Relationship between Lifestyle and Residence Area with 25(OH)D Levels in Older Adults
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camara, M.B.; Aidar, F.J.; Matos, D.G.; Gomes, A.A.B.; Barros, N.D.A.; De Souza, R.F.; Cabral, S.D.A.T. The relation between bone demineralization, physical activity and anthropometric standards. Motricidade 2017, 12, 45–55. [Google Scholar] [CrossRef]
- Santos, R.G.; Tribess, S.; Meneguci, J.; Bastos, L.L.A.; da, G.; Damião, R.; Virtuoso, J.J.S. Lower limb strength as an indicator of functional disability in older individuals. Motriz Rev. Educ. Fis. 2013, 19, S35–S42. [Google Scholar] [CrossRef]
- Amarante, T.R.P.; Gomes, A.R.S.; dos Santos, F.P.; Coelho, R.A.; Valderramas, S. Association between functional performance and falls in older women classified by age. Acta Fisiatr. 2015, 22, 181–185. [Google Scholar] [CrossRef]
- Amaral, S.M.; Santos, W.P.; Diniz, F.L.; Vinha, E.C.M. Evaluation of MIP and MEP in elderly women aged 60 to 70 years physical activity vs. sedentary sedentary practitioners. Id Line Rev. Mult. Psic. 2019, 13, 192–213. [Google Scholar]
- Pascotini, F.S.; Fedosse, E.; Ramos, M.C.; Ribeiro, V.V.; Trevisan, M.E. Respiratory muscle strength, pulmonary function and thoracoabdominal expansion in older adults and its relation with nutritional status. Fisioter. Pesqui. 2016, 23, 416–422. [Google Scholar] [CrossRef][Green Version]
- Mello, R.G.B.; Schneider, R.H.; Collares, F.M.; Dalacorte, R.R. Vitamin D and prevention of falls in the elderly: A systematic review. Sci Med. 2010, 20, 200–206. [Google Scholar]
- Silva, P.Z.; Schneider, R.H. Role of vitamin D in the neuro-muscular function. Acta Fisiatr. 2016, 23, 96–101. [Google Scholar] [CrossRef]
- Jorge, A.J.L.; Gonçalves, H.A.; Lima, L.P.; Ike, D.; Cancelliero, K.M.; Montebelo, M.I.L. Vitamin D deficiency and cardiovascular diseases. Int. J. Cardiovasc. Sci. 2018, 31, 422–432. [Google Scholar] [CrossRef]
