Microhabitat Conditions and Inter-Species Competition Predict the Successful Restoration of Declining Relict Species Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Reintroduction Experiment
2.3. Groundwater Quality
2.4. Data Analysis
3. Results
3.1. Changes in the Number of Individuals
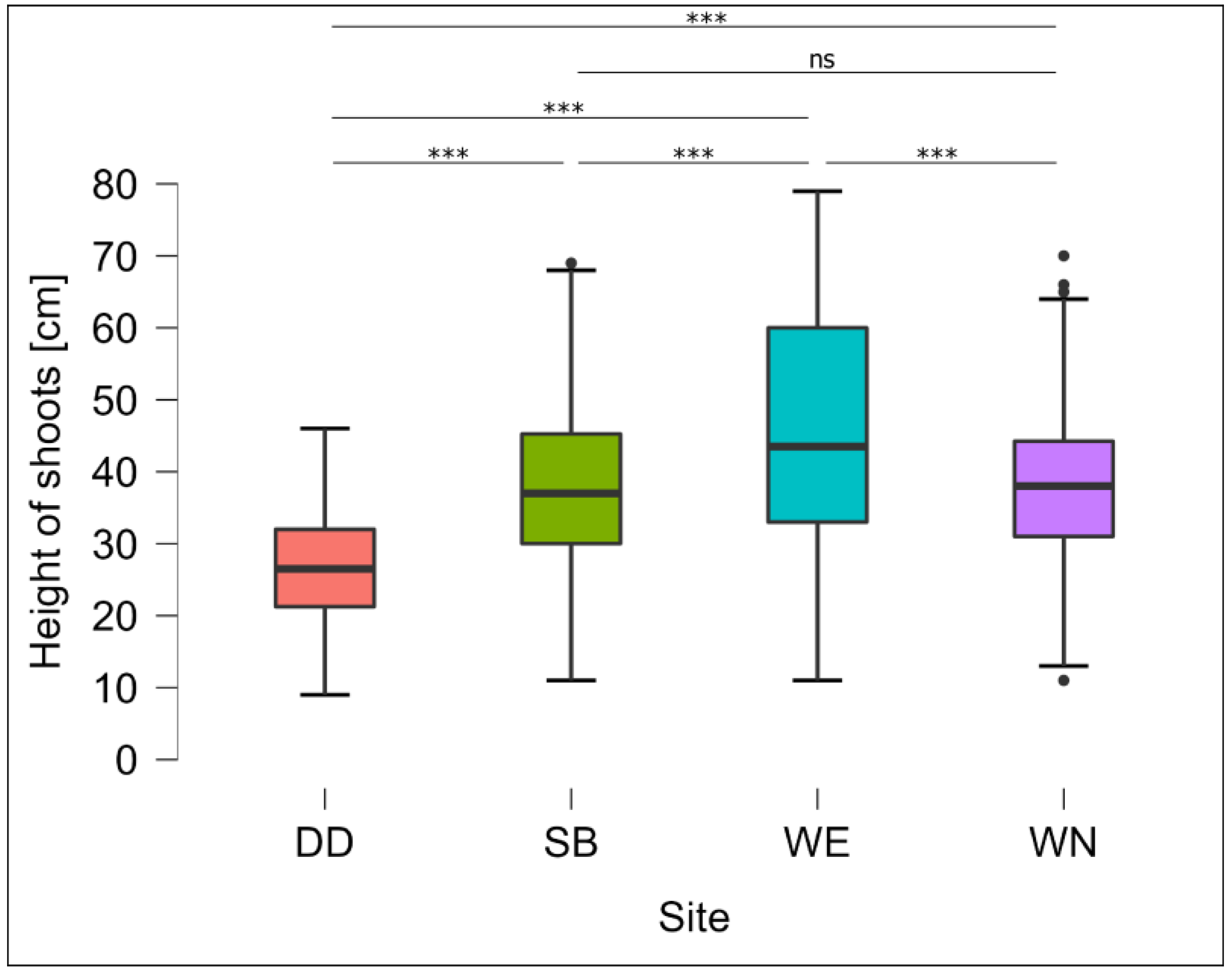
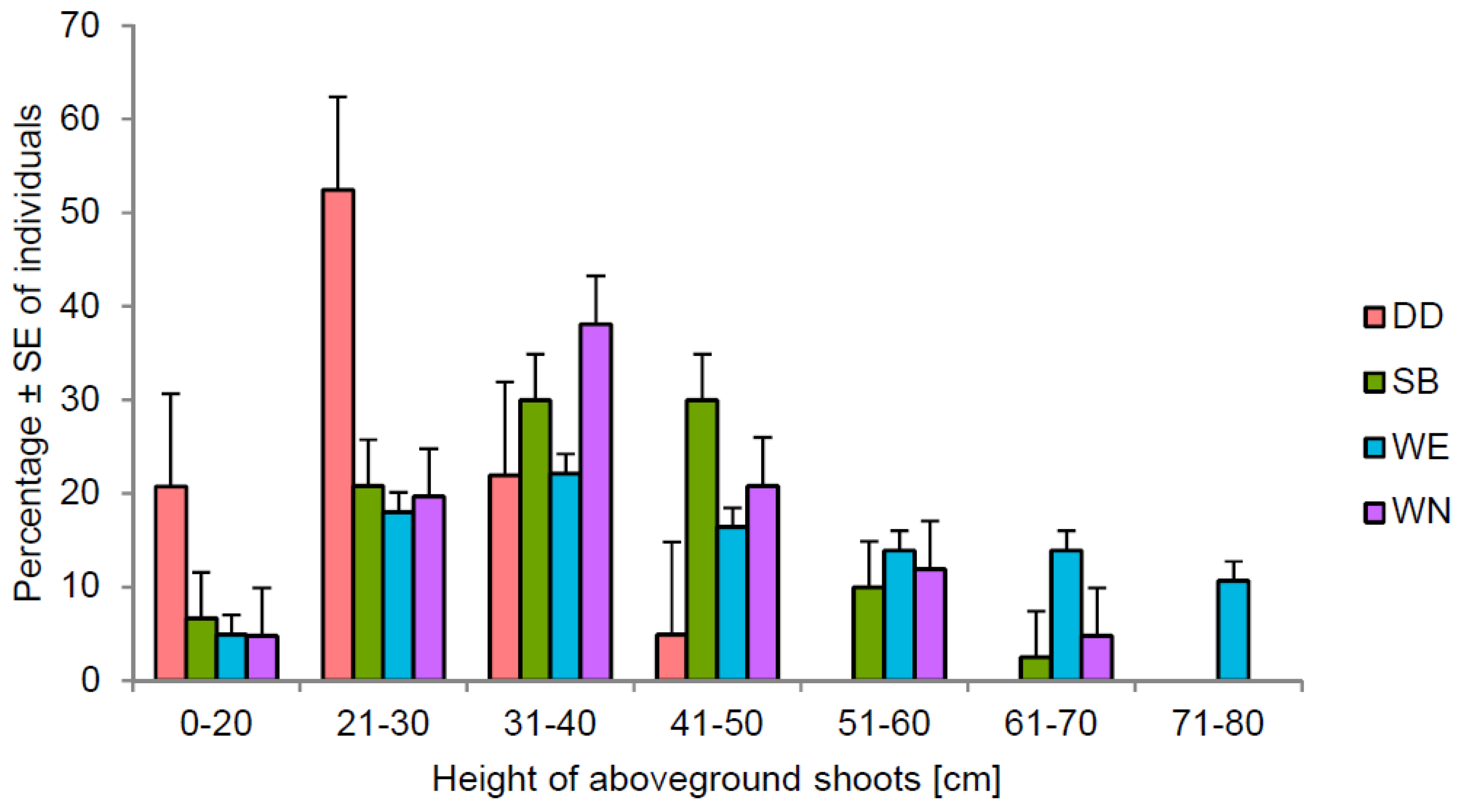
3.2. Changes in Shoot Height
3.3. Flowering Efficiency
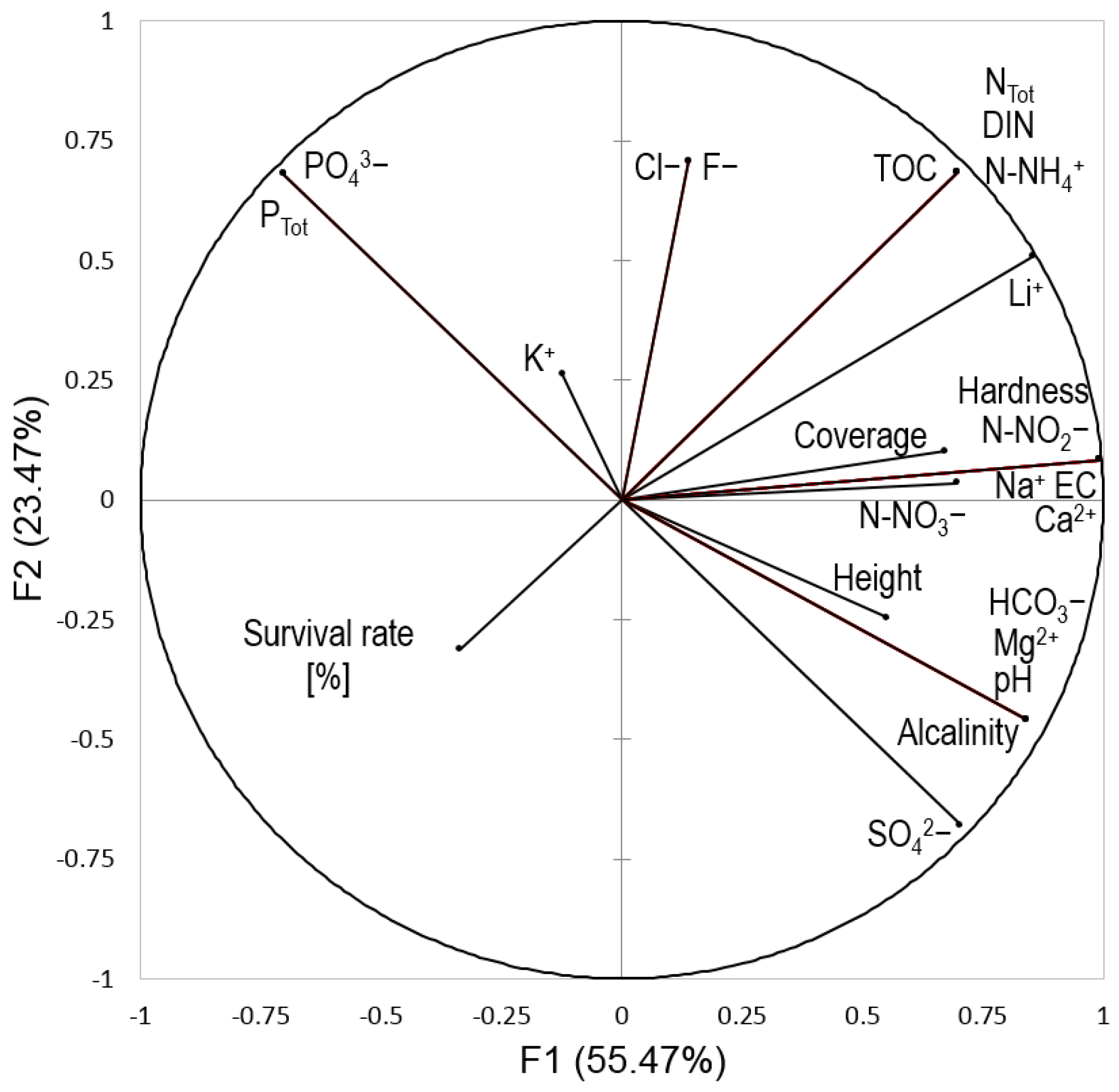
3.4. Interrelations between the Survival Rates of Salix lapponum, Acrotelm Water Chemistry, and Competition from Other Vascular Plants
4. Discussion
4.1. The Effects of Reintroduction
4.2. Effect of Competitor Abundance on Reintroduction Success and Fitness of Introduced Plants
4.3. Effect of Habitat Conditions on Reintroduction Success and Individual Growth and Fitness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banaszuk, P.; Kamocki, A. Effects of climatic fluctuations and land-use changes on the hydrology of temperate fluviogenous mire. Ecol. Eng. 2008, 32, 133–146. [Google Scholar] [CrossRef]
- Lesica, P.; McCune, B. Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming. J. Veg. Sci. 2004, 15, 679–690. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.P.; McIntyre, P.J.; Angert, A.L.; Rice, K.J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 415–436. [Google Scholar] [CrossRef]
- Comes, H.P.; Kadereit, J.W. The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci. 1998, 3, 432–438. [Google Scholar] [CrossRef]
