Quo Vadis, Amadeo Hand Robot? A Randomized Study with a Hand Recovery Predictive Model in Subacute Stroke
Abstract
1. Introduction
2. Materials and Methods
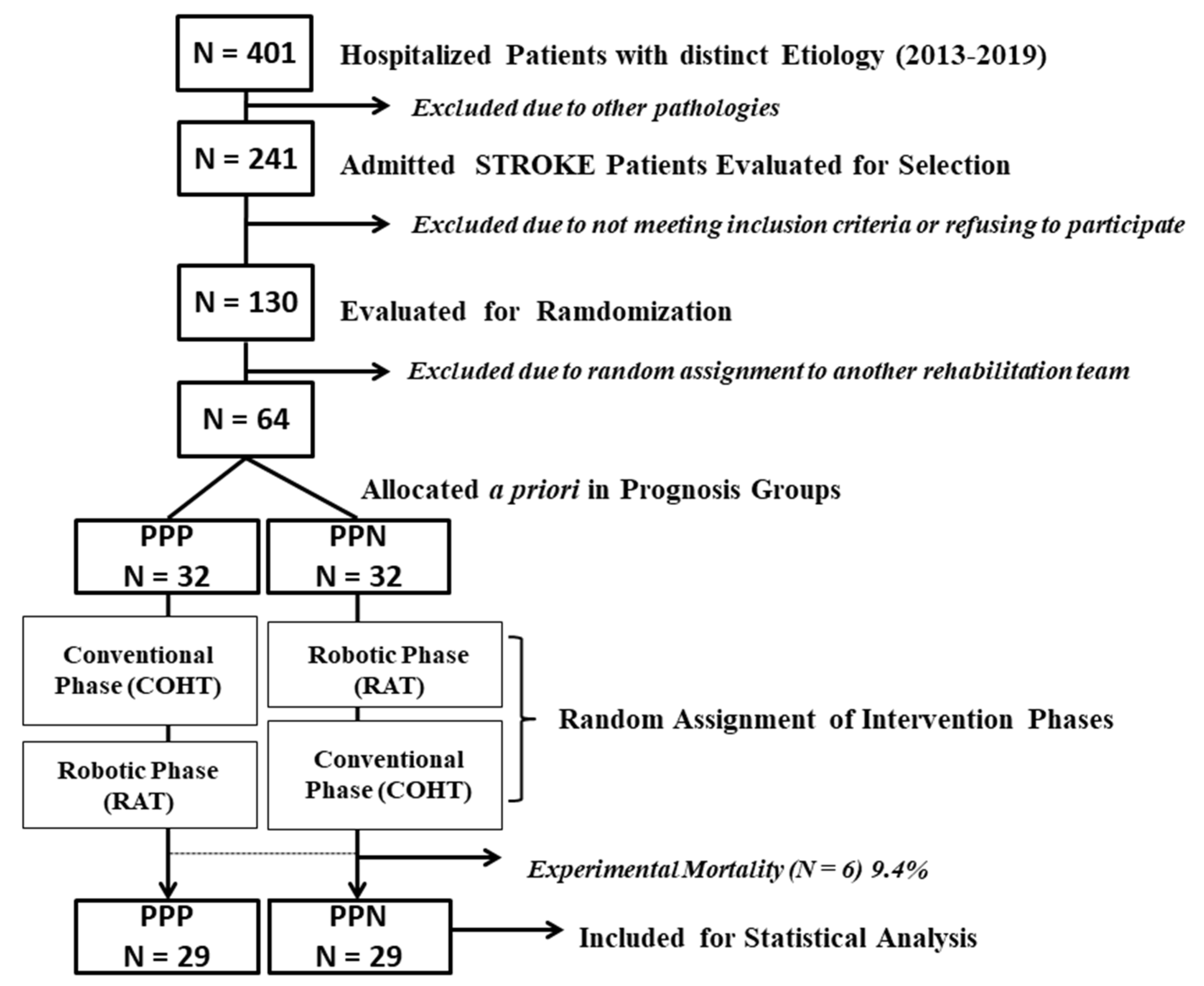
2.1. Study Design and Randomization
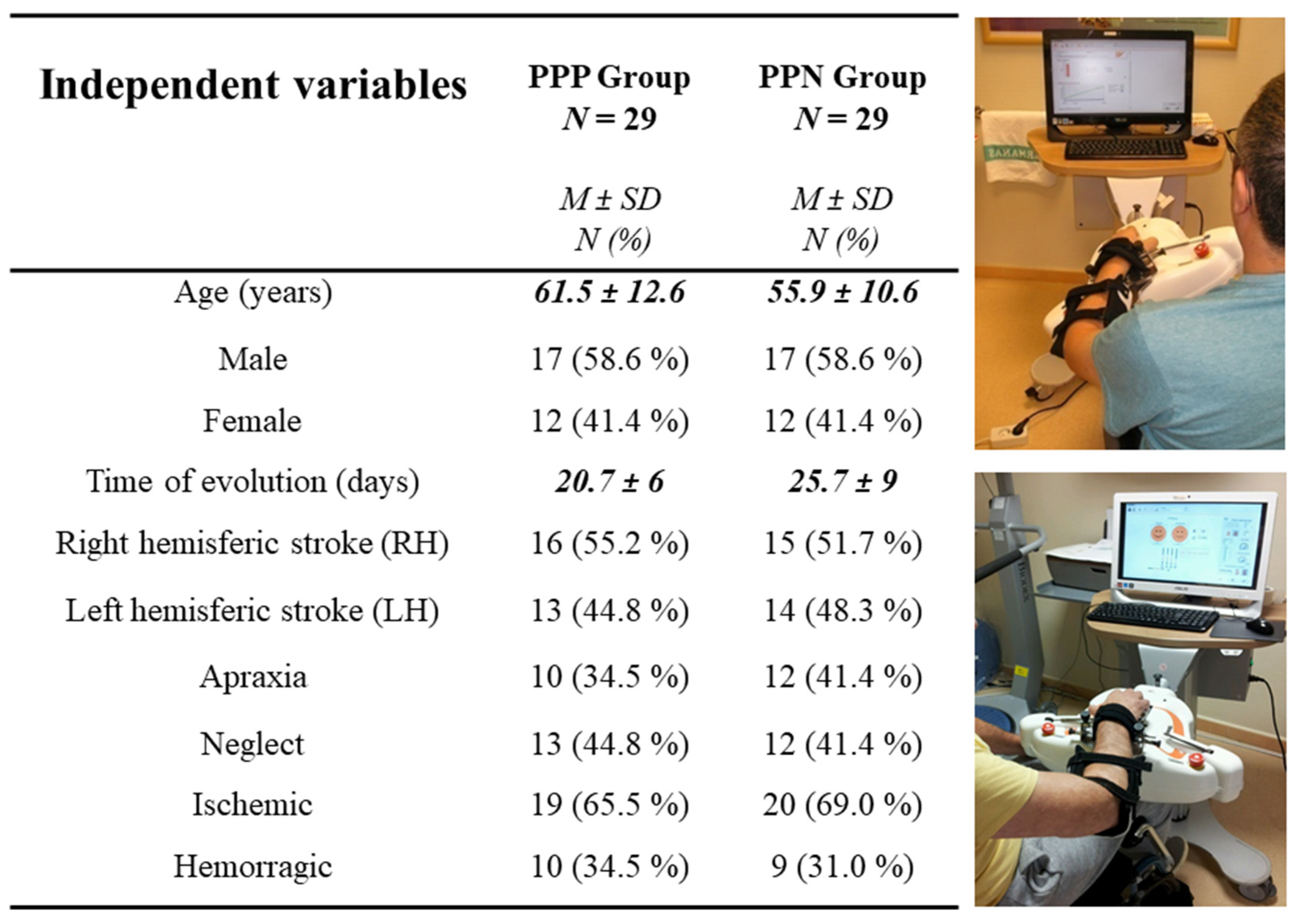
2.2. Participants
2.3. Procedures and Interventions
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Outcomes between Subacute Stroke Experimental and Healthy Control Groups
3.2. Changes between Groups at the End of the Treatment per Factors
