Melatonin Action in Type 2 Diabetic Parotid Gland and Dental Pulp: In Vitro and Bioinformatic Findings
Abstract
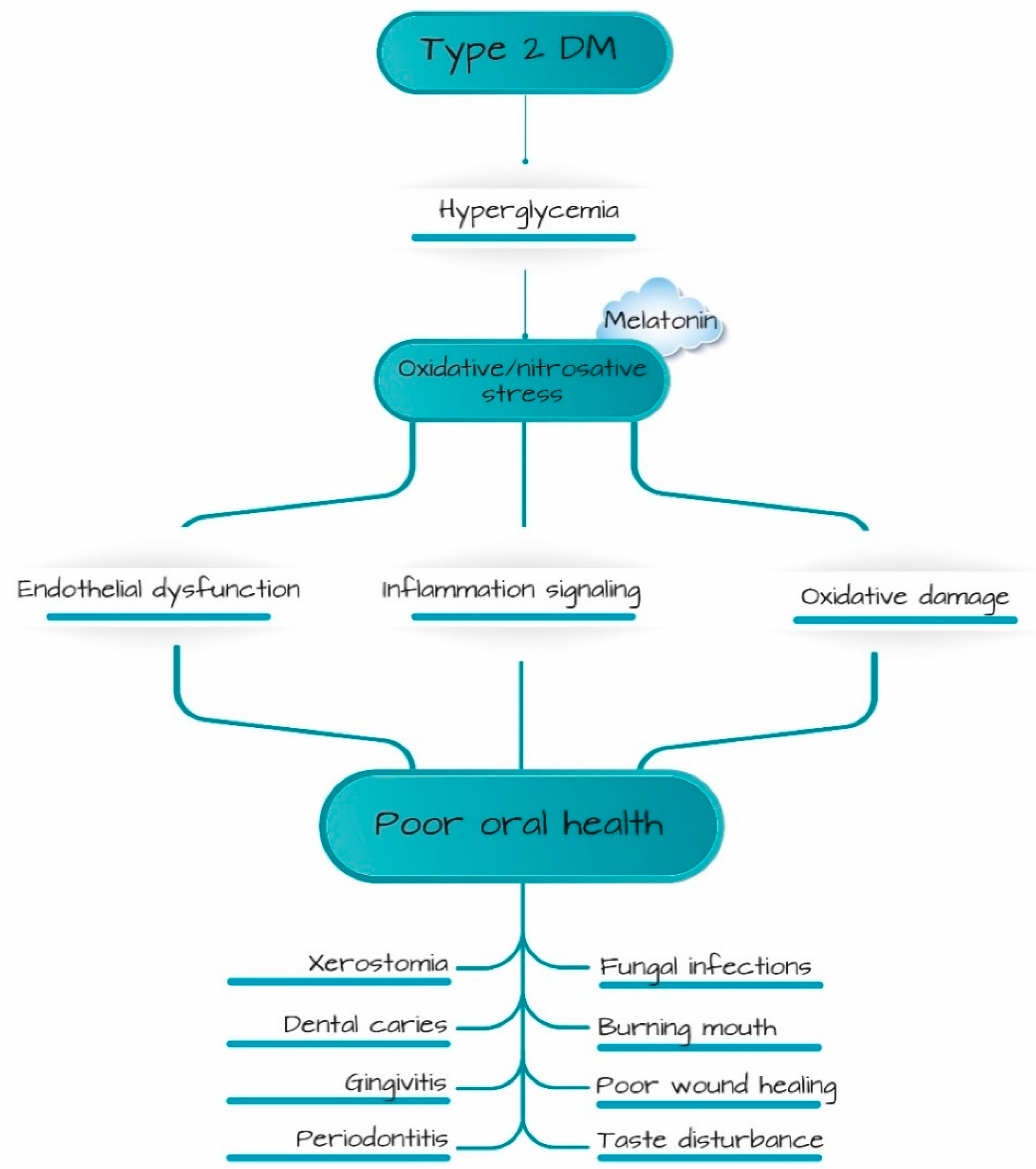
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Tissue Samples Collection
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Human Dental Pulp Cells (hDPCs) Culture
2.4. Human Parotid Gland Cells (hPGCs) Culture
2.5. Bioinformatic Analysis
2.6. Statistical Analyses
3. Results
3.1. Study Group Characteristics
3.2. Expression Levels of MEL, GDNF and iNOS in Parotid Gland and Dental Pulp Tissues of T2D Participants
3.3. Pearson’s Correlation Coefficient
3.4. GDNF Expression in hDPCs Treated with MEL
3.5. GDNF, iNOS Expression and SOD Activity in hPGCs Treated with MEL
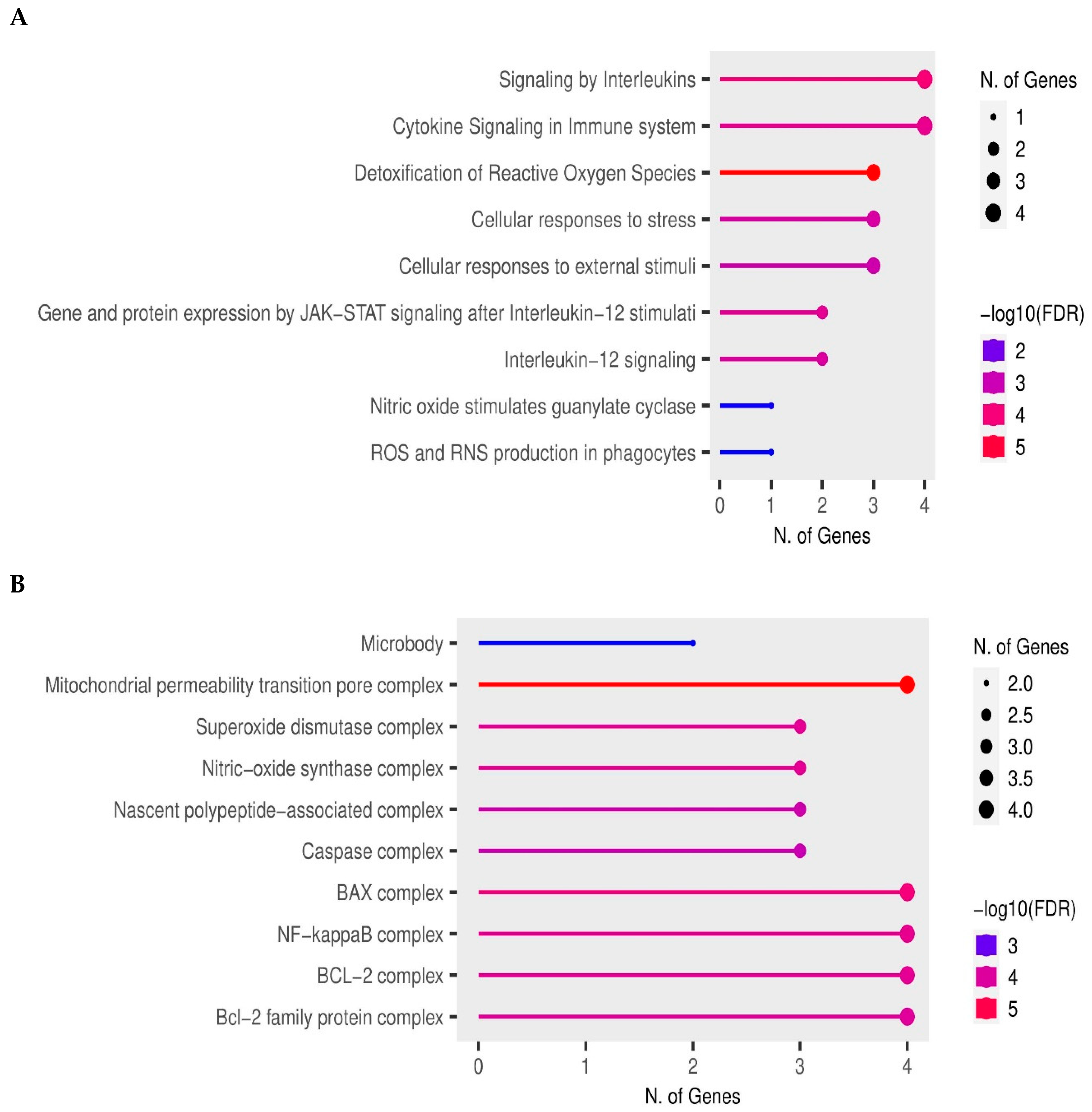
3.6. Enrichment Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ittichaicharoen, J.; Chattipakorn, N.; Chattipakorn, S.C. Is Salivary Gland Function Altered in Noninsulin-Dependent Diabetes Mellitus and Obesity–Insulin Resistance? Arch. Oral Biol. 2016, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Ebrahimpour, S.; Nourbakhsh, N.; Talebi, S.; Esmaeili, A. Protective Effect of Quercetin on Alteration of Antioxidant Genes Expression and Histological Changes in the Dental Pulp of the Streptozotocin-Diabetic Rats. Arch. Oral Biol. 2021, 125, 105088. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, A.; DJukić, L.; Toljić, B.; Milašin, J.; Dželetović, B.; Brković, B.; Roganović, J. Melatonin Levels in Human Diabetic Dental Pulp Tissue and Its Effects on Dental Pulp Cells under Hyperglycaemic Conditions. Int. Endod. J. 2018, 51, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Lilliu, M.A.; Solinas, P.; Cossu, M.; Puxeddu, R.; Loy, F.; Isola, R.; Quartu, M.; Melis, T.; Isola, M. Diabetes Causes Morphological Changes in Human Submandibular Gland: A Morphometric Study. J. Oral Pathol. Med. 2015, 44, 291–295. [Google Scholar] [CrossRef]
- Stewart, C.R.; Obi, N.; Epane, E.C.; Akbari, A.A.; Halpern, L.; Southerland, J.H.; Gangula, P.R. Effects of Diabetes on Salivary Gland Protein Expression of Tetrahydrobiopterin and Nitric Oxide Synthesis and Function. J. Periodontol. 2016, 87, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Bender, I.; Bender, A. Diabetes Mellitus and the Dental Pulp. J. Endod. 2003, 29, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, J.W.; Yeo, S.I.; Choi, B.J.; Suh, J.Y. Effects of High Glucose on Cellular Activity of Periodontal Ligament Cells in Vitro. Diabetes Res. Clin. Pract. 2006, 74, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Xiao, E.; Graves, D.T. Diabetes Mellitus Related Bone Metabolism and Periodontal Disease. Int. J. Oral Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef]