- Diggelen, R.v.; Middleton, B.; Bakker, J.; Grootjans, A.; Wassen, M. Fens and floodplains of the temperate zone: Present status, threats, conservation and restoration. Appl. Veg. Sci. 2006, 9, 157–162. [Google Scholar] [CrossRef]
- Joyce, C.B. Ecological consequences and restoration potential of abandoned wet grasslands. Ecol. Eng. 2014, 66, 91–102. [Google Scholar] [CrossRef]
- Anderson, H. Invasive European Black Alder (Alnus glutinosa) Best Management Practices in Ontario; Ontario Invasive Plant Council: Peterborough, ON, Canada, 2013; pp. 1–25. [Google Scholar]
- Douda, J.; Čejková, A.; Douda, K.; Kochánková, J. Development of alder carr after the abandonment of wet grasslands during the last 70 years. Ann. For. Sci. 2009, 66, 712. [Google Scholar] [CrossRef]
- Kamocki, A.K.; Kołos, A.; Banaszuk, P. Can we effectively stop the expansion of trees on wetlands? Results of a birch removal experiment. Wetl. Ecol. Manag. 2017, 25, 359–367. [Google Scholar] [CrossRef]
- Kamocki, A.K.; Banaszuk, P.; Kołos, A. Removal of European alder Alnus glutinosa—An active method of mire conservation. Ecol. Eng. 2018, 111, 44–50. [Google Scholar] [CrossRef]
- Kullman, L. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J. Ecol. 2002, 90, 68–77. [Google Scholar] [CrossRef]