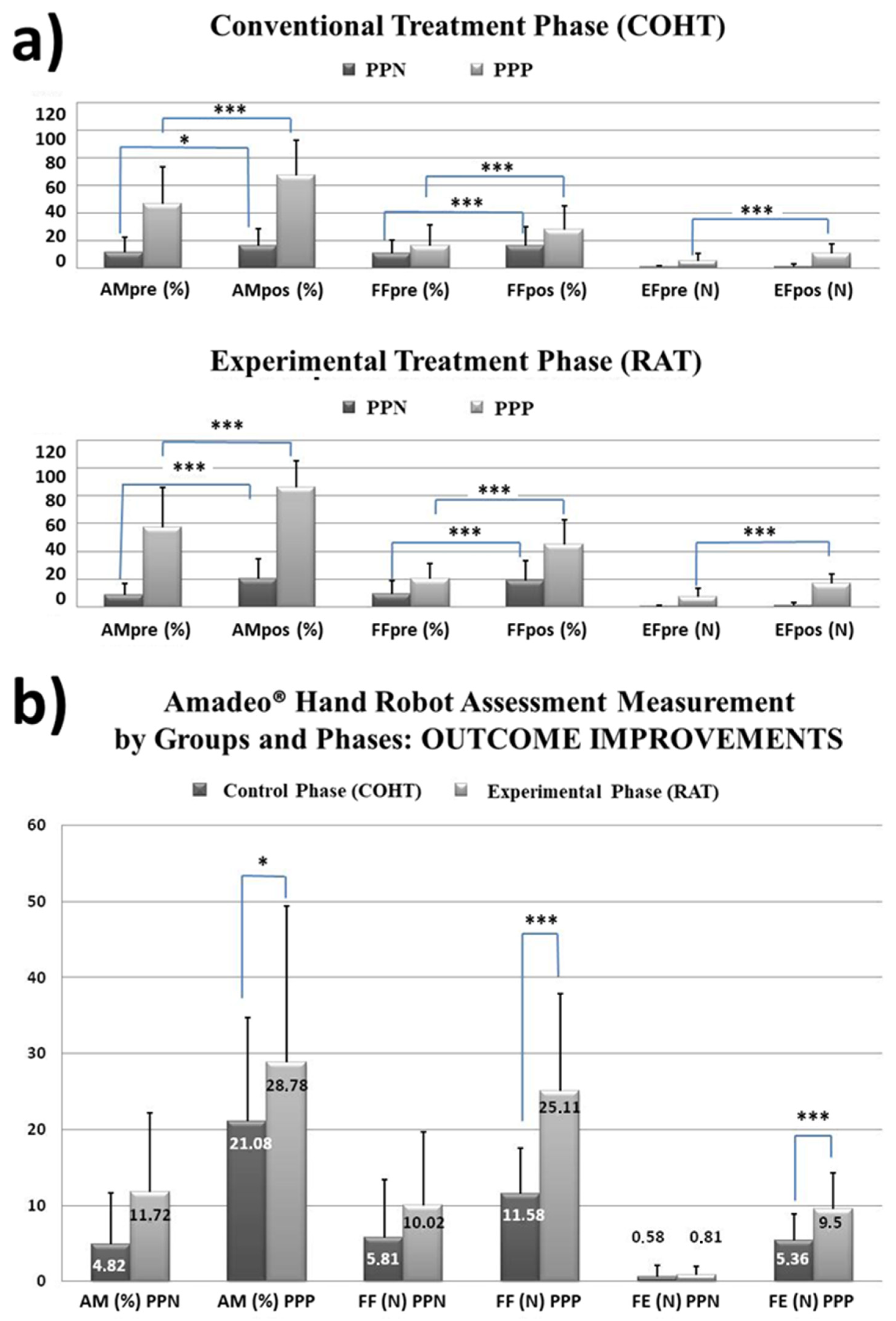
3.2.1. Factor 1 Ad Hoc: Severity of Sensorimotor Involvement at Admission
- (a)
- Kinetic and kinematic results. All statistical results can be seen in Table 1.
- (b)
- Functional sensorimotor results. Statistical results can be seen in Table 2
- (c)
- Functional performance in ADL. Statistics are shown in Table 3
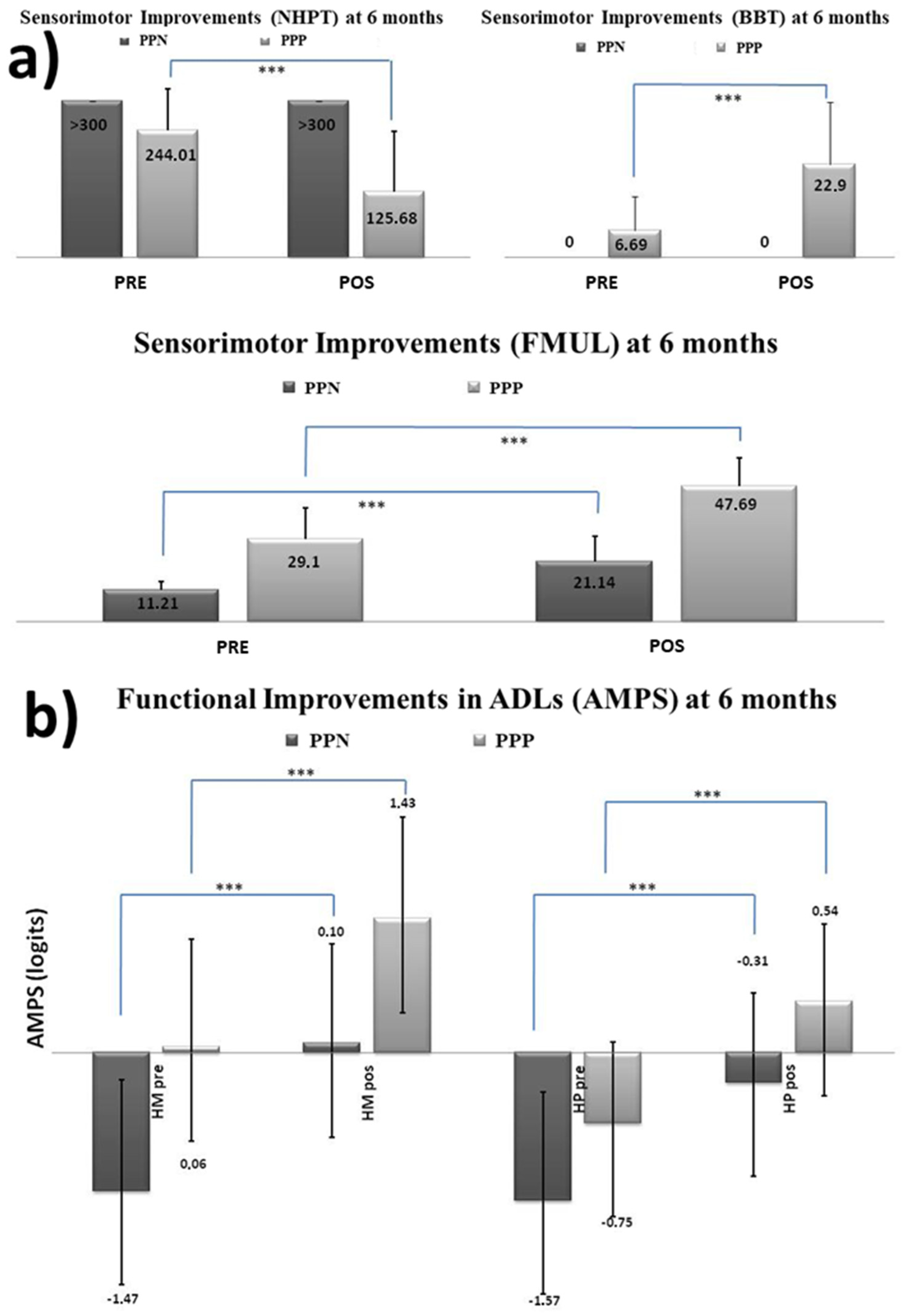
3.2.2. Factor 2 Ad Hoc: Laterality of Brain Injury and Its Cognition
- (a)
- Kinetic and kinematic results. All statistical results can be seen in Table 1.