- de Souza Bastos, A.; Leite, A.R.P.; Spin-Neto, R.; Nassar, P.O.; Massucato, E.M.S.; Orrico, S.R.P. Diabetes Mellitus and Oral Mucosa Alterations: Prevalence and Risk Factors. Diabetes Res. Clin. Pract. 2011, 92, 100–105. [Google Scholar] [CrossRef]
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jane-Salas, E.; Vinas, M.; Lopez-Lopez, J. Oral Manifestations of Diabetes Mellitus. A Systematic Review. Med. Oral. 2017, 22, e586–e594. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Fouani, M.; Basset, C.A.; Jurjus, A.R.; Leone, L.G.; Tomasello, G.; Leone, A. Salivary Gland Proteins Alterations in the Diabetic Milieu. J. Mol. Histol. 2021, 52, 893–904. [Google Scholar] [CrossRef]
- Roganović, J.; Djukić, L.; Kršljak, E.; Tanić, N.; Stojić, D. Reduced Muscarinic Parotid Secretion Is Underlain by Impaired NO Signaling in Diabetic Rabbits. Oral Dis. 2015, 21, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Daniszewska, I.; Klimiuk, A.; Matczuk, J.; Kołodziej, U.; Waszkiel, D.; Ładny, J.R.; Żendzian-Piotrowska, M.; Zalewska, A. Oxidative Damage to the Salivary Glands of Rats with Streptozotocin-Induced Diabetes-Temporal Study: Oxidative Stress and Diabetic Salivary Glands. J. Diabetes Res. 2016, 2016, 4583742. [Google Scholar] [CrossRef] [PubMed]
- Garber, S.E.; Shabahang, S.; Escher, A.P.; Torabinejad, M. The Effect of Hyperglycemia on Pulpal Healing in Rats. J. Endod. 2009, 35, 60–62. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Brown, J. The Association of Xerostomia and Inadequate Intake in Older Adults. J. Am. Diet. Assoc. 1990, 90, 1688–1692. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Konturek, S.J.; Loster, B.; Wisniewska, G.; Majewski, S. Melatonin and Its Role in Oxidative Stress Related Diseases of Oral Cavity. J. Physiol. Pharmacol. 2007, 58 (Suppl. S3), 5–19. [Google Scholar]
- Ilić, J.; Milosavljević, A.; Lazarević, M.; Milošević Marković, M.; Milašin, J.; Vučetić, M.; Chaurasia, A.; Miletić, V.; Roganović, J. Melatonin Mitigates iNOS-Related Effects of HEMA and Camphorquinone in Human Dental Pulp Cells: Relevance for Postoperative Sensitivity Mechanism in Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 2562. [Google Scholar] [CrossRef]