- Pajunen, A.M.; Oksanen, J.; Virtanen, R. Impact of shrub canopies on understorey vegetation in western Eurasian tundra. J. Veg. Sci. 2011, 22, 837–846. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Wojciechowska, J. The prospects for the survival of the population of a boreal relict species, Betula humilis Schrk., in a small isolated peat bog in the Łęczna—Włodawa Lakeland. Acta Agrobot. 2011, 64, 39–46. [Google Scholar] [CrossRef][Green Version]
- Kołos, A.; Wołkowycki, D.; Banaszuk, P.; Kamocki, A. Protection of relic plant species at the limit of geographical range: Response of downy willow Salix lapponum to removing a competitor. Ann. Bot. Fenn. 2015, 52, 303–314. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Mielniczuk, J. Habitat conditions of the endangered species Salix myrtilloides in Eastern Poland. Dendrobiology 2015, 73, 55–64. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Szczurowska, A.; Mielniczuk, J. Physico-chemical groundwater conditions at Salix lapponum stands in Eastern Poland. Dendrobiology 2015, 73, 65–74. [Google Scholar] [CrossRef]
- Serafin, A.; Urban, D.; Bronowicka-Mielniczuk, U.; Szczurowska, A. To what degree can the specifics of occurrence of glacial relic Betula humilis Schrank be an indicator of habitat conditions of moderate climate peatlands? Water 2018, 10, 1062. [Google Scholar] [CrossRef]
- Churski, M.; Danielewicz, W. Salix myrtilloides in the north central Poland. Distribution, threats and conservation. Dendrobiology 2008, 60, 3–9. [Google Scholar]