- (b)
- Functional sensorimotor results (See Table 2)
- (c)
- Functional performance in activities of daily living (see Table 3)
3.3. Outcome Clinical Predictive Model with Amadeo Hand Robot
3.3.1. Functional ADLs Outcome Predictive Model
3.3.2. Sensorimotor Outcome Predictive Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Acquired Brain Injury |
| ADLs | Activities of Daily Living |
| AHR | Amadeo Hand Robot |
| AM | Active range of Motion |
| AMPS | Assessment of Motor and Process Skills |
| BBT | Box and Block Test |
| COHT | Conventional Occupational Hand Therapy |
| EEG | Electroencephalography |
| EF | Extension Force |
| FMUL | Fugl Meyer Upper Limb |
| FF | Flexion Force |
| HM-AMPS | Motor Skills-AMPS |
| HP-AMPS | Process Skills-AMPS |
| NHPT | Nine Hole Peg Test |
| OT | Occupational Therapy |
| PT | Physiotherapy |
| RAT | Robot Assisted Therapy |
| sEMG | Surface Electromyography |
| UL | Upper limb |
| VR | Virtual Reality |
References
- Serrano, P.A.; Criado, T.; Aranda, V.; Fernández-Pinedo, N.; Riendas, A.; Sevilla, M.M.; Zafra, C.; Calvo-Vera, A.; Calvo-Arenillas, I. Robotics and Virtual Reality Exer-Games for the Neurorehabilitation of Children and Adults with Traumatic Brain Injury: The IS-BRAIN Model. In Engineering Biomaterials for Neural Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 243–276. [Google Scholar]
- Stinear, C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010, 9, 1228–1232. [Google Scholar] [CrossRef]
- Cunha, B.P.; de Freitas, S.M.S.F.; de Freitas, P.B. Assessment of the Ipsilesional Hand Function in Stroke Survivors: The Effect of Lesion Side. J. Stroke Cerebrovasc. Dis. 2017, 26, 1615–1621. [Google Scholar] [CrossRef]
- Mollayeva, T.; Xiong, C.; Hanafy, S.; Chan, V.; Hu, Z.J.; Sutton, M.; Escobar, M.; Colantonio, A. Comorbidity and outcomes in traumatic brain injury: Protocol for a systematic review on functional status and risk of death. BMJ Open 2017, 7, e018626. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, X.; Liu, W.; Liu, Z. Clinical Features, Etiology, and 6-Month Prognosis of Isolated Corpus Callosum Infarction. BioMed Res. Int. 2019, 2019, 9458039. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Yang, H.; Guo, K. Current State of Robotics in Hand Rehabilitation after Stroke: A Systematic Review. Appl. Sci. 2022, 12, 4540. [Google Scholar] [CrossRef]
- Khalid, S.; Alnajjar, F.; Gochoo, M.; Shimoda, S. Robotic Assistive and Rehabilitation Devices Leading to Motor Recovery in Upper Limb: A Systematic Review. In Disability and Rehabilitation: Assistive Technology; Taylor and Francis Ltd.: Milton Park, UK, 2021. [Google Scholar]
- Wattchow, K.A.; McDonnell, M.N.; Hillier, S.L. Rehabilitation Interventions for Upper Limb Function in the First Four Weeks Following Stroke: A Systematic Review and Meta-Analysis of the Evidence. Arch. Phys. Med. Rehabil. 2018, 99, 367–382. [Google Scholar] [CrossRef]
- Falzarano, V.; Marini, F.; Morasso, P.; Zenzeri, J. Devices and Protocols for Upper Limb Robot-Assisted Rehabilitation of Children with Neuromotor Disorders. Appl. Sci. 2019, 9, 2689. Available online: https://www.mdpi.com/2076-3417/9/13/2689 (accessed on 15 March 2022). [CrossRef]
- Held, J.P.O.; Van Duinen, J.; Luft, A.R.; Veerbeek, J.M. Eligibility screening for an early upper limb stroke rehabilitation study. Front. Neurol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Everard, G.; Declerck, L.; Detrembleur, C.; Leonard, S.; Bower, G.; Dehem, S.; Lejeune, T. New technologies promoting active upper limb rehabilitation after stroke–An overview and network meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 530–548. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-Based Hand Motor Therapy after Stroke. Brain 2008, 131 Pt 2, 425–437. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00628996/full?highlightAbstract=next%7Cvirtual%7Ccognit%7Cmemory%7Cstrok%7Ctraumat%7Cdiffuse%7Cinjuri%7Cinterface%7Cencephalopathy%7Cneuropsychological%7Cneuropsycholog%7Cexecutive%7Ctraumatic (accessed on 15 March 2022). [CrossRef]
- Jamin, P.; Duret, C.; Hutin, E.; Bayle, N.; Koeppel, T.; Gracies, J.-M.; Pila, O. Using Robot-Based Variables during Upper Limb Robot-Assisted Training in Subacute Stroke Patients to Quantify Treatment Dose. Sensors 2022, 22, 2989. [Google Scholar] [CrossRef] [PubMed]
- Aggogeri, F.; Mikolajczyk, T.; O’Kane, J. Robotics for rehabilitation of hand movement in stroke survivors. Adv. Mech. Eng. 2019, 11. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. In Cochrane Database of Systematic Reviews; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 2018. [Google Scholar]
- Serrano-Lopez-Terradas, P.A.; Seco-Rubio, R. Effectiveness of robotic therapy in the proximal and distal rehabilitationoftheupperlimb in patients after strokeusingthe Amadeo® and Armeo® devices: A systematic review of randomized clinical trials (Efectividad de la terapiarobóticaen la rehabilitación proximal y distal del miembro superior en personas tras un ictus con los dispositivos Amadeo® y Armeo®: Una revisiónsistemática de ensayosclínicosaleatorizados). Stud. Psychol. 2022, 43, 132–178. [Google Scholar]
- Heller, A.; Wade, D.T.; Wood, V.A.; Sunderland, A.; Hewer, R.L.; Ward, E. Arm function after stroke: Measurement and recovery over the first three months. J. Neurol. Neurosurg. Psychiatry 1987, 50, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Smania, N.; Paolucci, S.; Tinazzi, M.; Borghero, A.; Manganotti, P.; Fiaschi, A.; Moretto, G.; Bovi, P.; Gambarin, M. Active finger extension: A simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007, 38, 1088–1090. [Google Scholar] [CrossRef]
- Winters, C.; Van Wegen, E.E.H.; Daffertshofer, A.; Kwakkel, G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil. Neural Repair 2015, 29, 614–622. [Google Scholar] [CrossRef]
- Hoonhorst, M.H.; Nijland, R.H.; Van Den Berg, J.S.; Emmelot, C.H.; Kollen, B.J.; Kwakkel, G. How Do Fugl-Meyer Arm Motor Scores Relate to Dexterity According to the Action Research Arm Test at 6 Months Poststroke? Arch. Phys. Med. Rehabil. 2015, 96, 1845–1849. [Google Scholar] [CrossRef]
- Nijland, R.H.M.; van Wegen, E.E.H.; Harmeling-van der Wel, B.C.; Kwakkel, G. Presence of Finger Extension and Shoulder Abduction Within 72 Hours After Stroke Predicts Functional Recovery. Stroke 2010, 41, 745–750. [Google Scholar] [CrossRef]
- Kwah, L.K.; Harvey, L.A.; Diong, J.; Herbert, R.D. Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: An observational study. J. Physiother. 2013, 59, 189–197. [Google Scholar] [CrossRef]
