Control of Tongue Position in Patients with Obstructive Sleep Apnea: Concept and Protocol for a Randomized Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Participants
2.3.1. Inclusion Criteria
- (1)
- Patients who are scheduled to undergo a dental operation under intravenous sedation at Showa University Koto Toyosu Hospital.
- (2)
- Males aged > 20 years.
- (3)
- Patients diagnosed with OSA [10/h ≤ respiratory event index (REI) < 30/h] based on out of center sleep testing (OCST; also referred to as home sleep apnea testing or type III portable monitoring) (PMP-300E, Pacific Medico Co., Ltd., Tokyo, Japan) [12,13,14,15]. Assessment will be performed by registered polysomnographic technologists in accordance with the American Academy of Sleep Medicine Scoring Manual [16].
- (4)
- Patients who are healthy, or who have mild systemic disease in accordance with American Society of Anesthesiologists physical status I or II [17].
- (5)
- Patients who agree to participate in this study and provide their written consent to participate in the study.
2.3.2. Exclusion Criteria
- (1)
- Patients with cognitive dysfunction and/or sensor impairment.
- (2)
- Subjects assumed to be ineligible by the principal investigator or co-investigators.
- (3)
- Patients who cannot provide their written consent.
- (4)
- Patients in whom the tongue cannot be maintained in position by the TPR.
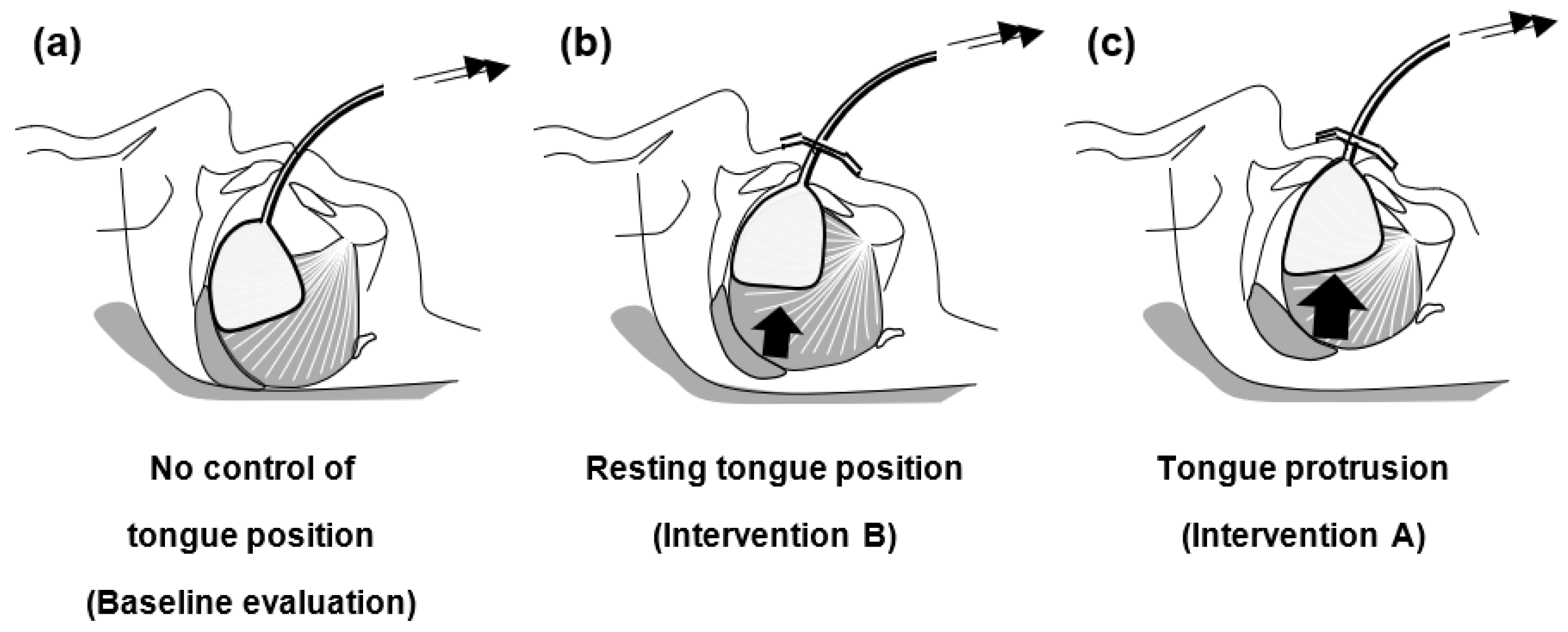
2.4. Intervention
2.5. Sample Size and Allocation Concealment Mechanism
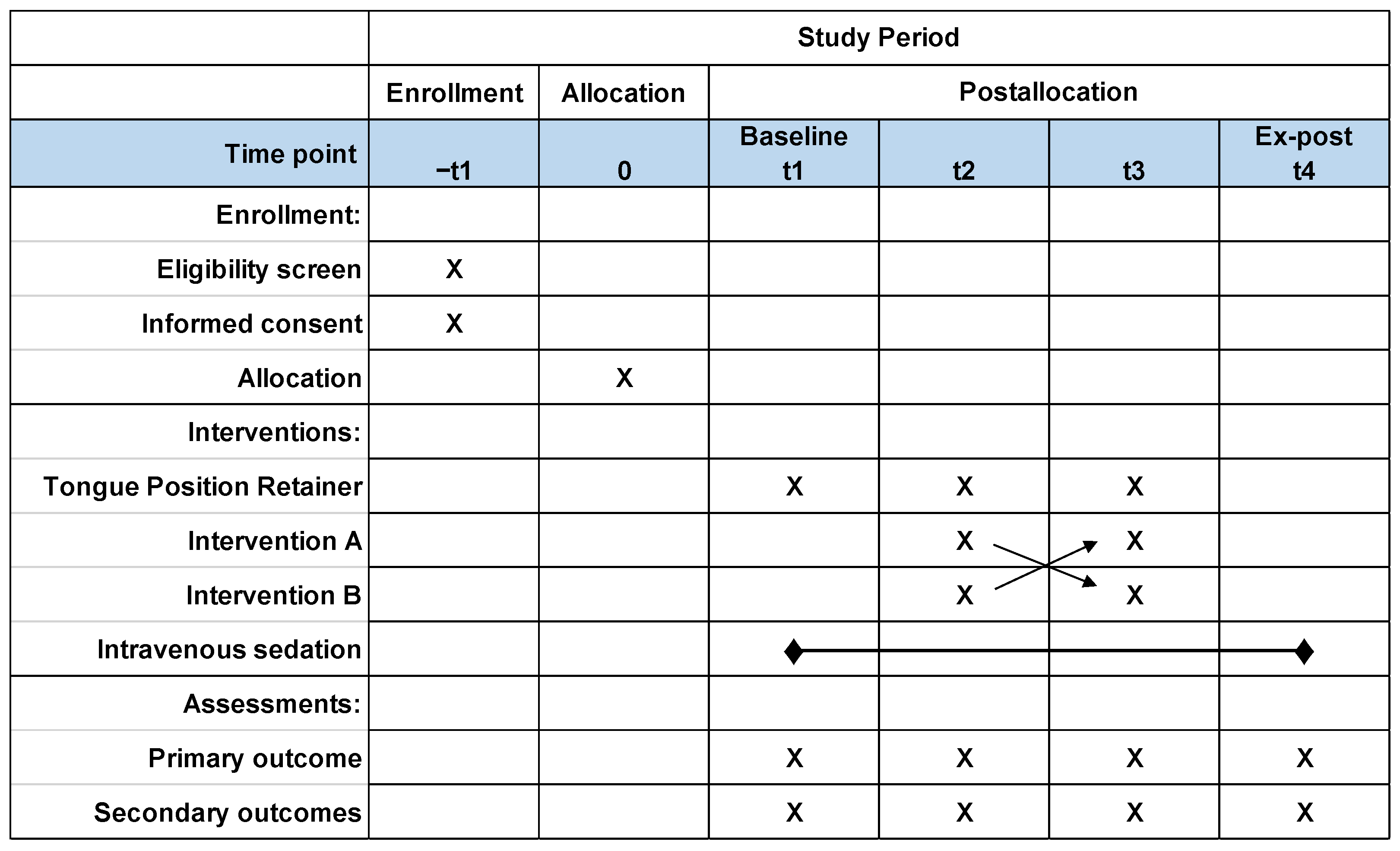
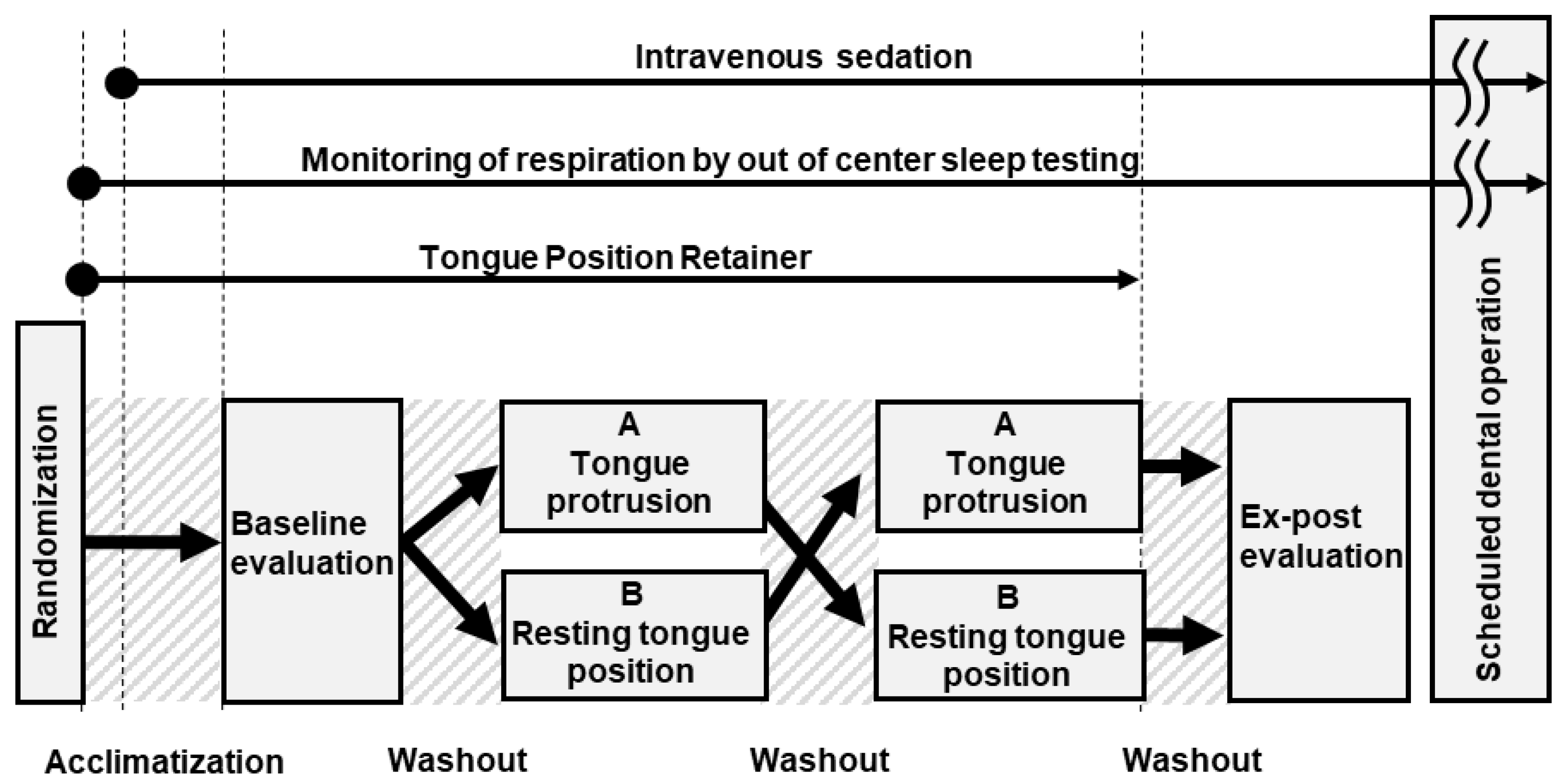
2.6. Study Protocol
2.7. Study Outcome
2.7.1. Primary Outcome
2.7.2. Secondary Outcome
2.8. Statistical Analysis
3. Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remmers, J.E.; De Groot, W.J.; Sauerland, E.K.; Anch, A.M. Pathogenesis of upper airway obstruction during sleep. J. Appl. Physiol. 1978, 44, 931–938. [Google Scholar] [CrossRef] [PubMed]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, S.; Isono, S.; Minamino, O.; Maeda, K.; Kobayashi, M.; Sasai, T.; Takahashi, Y.; Inoue, Y. Tongue position controller as an alternative treatment for obstructive sleep apnea. Sleep Breath 2012, 16, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Takei, Y.; Nakayama, H.; Inoue, Y.; Tsuiki, S. Continuous tongue suction as an alternative treatment for obstructive sleep apnea: A feasibility study. J. Dent. Sleep Med. 2020, 7. [Google Scholar] [CrossRef]
- Tsuiki, S.; Isono, S.; Ishikawa, T.; Yamashiro, Y.; Tatsumi, K.; Nishino, T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 2008, 108, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Tsuiki, S.; Maeda, K.; Okajima, I.; Inoue, Y. Oropharyngeal crowding closely relates to aggravation of OSA. Chest 2016, 150, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Nashi, N.; Kang, S.; Barkdull, G.C.; Lucas, J.; Davidson, T.M. Lingual fat at autopsy. Laryngoscope 2007, 117, 1467–1473. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X. The interaction of obesity and craniofacial deformity in obstructive sleep apnea. Dentomaxillofac. Radiol. 2021, 50, 20200425. [Google Scholar] [CrossRef]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Tsutsumi, I.; Tsutsumi, Y.; Yoshida, C.; Komeno, T.; Imanaka, Y. Impact of the Clinical Trials Act on Noncommercial Clinical Research in Japan: An Interrupted Time-series Analysis. J. Epidemiol. 2022, 32, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Cardell, C.; Black, S.; Khouzam, A. Comparison of a new type 3 portable monitor for OSA detection vs. in-lab polysomnography [abstract]. Sleep 2009, 32, A385. [Google Scholar]