- Pogorzelec, M. Influence of chosen environmental abiotic factors on Salix lapponum L. populations in Polesie Lubelskie Region. Pol. J. Environ. Stud. 2008, 17, 581–586. [Google Scholar]
- Kruszelnicki, J. Salix Lapponum L. In Polska Czerwona Księga Roślin. Paprotniki I Rośliny Kwiatowel, 3rd ed.; Kaźmierczakowa, R., Zarzycki, K., Mirek, Z., Eds.; Polska Akademia Nauk, Instytut Ochrony Przyrody: Kraków, Poland, 2014; pp. 86–88. [Google Scholar]
- Stroh, P.A.; Leach, S.J.; August, T.A.; Walker, K.J.; Pearman, D.A.; Rumsey, F.J.; Harrower, C.A.; Fay, M.F.; Martin, J.P.; Pankhurst, T.; et al. A Vascular Plant Red List for England; Botanical Society of Britain and Ireland: Bristol, UK, 2014; pp. 1–184. [Google Scholar]
- Smaliukas, D. Laplandinis Karklas Salix lapponum L. In Lietuvos Raudonoji Knyga; Rašomavičius, V., Ed.; Leidykla Lututė: Kaunas, Lithuania, 2007; p. 443. [Google Scholar]
- Didukh, Y. “Red Data Book of Ukraine. Vegetable Kingdom” Afterword. Biodivers. Res. Conserv. 2010, 19, 87–92. [Google Scholar] [CrossRef]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer; Koeltz Scientific Books: Königstein, Germany, 1986. [Google Scholar]
- eBiodiversity. Available online: https://elurikkus.ee/en (accessed on 21 October 2022).
- Evarts-Bunders, P. Some rare and unclear willow (Salix L.) species in Latvia. Acta Biol. Univ. Daugavp. 2001, 1, 103–105. [Google Scholar]
- Plants of Belarus. Available online: http://hbc.bas-net.by/plantae/eng/default.php (accessed on 21 October 2022).