- Resolução n.466, December 12, 2012. Aprova diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.html (accessed on 14 September 2021). (In Portuguese)
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; WHO: Geneva, Switzerland, 2000; 252p.
- Faria, F.R.; Borges, M.; Buratti, J.; Nogueira, C.D. Anthropometric and motor performance of cerebral palsy football athletes. Educ. Fís. Cienc. 2018, 20, e061. [Google Scholar]
- Thees, T.Y.P.; Pereira, P.M.L.; Bastos, M.G.; Cândido, A.P.C. Anthropometric and biochemical evaluation of patiens with carriers of chronic renal disease in conservative treatment. Nutr. Clín. Diet Hosp. 2018, 38, 75–81. [Google Scholar]
- Soares, P.G.; Pádua, T.V. Relationship between waist-hip ratio and body image in middle-aged women and older physically active. Rev. Kairos 2014, 17, 283–295. [Google Scholar]
- Costa, D.; Gonçalves, H.A.; Lima, L.P.; Ike, D.; Cancelliero, K.M.; Montebelo, M.I.L. New reference values for maximal respiratory pressures in the Brazilian population. J. Bras. Pneumol. 2010, 36, 306–312. [Google Scholar] [CrossRef]
- Dantas, M.L.V.; Costa, M.L.A.; Wanderley Filho, H.M.; Maia, T.S.A. Effects of a resisted exercises program on respiratory muscle strength in elderly. Fisioter. Bras. 2018, 19, S75–S82. [Google Scholar]
- Santos, R.M.G.; Pessoa-Santos, B.V.; Reis, I.M.M.; Labadessa, I.G.; Jamami, M. Manovacuometry performed by different length tracheas. Fisioter. Pesqui. 2017, 24, 9–14. [Google Scholar] [CrossRef][Green Version]
- Limberger, V.R.; Pastore, C.A.; Abib, R.T. Association between manual dynamometer, nutritional status and postoperative complications in oncologic patients. Rev. Bras. Cancerol. 2014, 60, 135–141. [Google Scholar]
- Bohannon, R.W.; Magasi, S.R.; Bubela, D.J.; Wang, Y.C.; Gershon, R.C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012, 46, 555–558. [Google Scholar] [CrossRef]
