.29 | 0.438 |
| Comparison | 2.1 (0.5) | 2.5 (0.9) | 2.3 (1.0) | (−0.23, 0.81) | (−0.23, 0.81) | ||||
| Social Support for PA from Family | 10–50 | Smart Walk | 24.9 (9.9) | 30.7 (15.9) | 31.4 (19.7) | 0.31 | 0.260 | 0.27 | 0.326 |
| Comparison | 22.3 (9.6) | 25.1 (11.3) | 24.8 (12.3) | (−0.21, 0.83) | (−0.25, 0.79) | ||||
| Social Support for PA from Friends | 8–40 | Smart Walk | 20.3 (9.8) | 21.3 (12.4) | 21.2 (13.4) | 0.12 | 0.879 | −0.17 | 0.750 |
| Comparison | 19.3 (8.6) | 20.0 (10.3) | 21.5 (10.7) | (−0.31, 0.73) | (−0.69, 0.35) | ||||
| Outcome Expectations | 1–5 | Smart Walk | 4.2 (0.5) | 4.3 (0.6) | 4.0 (1.0) | 0.34 | 0.260 | −0.17 | 0.868 |
| Comparison | 4.2 (0.4) | 4.1 (0.5) | 4.0 (0.7) | (−0.18, 0.86) | (−0.69, 0.35) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, R.P.; Todd, M.; Ainsworth, B.E.; Vega-López, S.; Adams, M.A.; Hollingshead, K.; Hooker, S.P.; Gaesser, G.A.; Keller, C. Smart Walk: A Culturally Tailored Smartphone-Delivered Physical Activity Intervention for Cardiometabolic Risk Reduction among African American Women. Int. J. Environ. Res. Public Health 2023, 20, 1000. https://doi.org/10.3390/ijerph20021000
Joseph RP, Todd M, Ainsworth BE, Vega-López S, Adams MA, Hollingshead K, Hooker SP, Gaesser GA, Keller C. Smart Walk: A Culturally Tailored Smartphone-Delivered Physical Activity Intervention for Cardiometabolic Risk Reduction among African American Women. International Journal of Environmental Research and Public Health. 2023; 20(2):1000. https://doi.org/10.3390/ijerph20021000
Chicago/Turabian StyleJoseph, Rodney P., Michael Todd, Barbara E. Ainsworth, Sonia Vega-López, Marc A. Adams, Kevin Hollingshead, Steven P. Hooker, Glenn A. Gaesser, and Colleen Keller. 2023. "Smart Walk: A Culturally Tailored Smartphone-Delivered Physical Activity Intervention for Cardiometabolic Risk Reduction among African American Women" International Journal of Environmental Research and Public Health 20, no. 2: 1000. https://doi.org/10.3390/ijerph20021000
APA StyleJoseph, R. P., Todd, M., Ainsworth, B. E., Vega-López, S., Adams, M. A., Hollingshead, K., Hooker, S. P., Gaesser, G. A., & Keller, C. (2023). Smart Walk: A Culturally Tailored Smartphone-Delivered Physical Activity Intervention for Cardiometabolic Risk Reduction among African American Women. International Journal of Environmental Research and Public Health, 20(2), 1000. https://doi.org/10.3390/ijerph20021000