- Fukuda, T.; Tsuiki, S.; Kobayashi, M.; Nakayama, H.; Inoue, Y. Selection of response criteria affects the success rate of oral appliance treatment for obstructive sleep apnea. Sleep Med. 2014, 15, 367–370. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Standards for Accreditation of Out of Center Sleep Testing (OCST) in Adult Patients. American Academy of Sleep Medicine, Darien. Available online: https://www.aasm.org/resources/pdf/ocststandards.pdf (accessed on 15 April 2023).
- Iber, C.; Ancoli-Israel, S.; Chesson, A.L.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine, Westchester 2007. Available online: http://aasm.org/resources/pdf/scoring-manual-preface.pdf (accessed on 7 March 2023).
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef]
- Yanagihara, M.; Tsuiki, S.; Setoguchi, Y.; Inoue, Y. Treatment of obstructive sleep apnea with a tongue-stabilizing device at a single multidisciplinary sleep center. J. Dent. Sleep Med. 2016, 3, 43–47. [Google Scholar] [CrossRef]
- Russel, D. Intravenous anaesthesia: Manual infusion schemes versus TCI systems. Anaesthesia 1988, 53 (Suppl. 1), 42–45. [Google Scholar] [CrossRef]
- Kohzuka, Y.; Isono, S.; Ohara, S.; Kawabata, K.; Kitamura, A.; Suzuki, T.; Almeida, F.R.; Sato, Y.; Iijima, T. Nasopharyngeal tube effects on breathing during sedation for dental procedures: A randomized controlled trial. Anesthesiology 2019, 130, 946–957. [Google Scholar] [CrossRef]
- Alshhrani, W.M.; Hamoda, M.M.; Okuno, K.; Kohzuka, Y.; Fleetham, J.A.; Ayas, N.T.; Comey, R.; Almeida, F.R. The efficacy of a titrated tongue-stabilizing device on obstructive sleep apnea: A quasi-experimental study. J. Clin. Sleep Med. 2021, 17, 1607–1618. [Google Scholar] [CrossRef]