- Serrano-López Terradas, P. Quo Vadis, Amadeo? Universidad de Salamanca (USAL): Salamanca, Spain, 2022. [Google Scholar]
- Demir, Y.; Köroğlu, Ö.; Tekin, E.; Adıgüzel, E.; Kesikburun, S.; Güzelküçük, Ü.; Yılmaz, B.; Alaca, R.; Yaşar, E. Factors affecting functional outcome in patients with traumatic brain injury sequelae: Our single-center experiences on brain injury rehabilitation. Turk. J. Phys. Med. Rehabil. 2019, 65, 67–73. [Google Scholar] [CrossRef]
- Byblow, W.D.; Stinear, C.M.; Barber, P.A.; Petoe, M.A.; Ackerley, S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015, 78, 848–859. [Google Scholar] [CrossRef]
- Ward, N.S.; Brander, F.; Kelly, K. Intensive upper limb neurorehabilitation in chronic stroke: Outcomes from the Queen Square programme. J. Neurol. Neurosurg. Psychiatry 2019, 90, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.; Alt Murphy, M.; Buurke, J.; Feys, P.; Keller, T.; Klamroth-Marganska, V.; Lamers, I.; McNicholas, L.; Prange, G.; Tarkka, I.; et al. A systematic review of international clinical guidelines for rehabilitation of people with neurological conditions: What recommendations are made for upperlimb assessment? Front. Neurol. 2019, 10, 567. [Google Scholar] [CrossRef]
- Kelly, G.; Moys, R.; Burrough, M.; Hyde, S.; Randall, S.; Wales, L. Rehabilitation in practice: Improving delivery of upper limb rehabilitation for children and young people with acquired brain injuries through the development and implementation of a clinical pathway. Disabil. Rehabil. 2020, 44, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Bishop, L.; Gillen, G.; Helbok, R. A pilot study of robotic-assisted exercise for hand weakness after stroke. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar]
- Sale, P.; Lombardi, V.; Franceschini, M. Hand robotics rehabilitation: Feasibility and preliminary results of a robotic treatment in patients with hemiparesis. Stroke Res. Treat. 2012, 2012, 820931. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Mazzoleni, S.; Lombardi, V.; Galafate, D.; Massimiani, M.P.; Posteraro, F.; Damiani, C.; Franceschini, M. Recovery of hand function with robot-assisted therapy in acute stroke patients: A randomized-controlled trial. Int. J. Rehabil. Res. 2014, 37, 236–242. [Google Scholar] [CrossRef]
- Serrano López-Terradas, P.A.; Moya Rosendo, D.; Ríos Lago, M. Hand Functional Recovery in Sub-acute Brain Injury Stage Patients using AMADEO® Robotic-assisted Therapy A Pilot Clinical Study with Apraxic and Neglect Patients. In Proceedings of the NEUROTECHNIX 2013-International Congress on Neurotechnology, Electronics and Informatics (Special Session on Virtual and Augmented Reality Systems for Upper Limbs Rehabilitation), Vilamoura, Algarve, Portugal, 18–20 September 2013; Rita Londral, A., Encarnação, P., Pons, J., Eds.; SCITEPRESS–Science and Technology Publications & SCITEPRESS Digital Library: Setúbal, Portugal, 2013; pp. 1–4. Available online: http://www.neurotechnix.org (accessed on 15 March 2022).
- Orihuela-Espina, F.; Roldán, G.F.; Sánchez-Villavicencio, I.; Palafox, L.; Leder, R.; Sucar, L.E.; Hernández-Franco, J. Robot training for hand motor recovery in subacute stroke patients: A randomized controlled trial. J. Hand Ther. 2016, 29, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.H.; Seong, J.W.; Son, D.-S. Individual finger synchronized robot-assisted hand rehabilitation in subacute to chronic stroke: A prospective randomized clinical trial of efficacy. Clin. Rehabil. 2012, 26, 696–704. Available online: http://journals.sagepub.com/doi/10.1177/0269215511431473 (accessed on 15 March 2022). [CrossRef]
- Celadon, N.; Dosen, S.; Paleari, M.; Farina, D.; Ariano, P. Individual finger classification from surface EMG: Influence of electrode set. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2015; pp. 7284–7287. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstem, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Portney, L.; Watkins, M. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Conneticut: Appleton & Lange; Prentice Hall: Hoboken, NJ, USA, 1993. [Google Scholar]
- Sani, F.; Todman, J. Experimental Design and Statistics for Psychology: A First Course; Blackwell Publishing Inc.: Oxford, UK, 2006. [Google Scholar]
- Prieto Valiente, L.; HerranzTejedor, I. Bioestadística Sin DificultadesMatemáticas. AnálisisEstadístico de DatosenInvestigaciónMédica y Sociológica; Diaz de Sa: Madrid, Spain, 2015. [Google Scholar]
- Kieran, D. Correlation and Simple Linear Regression. In Natural Resources Miometric; Open SUNY Textbooks: Geneseo, NY, USA, 2014. [Google Scholar]
- Fernández-López, J.A.; Fernández-Fidalgo, M.; Geoffrey, R.; Stucki, G.; Cieza, A. Funcionamiento y discapacidad: La ClasificaciónInternacional del Funcionamiento (CIF). Rev. EspSalud Publica 2009, 83, 775–783. [Google Scholar] [CrossRef]
- Germanotta, M.; Cruciani, A.; Pecchioli, C.; Loreti, S.; Spedicato, A.; Meotti, M.; Mosca, R.; Speranza, G.; Cecchi, F.; Giannarelli, G.; et al. Reliability, validity and discriminant ability of the instrumental indices provided by a novel planar robotic device for upper limb rehabilitation. J. Neuroeng. Rehabil. 2018, 15, 39. [Google Scholar] [CrossRef]
- Park, J.H. The effects of robot-assisted left-hand training on hemispatial neglect in older patients with chronic stroke: A pilot and randomized controlled trial. Medicine 2021, 100, e24781. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Accorinti, M.; Porcari, B.; Carioti, L.; Ciatto, L.; Billeri, L.; Andronaco, V.A.; Galletti, F.; Filoni, S.; Naro, A. Does hand robotic rehabilitation improve motor function by rebalancing interhemispheric connectivity after chronic stroke? Encouraging data from a randomised-clinical-trial. Clin. Neurophysiol. 2019, 130, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, S.E.; Adans-Dester, C.P. A paradigm shift: Rehabilitation robotics, cognitive skills training, and function after stroke. Front. Neurol. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanov, R.K.; Mokienko, O.A.; Aziatskaya, G.A.; Suponeva, N.A.; Piradov, M.A. Post Stroke Rehabilitation: Clinical Efficacy of BCI-Driven Hand Exoskeleton in Comparison with “AMADEO” Robotic Mechanotherapy. Phys. Rehabil. Med. Med Rehabil. 2019, 1, 63–72. [Google Scholar] [CrossRef]