- Lee, J.H.; O’keefe, J.H.; Bell, D. Vitamin D deficiency. An important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef]
- Lima-Costa, M.F.; Andrade, F.B.; Souza Junior, P.R.B.; Neri, A.L.; Duarte, Y.A.O.; Castro-Costa, E.; de Oliveira, C. The Brazilian longitudinal study of aging (ELSI-Brazil): Objectives and design. Am. J. Epidemiol. 2018, 187, 1345–1353. [Google Scholar] [CrossRef]
- Ribeiro, C.G.; Ferretti, F.; Sá, C.A. Quality of life based on level of physical activity among elderly residents of urban and rural areas. Rev. Bras. Geriatr. Gerontol. 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Deluga, A.; Kosicka, B.; Dobrowolska, B.; Chrzan-Rodak, A.; Jurek, K.; Wrońska, I.; Ksykiewicz-Dorota, A.; Jędrych, M.; Drop, B. Lifestyle of the elderly living in rural and urban areas measured by the FANTASTIC Life Inventory. Ann. Agric. Environ. Med. 2018, 25, 562–567. [Google Scholar] [CrossRef]
- Silva, E.F.; Paniz, V.M.V.; Laste, G.; Torres, I.L.S. The prevalence of morbidity and symptoms among the elderly: A comparative study between rural and urban areas. Ciênc. Saúde Colet. 2013, 18, 1029–1040. [Google Scholar] [CrossRef]
- Muzi, C.D.; Figueiredo, V.C.; Luiz, R.R. Gradiente urbano-rural no padrão de consumo e cessação do tabagismo no Brasil. Cad. Saúde Públ. 2018, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Garbaccio, J.L.; Tonaco, L.A.B.; Estevao, W.G.; Barcelos Bárbara, J. Envelhecimento e qualidade de vida de idosos residentes da zona rural. Rev. Bras. Enferm. 2018, 71, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, R.; Jiang, A.; Ding, Y.; Wu, M.; Ma, X.; Zhao, Y.; He, J. Abdominal obesity and its association with health-related quality of life in adults: A population-based study in five Chinese cities. Health Qual. Life Outcomes 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Brown, L.J.; Mathews, K.I.; Whatnall, M.C.; Hutchesson, M.J.; MacDonald-Wicks, L.K.; Patterson, A.J. Rural versus urban women: An examination of anthropometry and body composition. Aust. J. Rural Health 2019, 27, 70–77. [Google Scholar] [CrossRef]
- Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H.; Entezari, M.H. Effect of vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: A clinical trial study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar]
- Rontoyanni, V.G.; Avila, J.C.; Kaul, S.; Wong, R.; Veeranki, S.P. Association between Obesity and Serum 25(OH)D Concentrations in Older Mexican Adults. Nutrients 2017, 9, 97. [Google Scholar] [CrossRef]
- Prado, M.R.M.C.; Oliveira, F.C.C.; Assis, K.F.; Ribeiro, S.A.V.; Prado, P.P., Jr.; Sant’Ana, L.F.R.; Priore, S.E.; Franceschini, S.C.C. Prevalência de deficiência de vitamina D e fatores associados em mulheres e seus recém-nascidos no período pós-parto. Rev. Paul. Pediatr. 2015, 33, 286–293. (In Portuguese) [Google Scholar] [CrossRef]
- Matz, C.J.; Stieb, D.M.; Brion, O. Urban-rural differences in daily time-activity patterns, occupational activity and housing characteristics. Environ. Health 2015, 14, 88. [Google Scholar] [CrossRef]
- Patwardhan, V.G.; Mughal, Z.M.; Chiplonkar, S.A.; Webb, A.R.; Kift, R.; Khadilkar, V.V.; Padidela, R.; Khadilkar, A.V. Duration of casual sunlight exposure necessary for adequate vitamin d status in Indian Men. Indian J. Endocrinol. Metab. 2018, 22, 249–255. [Google Scholar]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Chiang, C.M.; Ismaeel, A.; Griffis, R.B.; Weems, S. Effects of vitamin D supplementation on muscle strength in athletes: A systematic review. J. Strength Cond. Res. 2017, 31, 566–574. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Gu, Y.; Liu, M.; Chi, V.T.Q.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; et al. Vitamin D is related to handgrip strength in adult men aged 50 years and over: A population study from the TCLSIH cohort study. Clin. Endocrinol. 2019, 90, 753–765. [Google Scholar] [CrossRef]