- Rabelo, F.A.; Braga, A.; Küpper, D.S.; De Oliveira, J.A.; Lopes, F.M.; de Lima Mattos, P.L.; Barreto, S.G.; Sander, H.H.; Fernandes, R.M.F.; Valera, F.C.P. Propofol-induced sleep: Polysomnographic evaluation of patients with obstructive sleep apnea and controls. Otolaryngol. Head Neck Surg. 2010, 142, 218–224. [Google Scholar] [CrossRef]
- Maddison, K.J.; Walsh, J.H.; Shepherd, K.L.; Bharat, C.; Lawther, B.K.; Platt, P.R.; Eastwood, P.R.; Hillman, D.R. Comparison of Collapsibility of the Human Upper Airway During Anesthesia and During Sleep. Anesth. Analg. 2020, 130, 1008–1017. [Google Scholar] [CrossRef]
- Kuna, S.T.; Remmers, J.E. Anatomy and Physiology of Upper Airway Obstruction. In Principles and Practice of Sleep Medicine, 3rd ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; WB Saunders: Philadelphia, PA, USA, 2000; pp. 840–858. [Google Scholar]
- Tsuiki, S. Anatomy and Function of Upper Airway during Sleep: What Are Essential Mechanisms Eliciting Apneas during Sleep? In Structure-Function Relationships in Various Respiratory Systems; Yamaguchi, K., Ed.; Springer: Singapore, 2020; pp. 55–65. [Google Scholar] [CrossRef]
- Isono, S.; Remmers, J.E.; Tanaka, A.; Sho, Y.; Sato, J.; Nishino, T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J. Appl. Physiol. 1997, 82, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Molnár, V.; Lakner, Z.; Molnár, A.; Tárnoki, D.L.; Tárnoki, Á.D.; Kunos, L.; Jokkel, Z.; Tamás, L. Ultrasound and Magnetic Resonance Imaging of the Tongue in Obstructive Sleep Apnoea. Appl. Sci. 2022, 12, 9583. [Google Scholar] [CrossRef]
- Akashiba, T.; Inoue, Y.; Uchimura, N.; Ohi, M.; Kasai, T.; Kawana, F.; Sakurai, S.; Takegami, M.; Tachikawa, R.; Tanigawa, T.; et al. Sleep Apnea Syndrome (SAS) Clinical Practice Guidelines 2020. Respir. Investig. 