- Zhang, B.; Kan, L.; Dong, A.; Zhang, J.; Bai, Z.; Xie, Y.; Liu, Q.; Peng, Y. The effects of action observation training on improving upper limb motor functions in people with stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221166. [Google Scholar] [CrossRef]
- Serrano, P.; Oliva-Navarrete, P.; Moreno-Barbás, J. Terapiaasistida por robot con Amadeo para la rehabilitación de la mano. In TerapiaOcupacionalEn Las DisfuncionesFísicas: Teoría y Práctica, 2nd ed.; Panamericana, M., Ed.; Panamericana: Madrid, Spain, 2015; pp. 449–457. [Google Scholar]
- Celadon, N.; Došen, S.; Binder, I.; Ariano, P.; Farina, D. Proportional estimation of finger movements from high-density surface electromyography. J. Neuroeng. Rehabil. 2016, 13, 73. [Google Scholar] [CrossRef]
- Emerson, J.R.; Binks, J.A.; Scott, M.W.; Ryan, R.P.; Eaves, D.L. Combined action observation and motor imagery therapy: A novel method for post-stroke motor rehabilitation. AIMS Neurosci. 2018, 5, 236–252. [Google Scholar] [CrossRef]
- Errante, A.; Saviola, D.; Cantoni, M.; Iannuzzelli, K.; Ziccarelli, S.; Togni, F.; Simonini, M.; Malchiodi, C.; Bertoni, D.; Inzaghi, M.G.; et al. Effectiveness of action observation therapy based on virtual reality technology in the motor rehabilitation of paretic stroke patients: A randomized clinical trial. BMC Neurol. 2022, 22, 109. [Google Scholar] [CrossRef]
- Schieber, M.H.; Lang, C.E.; Reilly, K.T.; McNulty, P.; Sirigu, A. Selective activation of human finger muscles after stroke or amputation. Adv. Exp. Med. Biol. 2009, 629, 559–575. [Google Scholar]
- Woodbury, M.; Velozo, C.; Rochards, L.; Duncan, P.; Studenski, S.; Lai, S. Longitudinal Stability of the Fugl Meyer Assessmeent of the Upper Extremity. Arch. Phys. Med. Rehabil. 2008, 89, 1563–1569. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; Van Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb after Stroke. Neurorehabilit. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Brackenridge, J.; Bradnam, L.V.; Lennon, S.; Costi, J.J.; Hobbs, D.A. A review of rehabilitation devices to promote upper limb function following stroke. Neurosci. Biomed. Eng. 2016, 4, 25–42. [Google Scholar] [CrossRef]
- Padua, L.; Imbimbo, I.; Aprile, I.; Loreti, C.; Germanotta, M.; Coraci, D.; Piccinini, G.; Pazzaglia, C.; Santilli, C.; Cruciani, A.; et al. Cognitive reserve as a useful variable to address robotic or conventional upper limb rehabilitation treatment after stroke: A multicentre study of the Fondazione Don Carlo Gnocchi. Eur. J. Neurol. 2020, 27, 392–398. [Google Scholar] [CrossRef]
- Archambault, P.S.; Norouzi-Gheidari, N.; Kairy, D.; Levin, M.F.; Milot, M.-H.; Monte-Silva, K.; Sveistrup, H.; Trivino, M. Upper extremity intervention for stroke combining virtual reality, robotics and electrical stimulation. In Proceedings of the International Conference on Virtual Rehabilitation, ICVR, Tel Aviv, Israel, 21–24 July 2019. [Google Scholar]
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation after Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Kumar, P.; Kathuria, P.; Nair, P.; Prasad, K. Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: A systematic review and meta-analysis. J. Stroke 2016, 18, 50–59. [Google Scholar] [CrossRef]
- Jakob, I.; Kollreider, A.; Germanotta, M.; Benetti, F.; Cruciani, A.; Padua, L.; Aprile, I. Robotic and Sensor Technology for Upper Limb Rehabilitation. PMR 2018, 10, S189–S197. [Google Scholar] [CrossRef]
- Aprile, I.; Cruciani, A.; Germanotta, M.; Gower, V.; Pecchioli, C.; Cattaneo, D.; Vannetti, F.; Padua, L.; Gramatica, F. Upper limb robotics in rehabilitation: An approach to select the devices, based on rehabilitation aims, and their evaluation in a feasibility study. Appl. Sci. 2019, 9, 3920. [Google Scholar] [CrossRef]
- Rexroth, P.; Fisher, A.G.; Merritt, B.K.; Gliner, J. ADL differences in individuals with unilateral hemispheric stroke. Can. J. Occup. Ther. 2005, 72, 212–221. [Google Scholar] [CrossRef]
- Fisher, A.G.; Bray Jones, K. Assessment of Motor and Process Skills, 8th ed.; User manual; Three Star Press: Fort Collins, CO, USA, 2014; Volume 2. [Google Scholar]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: Systematic review and meta-analysis. Top. Stroke Rehabil. 2021, 29, 539–550. [Google Scholar] [CrossRef]
- Baldan, F.; Turolla, A.; Rimini, D.; Pregnolato, G.; Maistrello, L.; Agostini, M.; Jakob, I. Robot-assisted rehabilitation of hand function after stroke: Development of prediction models for reference to therapy. J. Electromyogr. Kinesiol. 2021, 57, 102534. [Google Scholar] [CrossRef]
- Scherer, R.; Grieshofer, P.; Enzinger, C.; Müller-Putz, G.R. Predicting Functional Stroke-Rehabilitation Outcome by means of Brain-Computer Interface Technology: The BCI4REHAB Project. In Proceedings of the World Congress for NeuroRehabilitation, Melbourne, Australia, 16–19 May 2012. [Google Scholar]
- Dziemian, K.; Kiper, A.; Baba, A.; Baldan, F.; Alhelou, M.; Agostini, M.; Turolla, A.; Kiper, P. The effect of robot therapy assisted by surface EMG on hand recovery in post-stroke patients. A pilot study. Rehabil. Med. 2017, 21, 4–10. [Google Scholar] [CrossRef]
- Kim, G.; O’Dell, M.; Stein, J.; Taub, M.; Creelman, C.; Cahalan, C.; Rivera, L. The Feasibility of Utilizing an EMG-triggered Hand Robot for Individuals After Chronic Stroke. Arch. Phys. Med. Rehabil. 2016, 97, e35–e36. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, G.; Shuai, S.; Guo, Z.; Chen, H.; McClure, M.A.; Chen, X.; Mu, Q. Low-Frequency Repetitive Transcranial Magnetic Stimulation for Stroke-Induced Upper Limb Motor Deficit: A Meta-Analysis. Neural Plast. 2017, 2017, 2758097. [Google Scholar] [CrossRef]
- Sandrini, M.; Cohen, L. Non invasive brain stimulation in neurorehabilitation. Handb. Clin. Neurol. 2013, 116, 499–524. [Google Scholar]
- Romero-Muñoz, J.P.; Sanchez-Cuesta, F.J.; Arroyo Ferrer, A.; Rocón, E.; Castillo, M.D.; Serrano, I.; Ríos Lago, M.; Useros, A.; Serrano, P.; Oliva, P.; et al. Use of repetitive transcranial magnetic stimulation as an adjunctive therapy in upper limb rehabilitation in stroke; A research proposal for a double blind and crossed controlled study. In Proceedings of the World Congress for Neurorehabilitation (WCNR) and WFNR–Stroke Session, Lyon, France, 7–11 October 2020; Available online: https://programm.conventus.de/index.php?id=wcnr2020&tx_coprogramm_programm%5Bautor%5D=3104&tx_coprogramm_programm%5Baction%5D=autor&tx_coprogramm_programm%5Bcontroller%5D=Source&cHash=37a139e5e11660541e5229af0f441b61 (accessed on 15 March 2022).