- Himozuma, M.; Tokuyama, R.; Tatehara, S.; Umeki, H.; Ide, S.; Mishima, K.; Saito, I.; Satomura, K. Expression and cellular localizaion of melatonin-synthesizing enzymes in rat and human salivary glands. Histochem. Cell Biol. 2011, 135, 389–396. [Google Scholar] [CrossRef]
- Janebodin, K.; Buranaphatthana, W.; Ieronimakis, N.; Hays, A.L.; Reyes, M. An In Vitro Culture System for Long-Term Expansion of Epithelial and Mesenchymal Salivary Gland Cells: Role of TGF-β 1 in Salivary Gland Epithelial and Mesenchymal Differentiation. BioMed Res. Int. 2013, 2013, 815895. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-P.; Wu, C.; Miao, H.; Wu, H. RegNetwork: An Integrated Database of Transcriptional and Post-Transcriptional Regulatory Networks in Human and Mouse. Database 2015, 2015, bav095. [Google Scholar] [CrossRef]
- Binder, J.X.; Pletscher-Frankild, S.; Tsafou, K.; Stolte, C.; O’Donoghue, S.I.; Schneider, R.; Jensen, L.J. COMPARTMENTS: Unification and Visualization of Protein Subcellular Localization Evidence. Database 2014, 2014, bau012. [Google Scholar] [CrossRef]
- Sidiropoulos, K.; Viteri, G.; Sevilla, C.; Jupe, S.; Webber, M.; Orlic-Milacic, M.; Jassal, B.; May, B.; Shamovsky, V.; Duenas, C.; et al. Reactome Enhanced Pathway Visualization. Bioinformatics 2017, 33, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Pevet, P.; Challet, E. Melatonin: Both Master Clock Output and Internal Time-Giver in the Circadian Clocks Network. J. Physiol.-Paris 2011, 105, 170–182. [Google Scholar] [CrossRef]
- Chibly, A.M.; Patel, V.N.; Aure, M.H.; Pasquale, M.C.; NIDCD/NIDCR Genomics and Computational Biology Core; Morell, R.J.; Izquierdo, D.M.; Boger, E.; Martin, G.E.; Ghannam, M.; et al. Neurotrophin Signaling Is a Central Mechanism of Salivary Dysfunction after Irradiation That Disrupts Myoepithelial Cells. NPJ Regen. Med. 2023, 8, 17. [Google Scholar] [CrossRef]
- Xiao, N.; Lin, Y.; Cao, H.; Sirjani, D.; Giaccia, A.J.; Koong, A.C.; Kong, C.S.; Diehn, M.; Le, Q.-T. Neurotrophic Factor GDNF Promotes Survival of Salivary Stem Cells. J. Clin. Investig. 2014, 124, 3364–3377. [Google Scholar] [CrossRef]
- Gale, Z.; Cooper, P.R.; Scheven, B.A.A. Effects of Glial Cell Line-Derived Neurotrophic Factor on Dental Pulp Cells. J. Dent. Res. 2011, 90, 1240–1245. [Google Scholar] [CrossRef]