- Zając, A.; Zając, M. (Eds.) Atlas rozmieszczenia roślin naczyniowych w Polsce; Instytut Botaniki Uniwersytetu Jagiellońskiego: Kraków, Poland, 2001; pp. 1–714. [Google Scholar]
- Pogorzelec, M. Charakterystyka populacji i stanowisk Salix lapponum L. w Poleskim Parku Narodowym. Acta Agrophys. 2003, 1, 145–151. [Google Scholar]
- Koči, M. Subalpine tall-forb vegetation (Mulgedio-Aconitetea) in the Czech Republic: Syntaxonomical revision. Preslia 2001, 73, 289–331. [Google Scholar]
- Kołos, A. Współczesna roślinność i flora rezerwatów przyrody Bagno Wizna I i Bagno Wizna II jako efekt długotrwałego odwodnienia torfowisk w dolinie środkowej Narwi. Parki Nar. Rez. Przyr. 2004, 23, 61–91. [Google Scholar]
- Kruszelnicki, J. Stanowiska rzadszych roślin naczyniowych na terenie Mazurskiego Parku Krajobrazowego i jego okolic (Pojezierze Mazurskie). Fragm. Flor. Geobot. Pol. 2008, 15, 61–67. [Google Scholar]
- Pogorzelec, M.; Banach-Albińska, B.; Serafin, A.; Szczurowska, A. Population resources of an endangered species Salix lapponum L. in Polesie Lubelskie Region (Eastern Poland). Acta Agrobot. 2014, 67, 81–86. [Google Scholar] [CrossRef]
- Elven, R.; Karlsson, T. Salicaceae Mirbel. In Flora Nordica 1; Jonsell, B., Ed.; Bergius Foundation: Stockholm, Sweden, 2000; pp. 118–195. [Google Scholar]
- Pogorzelec, M.; Nowosielski, J. The Salix lapponum L. (downy willow) among-population genetic diversity in the Polesie Lubelskie Region. Ann. UMCS 2006, 61, 99–106. [Google Scholar]
- Hroneš, M.; Macurová, S.H.; Hradílek, Z.; Hekera, P.; Duchoslav, M. Female-biased sex ratio despite the absence of spatial and niche segregation between sexes in alpine populations of dioecious Salix lapponum (Salicaceae). Alp. Bot. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Sarrazin, F.; Barbault, R. Reintroduction: Challenges and lessons for basic ecology. Trends Ecol. Evol. 1996, 11, 474–478. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Polak, T.; Saltz, D. Reintroduction as an ecosystem restoration technique. Conserv. Biol. 2011, 25, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, N.; Buisson, E.; Dutoit, T. Species introduction—a major topic in vegetation restoration. Appl. Veg. Sci. 2012, 15, 161–165. [Google Scholar] [CrossRef]
- Ren, H.; Jian, S.G.; Liu, H.X.; Zhang, Q.M.; Lu, H.F. Advances in the reintroduction of rare and endangered wild plant species. Sci. China Life Sci. 2014, 57, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.A.; Crawley, M.J.; Rees, M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos 2000, 88, 225–238. [Google Scholar] [CrossRef]
- Clark, C.J.; Poulsen, J.R.; Levey, D.J.; Osenberg, C.W. Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am. Nat. 2007, 170, 128–142. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Q.; Lu, H.; Liu, H.; Guo, Q.; Wang, J.; Jian, S.; Bao, H. Wild plant species with extremely small populations require conservation and reintroduction in China. Ambio 2012, 41, 913–917. [Google Scholar] [CrossRef]
- Godefroid, S.; Piazza, C.; Rossi, G.; Buord, S.; Stevens, A.-D.; Aguraiuja, R.; Cowell, C.; Weekley, C.W.; Vogg, G.; Iriondo, J.M.; et al. How successful are plant species reintroductions? Biol. Conserv. 2011, 144, 672–682. [Google Scholar] [CrossRef]
- Maschinski, J.; Haskins, K.E. Plant Reintroduction in a Changing Climate: Promises and Perils; Island Press: Washington, DC, USA, 2012; pp. 1–432. [Google Scholar]
- Gilbert, D. (Ed.) Montane Scrub: The Challenge above the Treeline; Highland Birchwoods: Munlochy, UK, 2002; pp. 1–37. [Google Scholar]