- Lang, C.E.; Edwards, D.F.; Birkenmeier, R.L.; Dromerick, A.W. Estimating Minimal Clinically Important Differences of Upper-Extremity Measures Early After Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1693–1700. [Google Scholar] [CrossRef]

| Hand Hemiparesis at Admission Factor | Lateralization-Cognition Factor | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Kinetic & Kinematic Parameters of Amadeo® | PPP Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | PPN Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) | RH Group n = 27 M(SD) | F(1,56) | p Value | η2 | OP | LH Group n = 31 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) |
| Experimental Treatment (A) RAT (3 months) | AM pre (%) AM pos (%) | 57.2(29.2) 86(19.3) | 89.75 | <0.001 *** | 0.6 | 1 | 8.4(8.3) 20.1(14.9) | 14.88 | <0.001 *** | 0.2 | 0.97 | 32.8(32.5) 53.1(37.3) | 29.7(6.3) 47.2(7.2) | 24.54 | <0.001 *** | 0.3 | 1 | 35.5(5.7) 58.2(6.7) | 47.39 | <0.001 *** | 0.5 | 1 | 32.6(4.3) 52.7(4.9) |
| FF pre (N) FF pos (N) | 20.3(11.3) 45.4(17.5) | 142.38 | <0.001 *** | 0.7 | 1 | 9.1(9.9) 19.1(14.3) | 22.68 | <0.001 *** | 0.3 | 1 | 14.7(11.9) 32.3(20.7) | 14.6(2.3) 29.7(4) | 33.80 | <0.001 *** | 0.4 | 1 | 14.7(2.2) 34.5(3.7) | 66.33 | <0.001 *** | 0.5 | 1 | 14.7(1.6) 32.1(2.7) | |
| EF pre (N) EF pos (N) | 7.14(6.37) 16.6(7.4) | 211.7 | <0.001 *** | 0.8 | 1 | 0.28(1.03) 1.1(1.9) | 1.55 | 0.22 ns | 0.03 | 0.2 | 3.7(5.7) 8.9(9.5) | 3(1.1) 7.7(1.8) | 19.06 | <0.001 *** | 0.3 | 1 | 4.4(1) 9.9(1.7) | 29.82 | <0.001 *** | 0.04 | 1 | 3.7(0.8) 8.8(1.3) | |
| Control Treatment (B) COHT (3 months) | AM pre (%) AMpos (%) | 46.2(27.1) 67.2(25.7) | 111.02 | <0.001 *** | 0.7 | 1 | 11.1(11.1) 15.9(12.7) | 5.80 | 0.019 * | 0.1 | 0.7 | 28.6(27.1) 41.6(32.8) | 25(5.2) 39.6(6.3) | 31.77 | <0.001 *** | 0.4 | 1 | 31.8(4.8) 43.3(5.9) | 22.45 | <0.001 *** | 0.3 | 1 | 28.4(3.6) 41.5(4.3) |
| FF pre (N) FF pos (N) | 16.2(14.8) 27.8(17.4) | 83.11 | <0.001 *** | 0.6 | 1 | 10.1(10.1) 15.9(14.1) | 20.91 | <0.001 *** | 0.3 | 1 | 13.2(12.9) 21.9(16.8) | 12.7(2.5) 22.8(3.3) | 52.10 | <0.001 *** | 0.5 | 1 | 13.6(2.3) 21(3) | 31.90 | <0.001 *** | 0.4 | 1 | 13.1(1.7) 21.9(2.2) | |
| EF pre (N) EF pos (N) | 5(5.4) 10.4(6.9) | 109.4 | <0.001 *** | 0.7 | 1 | 0.44(0.89) 1(2.1) | 1.29 | 0.26 ns | 0.02 | 0.2 | 2.7(4.5) 5.7(6.9) | 2.1(.8) 4.8(1.3) | 14.16 | <0.001 *** | 0.2 | 0.9 | 3.2(0.8) 6.4(1.2) | 24.34 | <0.001 *** | 0.03 | 1 | 2.7(0.6) 5.6(0.9) | |
| Hand Hemiparesis at Admission Factor | Lateralization-Cognition Factor | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Sensorimotor Variables by Scale | PPP Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | PPN Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) | RH Group n = 27 M(SD) | F(1,56) | p Value | η2 | OP | LH Group n = 31 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) |
| Experimental Treatment (A) RAT (3 months) | FMUL pre FMUL pos | 36(1.5) 47.7(1.7) | 194.97 | <0.001 *** | 0.8 | 1 | 15.2(1.5) 21.1(1.7) | 51.07 | <0.001 *** | 0.5 | 1 | 25.6(1.1) 34.4(1.2) | 25.8(2.6) 35.7(3.2) | 94.92 | <0.001 *** | 0.6 | 1 | 25.4(2.4) 33.3(3) | 70.13 | <0.001 *** | 0.6 | 1 | 25.6(1.8) 34.5(2.2) |
| NHPT pre NHPT pos | 199(11) 126(15) | 81.47 | <0.001 *** | 0.6 | 1 | 300(11) 300(15) | 0 | 1 ns | 0 | 0.05 | 249(7.7) 213(10.7) | 254(15) 215(23) | 12.51 | <0.01 ** | 0.2 | 0.9 | 245(14) 211(22) | 11.20 | <0.01 ** | 0.02 | 0.9 | 250(10) 213(16) | |
| BBT pre BBT pos | 12.8(1.4) 22.9(2) | 114.99 | <0.001 *** | 0.7 | 1 | 0(1.4) 0(2) | 0.001 | 0.97 ns | 0 | 0.05 | 6.4(1) 11.4(1.4) | 6.5(1.9) 11.2(3) | 11.43 | <0.01 ** | 0.2 | 0.9 | 6.4(1.8) 11.7(2.9) | 16.81 | <0.001 *** | 0.2 | 1 | 6.4(1.3) 11.4(2.1) | |
| Control Treatment (B) COHT (3 months) | FMUL pre FMUL pos | 29.1(1.5) 36.2(1.5) | 218.78 | <0.001 *** | 0.8 | 1 | 11.2(1.5) 15.3(1.5) | 71.77 | <0.001 *** | 0.6 | 1 | 20.2(1) 25.7(1.1) | 19.4(2.3) 26(2.6) | 138.41 | <0.001 *** | 0.7 | 1 | 20.8(2.2) 25.5(2.4) | 85.25 | <0.001 *** | 0.6 | 1 | 20.1(1.6) 25.8(1.8) |
| NHPT pre NHPT pos | 244(10.3) 199(10.9) | 91.02 | <0.001 *** | 0.6 | 1 | 300(10.3) 300(10.9) | 0 | 1 ns | 0 | 0.05 | 272(7.3) 249.4(7.7) | 275(12) 254(15) | 10.43 | <0.01 ** | 0.2 | 0.9 | 269(11) 245(14) | 14.78 | <0.001 *** | 0.02 | 1 | 272(8) 250(10) | |