- Armstrong, K.J.; Niles, L.P. Induction of GDNF MRNA Expression by Melatonin in Rat C6 Glioma Cells. Neuroreport 2002, 13, 473–475. [Google Scholar] [CrossRef]
- Isola, M.; Lilliu, M.A.; Loy, F.; Isola, R. Diabetic Status Influences the Storage of Melatonin in Human Salivary Glands: Melatonin in salivary glands. Anat. Rec. 2018, 301, 711–716. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wang, Y.; Zhang, X.; Chen, L.; Sun, D. GDNF Enhances the Anti-Inflammatory Effect of Human Adipose-Derived Mesenchymal Stem Cell-Based Therapy in Renal Interstitial Fibrosis. Stem Cell Res. 2019, 41, 101605. [Google Scholar] [CrossRef]
- Saavedra, A.; Baltazar, G.; Santos, P.; Carvalho, C.M.; Duarte, E.P. Selective Injury to Dopaminergic Neurons Up-Regulates GDNF in Substantia Nigra Postnatal Cell Cultures: Role of Neuron–Glia Crosstalk. Neurobiol. Dis. 2006, 23, 533–542. [Google Scholar] [CrossRef]
- Niles, L.P.; Armstrong, K.J.; Rincón Castro, L.; Dao, C.V.; Sharma, R.; McMillan, C.R.; Doering, L.C.; Kirkham, D.L. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004, 5, 41. [Google Scholar] [CrossRef]
- Chen, K.-B.; Lin, A.M.-Y.; Chiu, T.-H. Oxidative Injury to the Locus Coeruleus of Rat Brain: Neuroprotection by Melatonin: Melatonin and Oxidative Injury in LC. J. Pineal Res. 2003, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Reiter, R.J.; Chen, Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020, 23, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Montilla, P.L.; Vargas, J.F.; Túnez, I.F.; Carmen, M.; Agueda, M.; Valdelvira, M.E.D.; Cabrera, E.S. Oxidative Stress in Diabetic Rats Induced by Streptozotocin: Protective Effects of Melatonin. J. Pineal Res. 1998, 25, 94–100. [Google Scholar] [CrossRef]
- Khoder-Agha, F.; Kietzmann, T. The Glyco-Redox Interplay: Principles and Consequences on the Role of Reactive Oxygen Species during Protein Glycosylation. Redox Biol. 2021, 42, 101888. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Kwon, H.-K.; Shin, H.-J.; Choi, Y.-M.; Anwar, M.A.; Choi, S. Activating Transcription Factor 3 Represses Inflammatory Responses by Binding to the P65 Subunit of NF-ΚB. Sci. Rep. 2015, 5, 14470. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, Y.J.; Bian, A.H.; Zuo, H.B.; Zhu, T.W.; Ji, S.X.; Kong, F.; Yin, D.Q.; Wang, C.B.; Wang, Z.F.; et al. The Regulatory Role of Activating Transcription Factor 2 in Inflammation. Mediat. Inflamm. 2014, 2014, 950472. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Dubinin, M.V. Diabetes Mellitus, Mitochondrial Dysfunction and Ca2+-Dependent Permeability Transition Pore. IJMS 2020, 21, 6559. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Nondiabetic n = 31 | Type 2 Diabetic n = 31 |