- de Lima, T.R.; Silva, D.A.S.; de Castro, J.A.C.; Christofaro, D.G.D. Handgrip strength and associated sociodemographic and lifestyle factors: A systematic review of the adult population. J. Bodyw. Mov. Ther. 2017, 21, 401–413. [Google Scholar] [CrossRef]
- Vasconcelos, K.S.; Dias, J.M.; Bastone Ade, C.; Vieira, R.A.; Andrade, A.C.; Perracini, M.R.; Guerra, R.O.; Dias, R.C. Handgrip Strength Cutoff Points to Identify Mobility Limitation in Community-dwelling Older People and Associated Factors. J. Nutr. Health Aging 2016, 20, 306–315. [Google Scholar] [CrossRef]
- Abrams, G.D.; Feldman, D.; Safran, M.R. Effects of Vitamin D on skeletal muscle and athletic performance. J. Am. Acad Orthop. Surg. 2018, 26, 278–285. [Google Scholar] [CrossRef]
- Furtado, G.E.; Santos, S.S.; Rocha, S.V.; Souza, N.R.; Santos, C.A.; Viana, H.P.S.; Vasconcelos, L.R.C.; Letieri, V. Association between nutritional status and handgrip strength in elderly people living in rural areas. Motricidade 2016, 12, 22–29. [Google Scholar]
- Lenardt, M.H.; Carneiro, N.H.K.; Betiolli, S.E.; Binotto, M.A.; Ribeiro, D.K.M.N.; Teixeira, F.F.R. Factors associated with decreased handgrip strength in the elderly. Esc. Anna Nery 2016, 20, 1–7. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Parentoni, A.N.; Lustosa, L.P.; Santos, K.D.; Sá, L.F.; Ferreira, F.O.; Mendonça, V.A. Comparison of respiratory muscle strength between fragility subgroups in community elderly. Fisioter. Pesqui. 2013, 20, 361–366. [Google Scholar] [CrossRef]
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef]
- Severin, R.; Arena, R.; Lavie, C.J.; Bond, S.; Phillips, S.A. Respiratory muscle performance screening for infectious disease management following COVID-19: A highly pressurized situation. Am. J. Med. 2020, 133, 1025–1032. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