| BBT pre BBT pos | 6.7(1.1) 11.9(1.3) | 69.58 | <0.001 *** | 0.6 | 1 | 0(1.1) 0.1(1.3) | 0.012 | 0.91 ns | 0 | 0.05 | 3.3(0.8) 6(0.9) | 3.6(1.3) 6.5(1.8) | 12.62 | <0.01 ** | 0.2 | 0.9 | 3.2(1.2) 5.6(1.7) | 9.91 | 0.003 ** | 0.15 | 0.9 | 3.4(0.9) 6(1.2) | |
| Synergistic Treatment (A + B) COHT + RAT (6 months) | FMUL pre FMUL pos | 29.1(10.8) 47.7(9.8) | 220.44 | <0.001 *** | 0.8 | 1 | 11.2(2.8) 21.1(8.9) | 62.94 | <0.001 *** | 0.5 | 1 | 20.2(11.9) 34.4(16.3) | 6.5(0.5) 9.8(1) | 25.84 | <0.000 *** | 0.3 | 1 | 4.8(0.5) 7.9(0.9) | 27.92 | <0.001 *** | 0.3 | 1 | 5.6(0.4) 8.9(0.7) |
| NHPT pre NHPT pos | 244(79) 126(115) | 135.04 | <0.001 *** | 0.7 | 1 | 300(0) 300(0) | 0 | 0.7 ns | 0 | 0.5 | 272(62) 213(119) | 21.3(6.6) 39(11) | 12.53 | <0.01 ** | 0.2 | 0.9 | 23.9(6.2) 34(10) | 5.03 | 0.029 * | 0.01 | 0.6 | 23(4.5) 37(7.6) | |
| BBT pre BBT pos | 6.7(8.4) 22.9(15.4) | 87.88 | <0.001 *** | 0.6 | 1 | 0.00(0.00) 0.00(0.00) | 0 | 0.5 ns | 0 | 0.5 | 3.3(6.8) 11.4(15.8) | 2.9(1) 4.9(1.4) | 4.77 | 0.033 * | 0.1 | 0.6 | 3.7(1) 5.5(1.3) | 4.64 | 0.036 * | 0.1 | 0.6 | 3.3(0.7) 5.2(0.9) | |
| Hand Hemiparesis at Admission | Lateralization-Cognition | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | AMPS© Measure Variables | PPP Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | PPN Group n = 29 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) | RH Group n = 27 M(SD) | F(1,56) | p Value | η2 | OP | LH Group n = 31 M(SD) | F(1,56) | p Value | η2 | OP | Total N = 58 M(SD) |
| Experimental Treatment (A) RAT (3 months) | HM-AMPSpre | 0.62(0.2) | 208.04 | <0.001 *** | 0.8 | 1 | −1.1(0.2) | 61.53 | <0.001 *** | 0.5 | 1 | −0.24(0.1) | −0.22(0.3) | 88.81 | <0.001 *** | 0.6 | 1 | −0.25(0.2) | 90.65 | <0.001 *** | 0.6 | 1 | −0.24(0.2) |
| HM-AMPSpos | 1.42(0.2) | −0.67(0.2) | 0.38(0.2) | 0.41(0.3) | 0.34(0.3) | 0.38(0.2) | |||||||||||||||||
| HP-AMPSpre | −0.12(0.2) | 116.38 | <0.001 *** | 0.7 | 1 | −0.99(0.2) | 121.41 | <0.001 *** | 0.7 | 1 | −0.55(0.1) | −0.65(0.2) | 106.56 | <0.001 *** | 0.7 | 1 | −0.47(0.2) | 131.49 | <0.001 *** | 0.7 | 1 | −0.56(0.1) | |
| HP-AMPSpos | 0.55(0.2) | −0.31(0.2) | 0.11(0.1) | 0.01(0.2) | −0.21(0.2) | 0.11(0.1) | |||||||||||||||||
| Control Treatment (B) COHT (3 months) | HM-AMPSpre HM-AMPSpos | 0.05(0.2) 0.62(0.2) | 421.09 | <0.001 *** | 0.9 | 1 | −1.42(0.2) −1.1(0.2) | 132.72 | <0.001 *** | 0.7 | 1 | −0.68(0.1) −0.24(0.1) | −0.69(0.3) −0.22(0.3) | 155.11 | <0.001 *** | 0.7 | 1 | −0.7(0.2) −0.25(0.2) | 147.11 | <0.001 *** | 0.7 | 1 | −0.68(0.8) −0.24(0.2) |
| HP-AMPSpre HP-AMPSpos | −0.7(0.2) −0.1(0.2) | 150.7 | <0.001 *** | 0.7 | 1 | −1.6(0.2) −1(0.2) | 127.8 | <0.001 *** | 0.7 | 1 | −1.2(0.1) −0.6(0.1) | −1.3(0.2) −0.6(0.2) | 129.60 | <0.001 *** | 0.7 | 1 | −1.1(0.2) −0.5(0.2) | 146.16 | <0.001 *** | 0.7 | 1 | −1.2(0.1) −0.6(0.1) | |
| Synergistic Treatment (A + B) COHT + RAT (6 months) | HM-AMPSpre HM-AMPSpos | 0.06(1.16) 1.4(1.07) | 182.9 | <0.001 *** | 0.7 | 1 | −1.5(1.2) 0.10(1.06) | 172.9 | <0.001 *** | 0.7 | 1 | −0.70(1.4) 0.76(1.2) | −0.69(0.3) 0.41(0.3) | 120.94 | <0.001 *** | 0.7 | 1 | −0.68(0.2) 0.34(0.28) | 119.69 | <0.001 *** | 0.7 | 1 | −0.68(0.2) 0.38(0.2) |
| HP-AMPSpre HP-AMPSpos | −0.75(0.86) 0.54(0.83) | 121.6 | <0.001 *** | 0.8 | 1 | −1.6(1.1) −0.31(0.95) | 160.9 | <0.001 *** | 0.8 | 1 | −1.1(1.1) 0.12(0.98) | −1.3(0.2) 0.01(0.2) | 162.91 | <0.001 *** | 0.7 | 1 | −1.1(0.2) 0.21(0.2) | 192.61 | <0.001 *** | 0.8 | 1 | −1.2(0.1) 0.11(0.1) | |
| Criterion Variables | Predictive Variables | N | Mean (SD) | Pearson Coefficient | p-Value |
|---|---|---|---|---|---|
| Amadeus—A Functional Models | |||||
| Amadeus-A1 Predictive Model (AMPS) | |||||
| HMAmpsImprov COHT (to 3 months) | 58 | 0.44(0.19) | |||
| AM_improv_1d | 58 | 3.67(3.53) | 0.64 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | 0.63 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | 0.58 | <0.001 *** | |
| Amadeus-A2 Predictive Model (AMPS) | |||||
| HMAmpsImprov RAT (to 3 months) | 58 | 0.60(0.33) | |||
| AM_improv_1d | 58 | 3.67(3.53) | 0.46 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | 0.49 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | 0.59 | <0.001 *** | |
| Amadeus-A3 Predictive Model (AMPS) | |||||
| HMAmpsImprov COHT + RAT (to 6 months) | 58 | 1.04(0.50) | |||
| AM_improv_1d | 58 | 3.67(3.53) | 0.55 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | 0.56 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | 0.61 | <0.001 *** | |