|---|---|---|
| Woman/man † | 15/16 | 16/15 |
| Age (years, mean ± SD) ‡ | 59.1 ± 11.6 | 67.1 ± 9.7 |
| Smokers (%) † | 37.5 | 31.25 |
| BMI (kg/m2, mean ± SD) § | 23.4 ± 2.8 | 26.1 ± 3.3 |
| Disease duration (years, mean ± SD) | 6.1 ± 2.8 |
| Genes | Fold Enrichment | Pathways |
|---|---|---|
| GDNF, AANAT | 39.4 | ATF5 target gene |
| GDNF, AANAT | 39.3 | ATF7 target gene |
| GDNF, AANAT | 39.0 | ATF3 target gene |
| GDNF, AANAT | 36.8 | ATF4 target gene |
| GDNF, AANAT | 14.9 | CREB1 target gene |
| GDNF, AANAT | 13.0 | HIF-1-beta target gene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barać, M.; Petrović, M.; Petrović, N.; Nikolić-Jakoba, N.; Aleksić, Z.; Todorović, L.; Petrović-Stanojević, N.; Anđelić-Jelić, M.; Davidović, A.; Milašin, J.; et al. Melatonin Action in Type 2 Diabetic Parotid Gland and Dental Pulp: In Vitro and Bioinformatic Findings. Int. J. Environ. Res. Public Health 2023, 20, 6727. https://doi.org/10.3390/ijerph20186727
Barać M, Petrović M, Petrović N, Nikolić-Jakoba N, Aleksić Z, Todorović L, Petrović-Stanojević N, Anđelić-Jelić M, Davidović A, Milašin J, et al. Melatonin Action in Type 2 Diabetic Parotid Gland and Dental Pulp: In Vitro and Bioinformatic Findings. International Journal of Environmental Research and Public Health. 2023; 20(18):6727. https://doi.org/10.3390/ijerph20186727
Chicago/Turabian StyleBarać, Milena, Milan Petrović, Nina Petrović, Nataša Nikolić-Jakoba, Zoran Aleksić, Lidija Todorović, Nataša Petrović-Stanojević, Marina Anđelić-Jelić, Aleksandar Davidović, Jelena Milašin, and et al. 2023. "Melatonin Action in Type 2 Diabetic Parotid Gland and Dental Pulp: In Vitro and Bioinformatic Findings" International Journal of Environmental Research and Public Health 20, no. 18: 6727. https://doi.org/10.3390/ijerph20186727
APA StyleBarać, M., Petrović, M., Petrović, N., Nikolić-Jakoba, N., Aleksić, Z., Todorović, L., Petrović-Stanojević, N., Anđelić-Jelić, M., Davidović, A., Milašin, J., & Roganović, J. (2023). Melatonin Action in Type 2 Diabetic Parotid Gland and Dental Pulp: In Vitro and Bioinformatic Findings. International Journal of Environmental Research and Public Health, 20(18), 6727. https://doi.org/10.3390/ijerph20186727