| Variables | Rural Area 41 (100%) | Urban Area 43 (100%) |
|---|---|---|
| Age (years) (Mean ± SD) | 68 ± 6 | 70 ± 8 |
| Sex | ||
| Male | 16 (39) | 7 (16) # |
| Female | 25 (61) | 36 (84) |
| Ethnicity | ||
| White | 12 (29) | 14 (32) |
| Pardo | 27 (66) | 24 (56) |
| Black | 2 (5) | 4 (9.30) |
| Yellow | - | 1 (2) |
| Marital Status | ||
| Married/Stable union | 27 (66) | 8 (19) |
| Widow(er) | 10 (24) | 24 (56) |
| Separated | 1 (2) | 8 (19) |
| Unmarried | 3 (7) | 3 (7) |
| Education | ||
| Illiterate | 18 (44) | 16 (37) |
| Incomplete elementary education | 21 (51) | 25 (58) |
| Complete elementary education | 1 (2) | 1 (2) |
| Incomplete secondary education | 1 (2) | 1 (2) |
| Per capita income | ||
| Up to 1 minimum wage | 40 (98) | 41 (95) |
| Above one minimum wage | 1 (2) | 1 (2) |
| Blank | - | 1 (2) |
| Professional activity | ||
| Retired | 41 (100) | 40 (93) |
| Farmer | - | 1 (2) |
| Hawker | - | 1 (2) |
| Blank | - | 1 (2) |
| Variables | Rural Area 41 (100%) | Urban Area 43 (100%) |
|---|---|---|
| Diabetes mellitus | ||
| Yes | 5 (12) | 7 (16) |
| No | 36 (88) | 36 (84) |
| Systemic arterial hypertension | ||
| Yes | 23 (56) | 24 (56) |
| No | 18 (44) | 19 (44) |
| Practice of physical activity | ||
| Yes | 11 (29) | 10 (23) |
| No | 30 (73) | 33 (77) |
| Frequency of physical activity | ||
| Twice or more a week | 11 (27) | 10 (23) |
| Alcohol use | ||
| Yes | 1 (2) | 3 (7) |
| No | 40 (98) | 40 (93) |
| Smoking | ||
| Smoker | 3 (7) | 12 (28) # |
| Ex-smoker | 21 (51) | 16 (37) |
| Never smoked | 17 (41) | 15 (35) |
| Perceived health | ||
| Good | 33 (80) | 22 (51) # |
| Regular | 8 (19) | 21 (49) |
| Variables | Rural Area | Urban Area | ||
|---|---|---|---|---|
| Male 16 (100%) | Female 25 (100%) | Male 7 (100%) | Female 36 (100%) | |
| BMI (Kg/m2) | 25 ± 4 | 26 ± 4 | 27 ± 5 | 25 ± 5 |
| Waist/hip ratio | 0.98 ± 0.05 | 0.94 ± 0.07 | 0.91 ± 0.05 # | 0.88 ± 0.08 # |