- Skálová, D.; Navrátilová, B.; Richterová, L.; Knitl, M.; Sochor, M.; Vašut, R.J. Biotechnological methods of in vitro propagation in willows (Salix spp.). Cent. Eur. J. Biol. 2012, 7, 931–940. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Parzymies, M.; Banach-Albińska, B.; Serafin, A.; Szczurowska, A. Experimental reintroduction of the boreal species Salix lapponum L. to refuges at the southern limit of its range—Short-term results. Boreal Environ. Res. 2020, 25, 161–169. [Google Scholar]
- Stamati, K.; Hollingsworth, P.M.; Russell, J. Patterns of clonal diversity in three species of sub-arctic willow (Salix lanata, Salix lapponum and Salix herbacea). Plant Syst. Evol. 2007, 269, 75–88. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Torvell, L. Identifying suitable restoration sites for a scarce subarctic willow (Salix arbuscula) using different information sources and methods. Plant Ecol. Divers. 2008, 1, 105–114. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Bronowicka-Mielniczuk, U.; Banach, B.; Szczurowska, A.; Serafin, A. Relict boreal willows (Salix lapponum and Salix myrtilloides) as an element of phytocoenoses overgrowing the water bodies in eastern Poland. Appl. Ecol. Environ. Res. 2014, 12, 441–456. [Google Scholar] [CrossRef][Green Version]
- Pogorzelec, M.; Bronowicka-Mielniczuk, U.; Serafin, A.; Parzymies, M. The importance of habitat selection for the reintroduction of the endangered Salix lapponum L. in eastern Poland. J. Nat. Conserv. 2020, 54, 125785. [Google Scholar] [CrossRef]
- Głębocka, K.; Pogorzelec, M. Genetic diversity of the Salix lapponum L. population intended as a source of material for reintroduction. Dendrobiology 2017, 78, 136–145. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Bronowicka-Mielniczuk, U. Effect of the quality of shallow groundwaters on the occurrence of selected relic plant species of peatlands in the Łęczna-Włodawa Lakeland. J. Water Land Dev. 2020, 45, 133–142. [Google Scholar]
- Serafin, A.; Pogorzelec, M.; Banach-Albińska, B.; Zalewska, E.; Bronowicka-Mielniczuk, U.; Arciszewski, M. The importance of groundwater quality and other habitat parameters for effective active protection of an endangered plant species in Eastern Poland. Water 2022, 14, 1270. [Google Scholar] [CrossRef]
- Hroneš, M.; Macurová, S.H.; Hradílek, Z.; Hekera, P.; Duchoslav, M. Habitat conditions, stage structure and vegetation associations of geographically isolated subalpine populations of Salix lapponum L. (Salicaceae) in the Krkonoše Mts (Czech Republic). Biologia 2018, 73, 319–332. [Google Scholar] [CrossRef]
- Finger, A.; Rao, S.; Cowie, N.; MacDonell, T.; Beck, A.; Denny, B. Conservation genetics of montane willow populations in Scotland-limited natural recovery despite long-distance gene flow and high genetic diversity. Environ. Res. Ecol. 2023, 2, 015001. [Google Scholar] [CrossRef]
- Zieliński, P.; Ejsmont-Karabin, J.; Grabowska, M.; Karpowicz, M. Ecological status of shallow Lake Gorbacz (NE Poland) in its final stage before drying up. Oceanol. Hydrobiol. Stud. 2011, 40, 1–12. [Google Scholar] [CrossRef]
- Kołos, A.; Tarasewicz, A. Czynna ochrona zagrożonych ekosystemów jeziornych Niziny Północnopodlaskiej na przykładzie Jeziora Wiejki. Chrońmy Przyr. Ojcz. 2005, 61, 41–57. [Google Scholar]
- Gąsiorowski, M.; Kupryjanowicz, M. Lake—peat bog transformation recorded in the sediments of the Stare Biele mire (Northeastern Poland). Hydrobiologia 2009, 631, 143–154. [Google Scholar] [CrossRef]
- Czerwiński, A.; Kołos, A.; Matowicka, B. (Eds.) Dynamika Siedlisk i Roślinności Torfowisk Uroczyska Stare Biele w Puszczy Knyszyńskiej; Politechnika Białostocka: Białystok, Poland, 2000; pp. 1–225. [Google Scholar]