2022, 60, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Fernandez-Salvador, C.; Giambo, J.; Nesbitt, B.; Liu, S.Y.; Capasso, R.; Kushida, C.A.; Camacho, M. Tongue retaining devices for obstructive sleep apnea: A systematic review and meta-analysis. Am. J. Otolaryngol. 2017, 38, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi-Filho, G.; Almeida, F.R.; Strollo, P.J. Treating OSA: Current and emerging therapies beyond CPAP. Respirology 2017, 22, 1500–1507. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, T.; Kohzuka, Y.; Almeida, F.R.; Iijima, T.; Masuda, R.; Tsuiki, S. Control of Tongue Position in Patients with Obstructive Sleep Apnea: Concept and Protocol for a Randomized Controlled Crossover Trial. Int. J. Environ. Res. Public Health 2023, 20, 6026. https://doi.org/10.3390/ijerph20116026
Fukuda T, Kohzuka Y, Almeida FR, Iijima T, Masuda R, Tsuiki S. Control of Tongue Position in Patients with Obstructive Sleep Apnea: Concept and Protocol for a Randomized Controlled Crossover Trial. International Journal of Environmental Research and Public Health. 2023; 20(11):6026. https://doi.org/10.3390/ijerph20116026
Chicago/Turabian StyleFukuda, Tatsuya, Yuuya Kohzuka, Fernanda R. Almeida, Takehiko Iijima, Rikuo Masuda, and Satoru Tsuiki. 2023. "Control of Tongue Position in Patients with Obstructive Sleep Apnea: Concept and Protocol for a Randomized Controlled Crossover Trial" International Journal of Environmental Research and Public Health 20, no. 11: 6026. https://doi.org/10.3390/ijerph20116026
APA StyleFukuda, T., Kohzuka, Y., Almeida, F. R., Iijima, T., Masuda, R., & Tsuiki, S. (2023). Control of Tongue Position in Patients with Obstructive Sleep Apnea: Concept and Protocol for a Randomized Controlled Crossover Trial. International Journal of Environmental Research and Public Health, 20(11), 6026. https://doi.org/10.3390/ijerph20116026