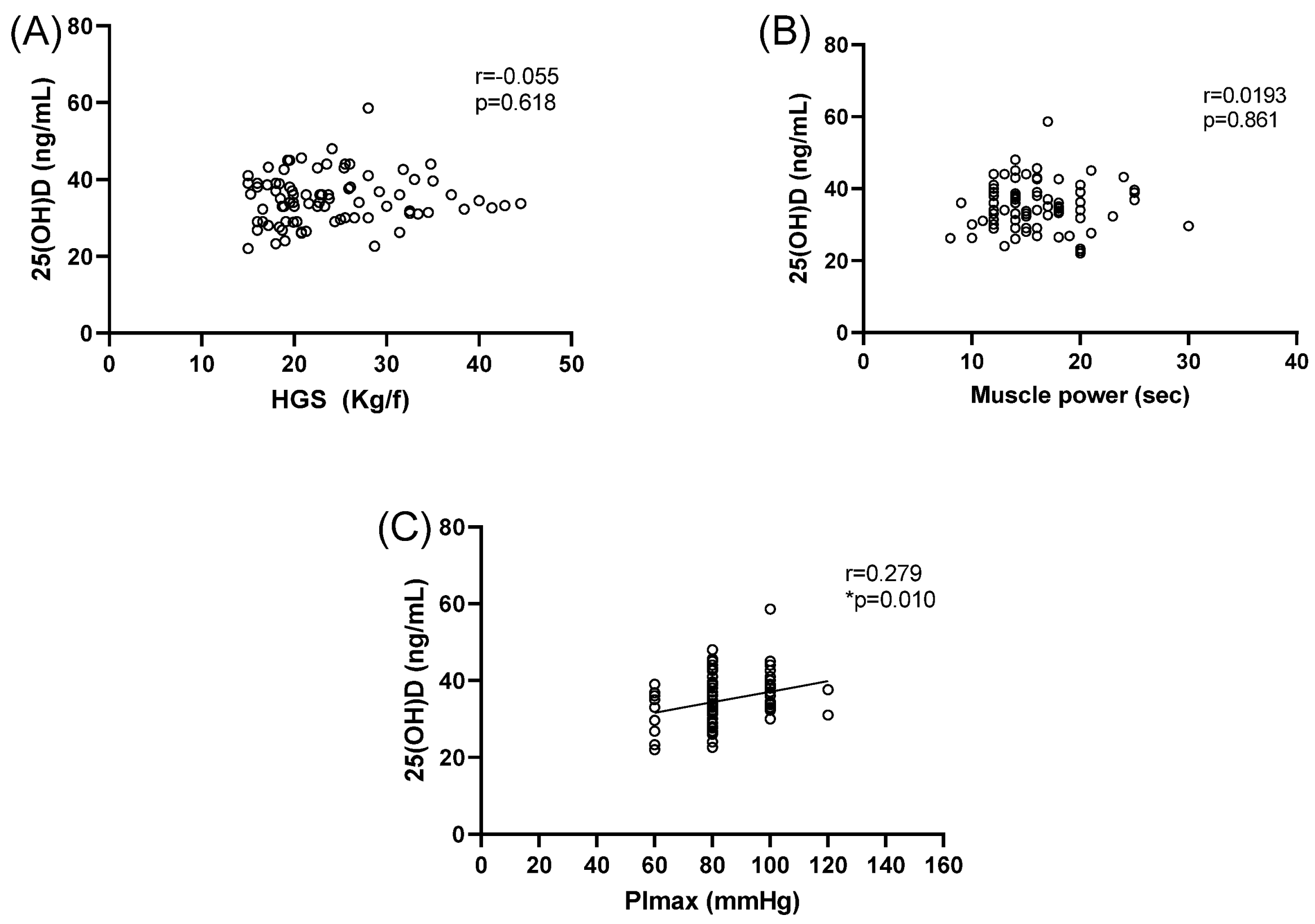
| Vit D (ng/mL) | 35 ± 8 | 34 ± 7 | 37 ± 5 | 35 ± 6 |
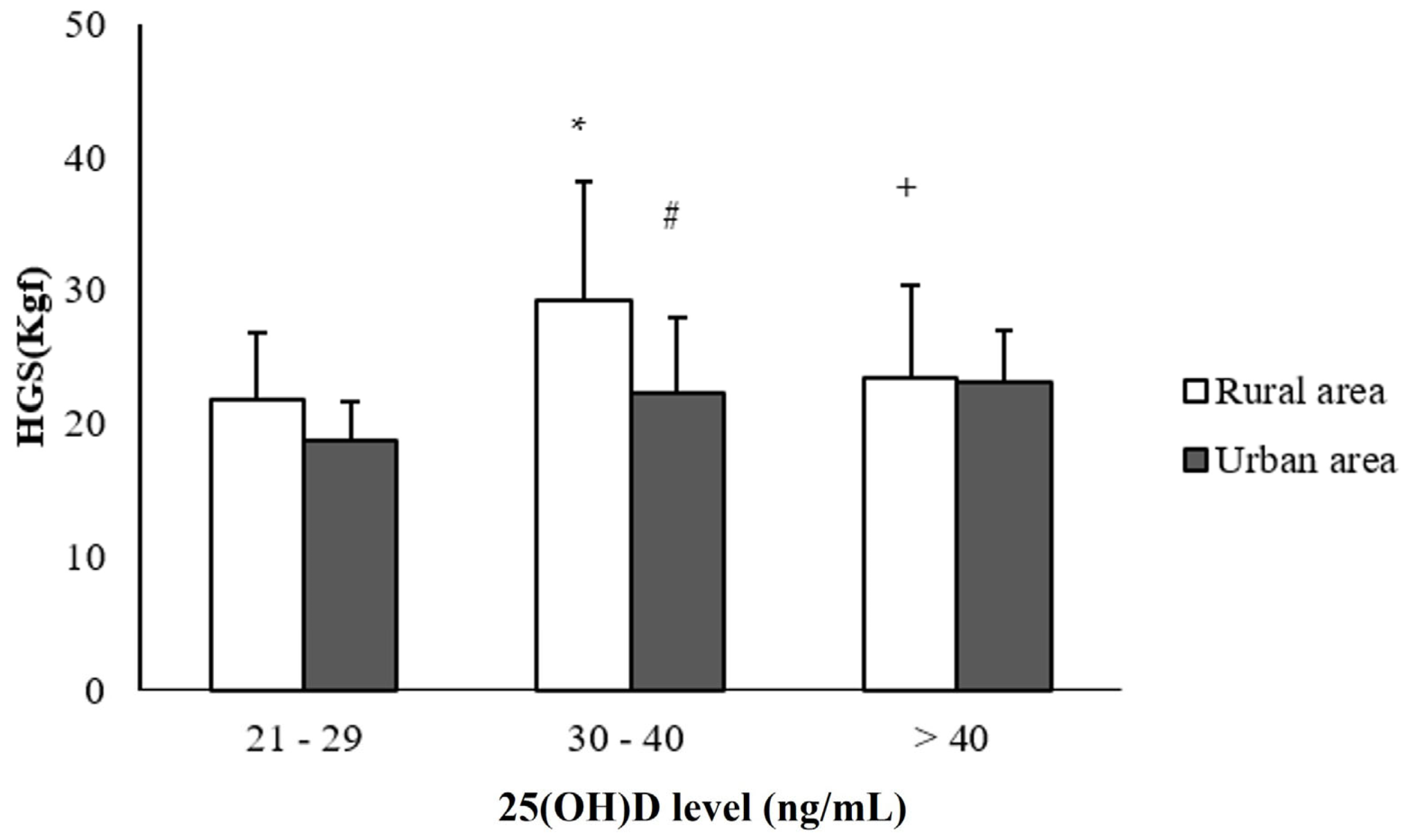
| Handgrip strength (Kgf) | 32 ± 7 | 22 ± 6 * | 29 ± 5 | 20 ± 4 * |
| Muscle power (s) | 16 ± 4 | 18 ± 5 | 13 ± 2 | 14 ± 2 # |
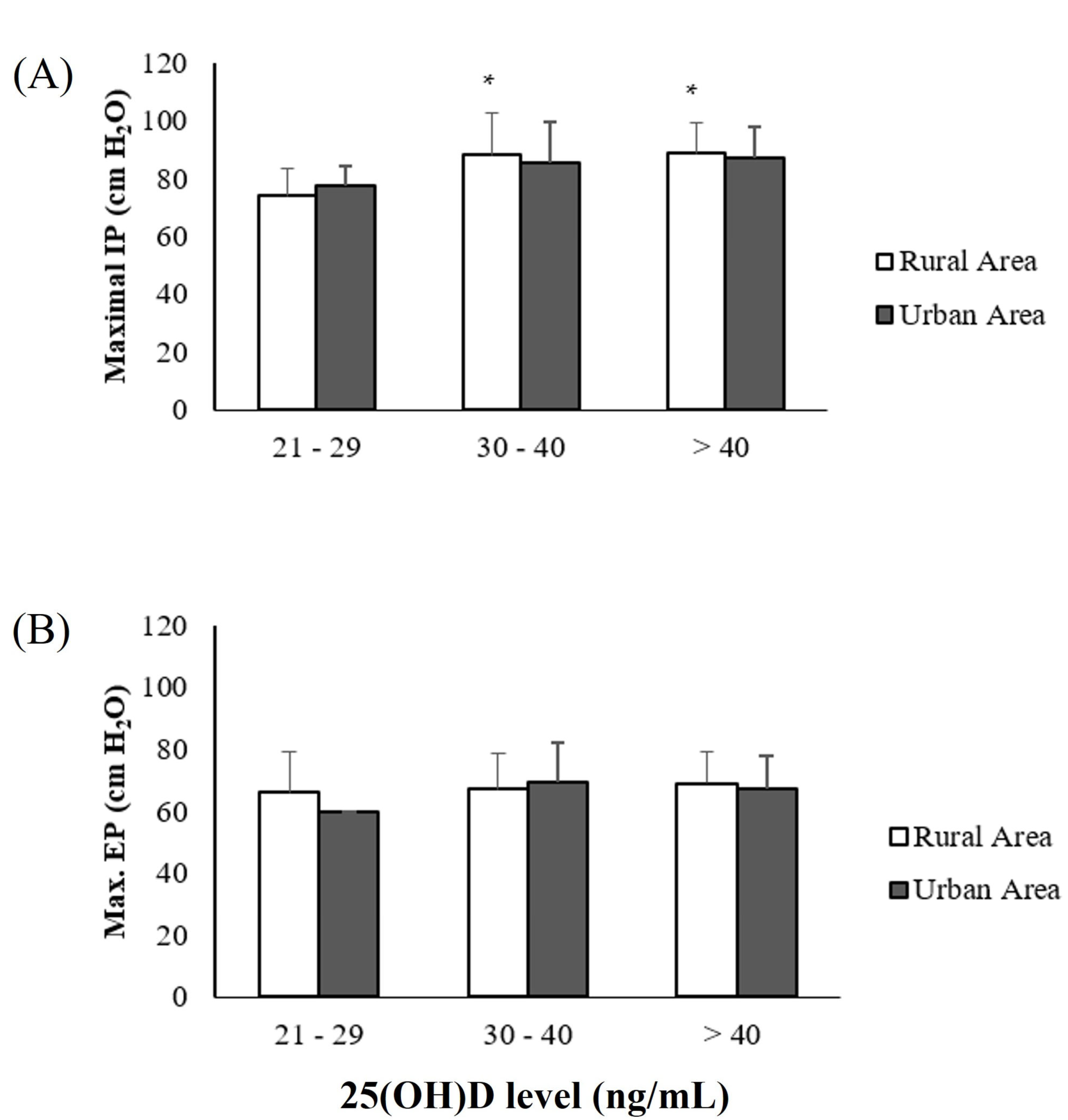
| MIP (mmHg) | 87 ± 14 | 83 ± 14 | 89 ± 16 | 83 ± 12 |
| MEP (mmHg) | 67 ± 13 | 66 ± 11 | 69 ± 15 | 67 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isidorio, U.d.A.; de Assis, E.V.; Lacerda, S.N.B.; Feitosa, A.d.N.A.; Alves, B.d.C.A.; Gascón, T.; da Veiga, G.L.; Fonseca, F.L.A. Relationship between Lifestyle and Residence Area with 25(OH)D Levels in Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 407. https://doi.org/10.3390/ijerph20010407
Isidorio UdA, de Assis EV, Lacerda SNB, Feitosa AdNA, Alves BdCA, Gascón T, da Veiga GL, Fonseca FLA. Relationship between Lifestyle and Residence Area with 25(OH)D Levels in Older Adults. International Journal of Environmental Research and Public Health. 2023; 20(1):407. https://doi.org/10.3390/ijerph20010407
Chicago/Turabian StyleIsidorio, Ubiraídys de Andrade, Elisangela Vilar de Assis, Sheylla Nadjane Batista Lacerda, Ankilma do Nascimento Andrade Feitosa, Beatriz da Costa Aguiar Alves, Thais Gascón, Glaucia Luciano da Veiga, and Fernando Luiz Affonso Fonseca. 2023. "Relationship between Lifestyle and Residence Area with 25(OH)D Levels in Older Adults" International Journal of Environmental Research and Public Health 20, no. 1: 407. https://doi.org/10.3390/ijerph20010407
APA StyleIsidorio, U. d. A., de Assis, E. V., Lacerda, S. N. B., Feitosa, A. d. N. A., Alves, B. d. C. A., Gascón, T., da Veiga, G. L., & Fonseca, F. L. A. (2023). Relationship between Lifestyle and Residence Area with 25(OH)D Levels in Older Adults. International Journal of Environmental Research and Public Health, 20(1), 407. https://doi.org/10.3390/ijerph20010407