- Kołos, A.; Chmielewska–Nowik, E. Struktura populacji Salix lapponum (Salicaceae) na izolowanych stanowiskach w Puszczy Knyszyńskiej i Puszczy Białowieskiej. Fragm. Flor. Geobot. Pol. 2007, 14, 123–137. [Google Scholar]
- Żurek, S. Stratygrafia, Geneza I Wiek Torfowiska. In Dynamika Siedlisk i Roślinności Torfowisk Uroczyska Stare Biele w Puszczy Knyszyńskiej; Czerwiński, A., Kołos, A., Matowicka, B., Eds.; Politechnika Białostocka: Białystok, Poland, 2000; pp. 40–69. [Google Scholar]
- Pogorzelec, M.; Parzymies, M.; Bronowicka-Mielniczuk, U.; Banach, B.; Serafin, A. Pollen viability and tissue culture initiation of Salix lapponum, an endangered species in Poland. Acta Sci. Pol. Hortorum Cultus 2015, 14, 151–161. [Google Scholar]
- Kaye, T.N. Toward successful reintroductions: The combined importance of species traits, site quality, and restoration technique. In Proceedings of the CNPS Conservation Conference, Sacramento, CA, USA, 17–19 January 2009; pp. 99–106. [Google Scholar]
- Guerrant, E.O.; Kaye, T.N. Reintroduction of rare and endangered plants: Common factors questions and approaches. Aust. J. Bot. 2007, 55, 362–370. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Williams, S.L.; Rice, K.J.; Buchmann, S.L.; Cory, C.; Handel, S.N.; Nabhan, G.P.; Primack, R.; Robichaux, R.H. Restoration biology: A population biology perspective. Restor. Ecol. 1997, 5, 277–290. [Google Scholar] [CrossRef]
- Mardon, D.K. Conserving montane willow scrub on Ben Lawers NNR. Bot. J. Scotl. 2003, 55, 189–203. [Google Scholar] [CrossRef]
- Drayton, B.; Primack, R.B. Success rates for reintroductions of eight perennial plant species after 15 years. Restor. Ecol. 2012, 20, 299–303. [Google Scholar] [CrossRef]
- Bertoncello, R.; Oliveira, A.A.; Holl, K.D.; Pansonato, M.P.; Martini, A.M.Z. Cluster planting facilitates survival but not growth in early development of restored tropical forest. Basic Appl. Ecol. 2016, 17, 489–496. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Hawrylak-Nowak, B.; Banach-Albińska, B.; Szczurowska, A.; Parzymies, M.; Spólna, K. From ex situ cultivation to stands in natural habitats: Critical periods for plants during the reintroduction of Salix lapponum L. in Eastern Poland. J. Nat. Conserv. 2022, 67, 126172. [Google Scholar] [CrossRef]
- Hughes, F.M.R.; Johansson, M.; Xiong, S.; Carlborg, E.; Hawkins, D.; Svedmark, M.; Hayes, A.; Goodall, A.; Richards, K.S.; Nilsson, C. The influence of hydrological regimes on sex ratios and spatial segregation of the sexes in two dioecious riparian shrub species in northern Sweden. Plant Ecol. 2010, 208, 77–92. [Google Scholar] [CrossRef]
- Shaw, R.F.; Iason, G.R.; Pakeman, R.J.; Young, M.R. Regeneration of Salix arbuscula and Salix lapponum within a large mammal exclosure: The impacts of microsite and herbivory. Restor. Ecol. 2010, 18, 1–9. [Google Scholar] [CrossRef]
- Herder, M.D.; Virtanen, R.; Roininen, H. Effects of reindeer browsing on tundra willow and its associated insect herbivores. J. Appl. Ecol. 2004, 41, 870–879. [Google Scholar] [CrossRef]
- Kitti, H.; Forbes, B.C.; Oksanen, J. Long- and short-term effects of reindeer grazing on tundra wetland vegetation. Polar Biol. 2009, 32, 253–261. [Google Scholar] [CrossRef]
- Kolari, T.H.M.; Kumpula, T.; Verdonen, M.; Forbes, B.C.; Tahvanainen, T. Reindeer grazing controls willows but has only minor effects on plant communities in Fennoscandian oroarctic mires. Arct. Antarct. Alp. Res. 2019, 51, 506–520. [Google Scholar] [CrossRef]
- Kołos, A.; Banaszuk, P. Mowing as a tool for wet meadows restoration: Effect of long-term management on species richness and composition of sedge-dominated wetland. Ecol. Eng. 2013, 55, 23–28. [Google Scholar] [CrossRef]