| AMADEUS-B SENSORIMOTOR MODELS | |||||
| Amadeus-B1 Predictive Model (FMUL) | |||||
| FMUL_6m COHT + RAT (to 6 months) | 58 | 34.41(16.29) | |||
| AM_improv_1d | 58 | 3.67(3.53) | 0.56 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | 0.64 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | 0.77 | <0.001 *** | |
| Amadeus-B2 Predictive Model (BBT) | |||||
| BBT_6m COHT + RAT (to 6 months) | 58 | 11.45(15.81) | |||
| AM_improv_1d | 58 | 3.67(3.53) | 0.44 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | 0.60 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | 0.78 | <0.001 *** | |
| Amadeus-B3 Predictive Model (NHPT) | |||||
| NHPT_6m COHT + RAT (to 6 months) | 58 | 212.84(119.37) | |||
| AM_improv_1d | 58 | 3.67(3.53) | −0.48 | <0.001 *** | |
| FF_improv_1d | 58 | 2.06(1.77) | −0.63 | <0.001 *** | |
| EF_improv_1d | 58 | 0.45(0.52) | −0.78 | <0.001 *** | |
| Regression Coefficient (B) | Standarized Coefficient (β) | Overall Model | Analysis of Residuals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictive Models | p-Value | VIF | R | Adjusted R2 | F | Durbin-Watson | D | |||
| Amadeus-A Functional models | ||||||||||
| Amadeus-A1 Predictive Model (AMPS) | ||||||||||
| VD: HMAmps COHT(to 3months) | <0.001 * | 0.72 | 0.51 | 29.03 | 1.7 | 0.49 | ||||
| VP: AMimprov1d FFimprov1d | 0.23 0.43 | 0.41 0.39 | 0.001 * 0.001 * | 1.50 1.50 | ||||||
| VE: EFimprov1d | 0.22 | 0.071 | 1.68 | |||||||
| Amadeus-A2 Predictive Model (AMPS) | ||||||||||
| VD: HMAmps RAT(to 3months) | <0.001 * | 0.59 | 0.34 | 30.32 | 1.7 | 0.19 | ||||
| VP: | ||||||||||
| EFimprov1d | 0.37 | 0.59 | <0.001 * | 1.00 | ||||||
| VE: | ||||||||||
| AMimprov1d FFimprov1d | 0.20 0.22 | 0.12 0.10 | 1.42 1.51 | |||||||
| Amadeus-A3 Predictive Model (AMPS) | ||||||||||
| VD: HMAmps COHT + RAT(to 6months) | <0.001 * | 0.67 | 0.43 | 22.23 | 1.8 | 0.38 | ||||
| VP: | ||||||||||
| EFimprov1d | 0.43 | 0.45 | <0.001 * | 1.42 | ||||||
| AMimprov1d | 0.04 | 0.31 | 0.012 * | 1.42 | ||||||
| VE: | ||||||||||
| FFimprov1d | 0.22 | 0.09 | 1.77 | |||||||
| AMADEUS-B Sensorimotor Models | ||||||||||
| Amadeus-B1 Predictive Model (FMUL) | ||||||||||
| VD: FMUL_6m (to 6 months) | <0.001 * | 0.80 | 0.64 | 51.09 | 1.4 | 0.12 | ||||
| VP: | ||||||||||
| FFimprov1d | 2.7 | 0.29 | 0.004 * | 1.51 | ||||||
| EFimprov1d | 18.6 | 0.60 | <0.001 * | 1.51 | ||||||
| VE: | ||||||||||
| AMimprov1d | 0.10 | 0.32 | 1.66 | |||||||
| Amadeus-B2 Predictive Model (BBT) | ||||||||||
| VD:BBT_6m (to 6 months) | <0.001 * | 0.80 | 0.62 | 48.11 | 1.5 | 0.22 | ||||
| VP: | ||||||||||
| FFimprov1d | 2.02 | 0.23 | 0.028 * | 1.51 | ||||||
| EFimprov1d | 19.48 | 0.65 | <0.001 * | 1.51 | ||||||
| VE: | ||||||||||
| AMimprov1d | −0.07 | 0.51 | 1.67 | |||||||
| Amadeus-B3 Predictive Model (NHPT) | ||||||||||
| VD:NHPT_6m (to 6 months) | <0.001 * | 0.81 | 0.65 | 53.05 | 1.6 | 0.25 | ||||
| VP: | ||||||||||
| FFimprov1d | −18.36 | −0.27 | 0.007 * | 1.51 | ||||||
| EFimprov1d | −141.86 | −0.62 | <0.001 * | 1.51 | ||||||
| VE: | ||||||||||
| AMimprov1d | 0.26 | 0.78 | 1.67 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-López Terradas, P.A.; Criado Ferrer, T.; Jakob, I.; Calvo-Arenillas, J.I. Quo Vadis, Amadeo Hand Robot? A Randomized Study with a Hand Recovery Predictive Model in Subacute Stroke. Int. J. Environ. Res. Public Health 2023, 20, 690. https://doi.org/10.3390/ijerph20010690
Serrano-López Terradas PA, Criado Ferrer T, Jakob I, Calvo-Arenillas JI. Quo Vadis, Amadeo Hand Robot? A Randomized Study with a Hand Recovery Predictive Model in Subacute Stroke. International Journal of Environmental Research and Public Health. 2023; 20(1):690. https://doi.org/10.3390/ijerph20010690
Chicago/Turabian StyleSerrano-López Terradas, Pedro Amalio, Teresa Criado Ferrer, Iris Jakob, and Jose Ignacio Calvo-Arenillas. 2023. "Quo Vadis, Amadeo Hand Robot? A Randomized Study with a Hand Recovery Predictive Model in Subacute Stroke" International Journal of Environmental Research and Public Health 20, no. 1: 690. https://doi.org/10.3390/ijerph20010690
APA StyleSerrano-López Terradas, P. A., Criado Ferrer, T., Jakob, I., & Calvo-Arenillas, J. I. (2023). Quo Vadis, Amadeo Hand Robot? A Randomized Study with a Hand Recovery Predictive Model in Subacute Stroke. International Journal of Environmental Research and Public Health, 20(1), 690. https://doi.org/10.3390/ijerph20010690