- Kołos, A.; Banaszuk, P. How to remove expansive perennial species from sedge-dominated wetlands: Results of a long-term experiment in lowland river valleys. Rend. Lincei Sci. Fis. Nat. 2021, 32, 881–897. [Google Scholar] [CrossRef]
- Alford, A.L.; Hellgren, E.C.; Limb, R.; Engle, D.M. Experimental tree removal in tallgrass prairie: Variable responses of flora and fauna along a woody cover gradient. Ecol. Appl. 2012, 22, 947–958. [Google Scholar] [CrossRef]


| Location | Aggregation Number | Number of Individuals Introduced | Number of Surviving Individuals | Percentage of Surviving Individuals [%] | Number of Flowering Individuals | Percentage of Flowering Individuals [%] | Time Since Reintroduction [months] | |
|---|---|---|---|---|---|---|---|---|
| Stare Biele (SB) | 1SB | 24 | 20 | 83.33 | 0 | 0 | 34 | |
| 2SB | 24 | 11 | 45.83 | 0 | 0 | 34 | ||
| 3SB | 24 | 20 | 83.33 | 0 | 0 | 34 | ||
| 4SB | 24 | 15 | 62.50 | 0 | 0 | 34 | ||
| 5SB | 24 | 9 | 37.50 | 0 | 0 | 34 | ||
| 6SB | 24 | 13 | 54.17 | 0 | 0 | 34 | ||
| 7SB | 24 | 10 | 41.67 | 0 | 0 | 34 | ||
| 8SB | 24 | 18 | 75.00 | 0 | 0 | 34 | ||
| 9SB | 24 | 4 | 16.67 | 0 | 0 | 34 | ||
| Derazina valley (DD) | 10DD | 48 | 48 | 100.00 | 0 | 0 | 36 | |
| 11DD | 24 | 15 | 62.50 | 0 | 0 | 36 | ||
| 12DD | 24 | 19 | 79.17 | 0 | 0 | 36 | ||
| Lake Wiejki–east (WE) | 1WE | 24 | 0 | 0 | - | - | 36 | |
| 2WE | 24 | 8 | 33.33 | 0 | 0 | 36 | ||
| 3WE | 75 | 29 | 38.67 | 9 | 31.03 | 36 | ||
| 4WE | 38 | 36 | 94.74 | 0 | 0 | 46 | ||
| 5WE | 18 | 17 | 94.44 | 0 | 0 | 46 | ||
| 6WE | 33 | 32 | 96,97 | 1 | 3.13 | 46 | ||
| Lake Wiejki–north (WN) | 7WN | 52 | 37 | 71.15 | 0 | 0 | 33 | |
| 8WN | 51 | 35 | 68.63 | 6 | 17.14 | 33 | ||
| 16WN | 52 | 49 | 94.23 | 15 | 30.61 | 33 | ||
| 17WN | 51 | 47 | 92.16 | 6 | 12.77 | 33 | ||
| SB total | 216 | 120 | 55.56 | 0 | 0 | |||
| DD total | 96 | 82 | 85.42 | 0 | 0 | |||
| WE total | 212 | 122 | 57.55 | 10 | 8.20 | |||
| WN total | 206 | 168 | 81.55 | 27 | 16.07 | |||
| F1 | F2 | F3 | F4 | F5 | F6 | |
|---|---|---|---|---|---|---|
| Eigenvalue | 13.313 | 5.634 | 3.152 | 1.440 | 0.374 | 0.088 |
| Variability [%] | 55.471 | 23.474 | 13.132 | 6.000 | 1.556 | 0.366 |
| Cumulative [%] | 55.471 | 78.945 | 92.078 | 98.078 | 99.634 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamocki, A.K.; Kołos, A.; Pogorzelec, M.; Ożgo, M. Microhabitat Conditions and Inter-Species Competition Predict the Successful Restoration of Declining Relict Species Populations. Int. J. Environ. Res. Public Health 2023, 20, 608. https://doi.org/10.3390/ijerph20010608
Kamocki AK, Kołos A, Pogorzelec M, Ożgo M. Microhabitat Conditions and Inter-Species Competition Predict the Successful Restoration of Declining Relict Species Populations. International Journal of Environmental Research and Public Health. 2023; 20(1):608. https://doi.org/10.3390/ijerph20010608
Chicago/Turabian StyleKamocki, Andrzej K., Aleksander Kołos, Magdalena Pogorzelec, and Małgorzata Ożgo. 2023. "Microhabitat Conditions and Inter-Species Competition Predict the Successful Restoration of Declining Relict Species Populations" International Journal of Environmental Research and Public Health 20, no. 1: 608. https://doi.org/10.3390/ijerph20010608
APA StyleKamocki, A. K., Kołos, A., Pogorzelec, M., & Ożgo, M. (2023). Microhabitat Conditions and Inter-Species Competition Predict the Successful Restoration of Declining Relict Species Populations. International Journal of Environmental Research and Public Health, 20(1), 608. https://doi.org/10.3390/ijerph20010608







