When Reality Does Not Meet Expectations—Experiences and Perceived Attitudes of Dutch Stakeholders Regarding Payment and Reimbursement Models for High-Priced Hospital Drugs
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Survey Objective and Design
2.3. Survey Dissemination and Analysis
3. Results
3.1. Sample Characteristics
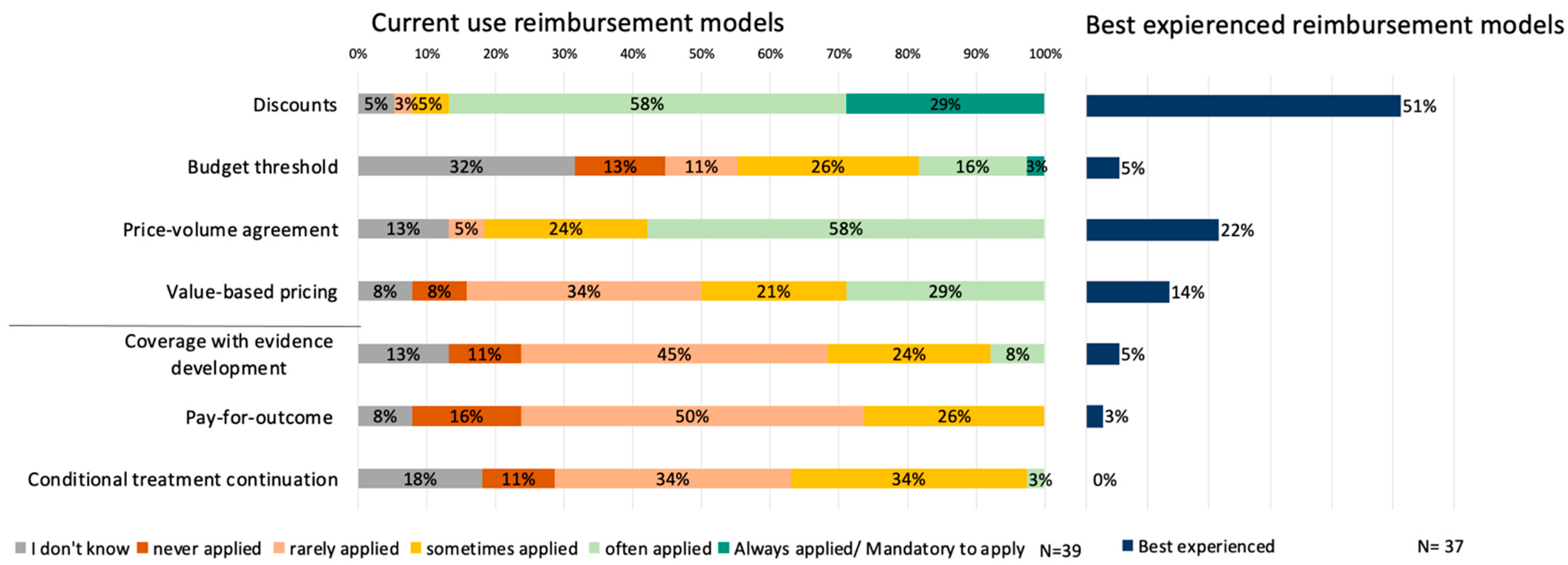
3.2. Current Use of Reimbursement Models
3.3. Future Preferences and Barriers of Reimbursement Models
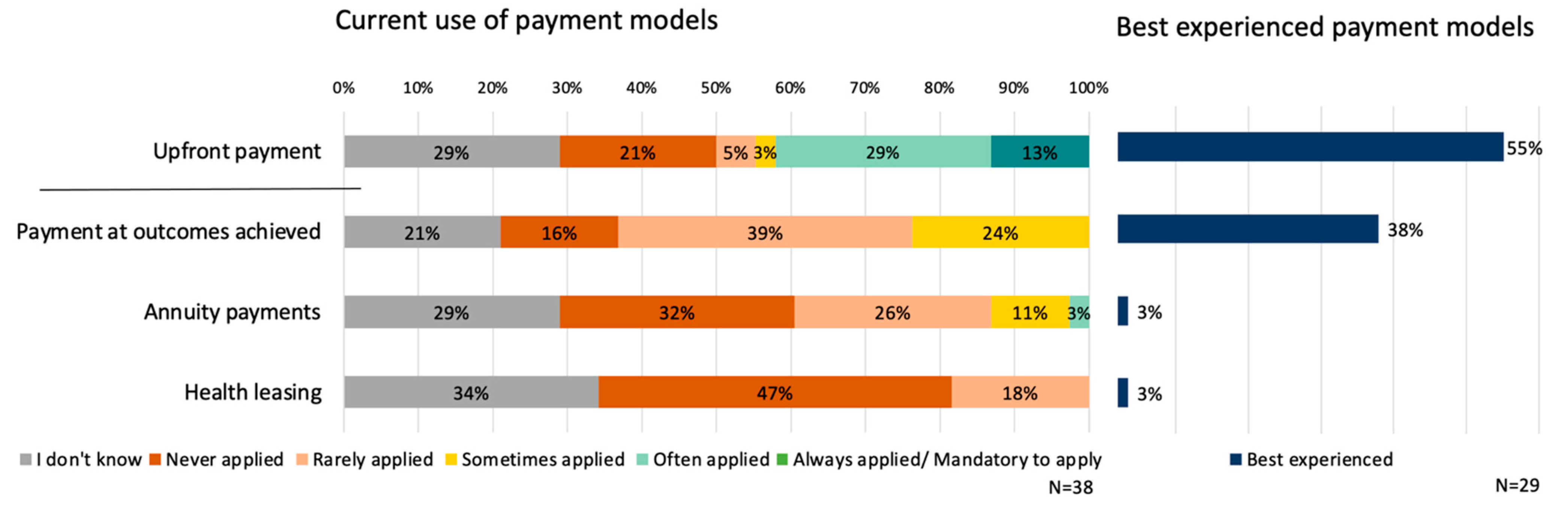
3.4. Current Use of Payment Models
3.5. Future Preferences and Barriers of Payment Models
3.6. Reimbursement and Payment Models within the Dutch Policy Setting
4. Discussion
4.1. Implications and Directions for Future Research
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wirtz, V.J.; Hogerzeil, H.V.; Gray, A.L.; Bigdeli, M.; de Joncheere, C.P.; Ewen, M.; Gyansa-Lutterodt, M.; Jing, S.; Luiza, V.L.; Mbindyo, R.M.; et al. Essential medicines for universal health coverage. Lancet 2017, 389, 403–476. Available online: https://pubmed.ncbi.nlm.nih.gov/27832874/ (accessed on 8 January 2021). [CrossRef] [PubMed]
- Lee, I.H.; Bloor, K.; Hewitt, C.; Maynard, A. International experience in controlling pharmaceutical expenditure: Influencing patients and providers and regulating industry-a systematic review. J. Health Serv. Res Policy 2015, 20, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Tordrup, D.; Ham, H.A.V.D.; Glanville, J.; Mantel-Teeuwisse, A.K. Systematic reviews of ten pharmaceutical pricing policies—A research protocol. J. Pharm. Policy Practice. BioMed Cent. 2020, 13, 22. Available online: https://joppp.biomedcentral.com/articles/10.1186/s40545-020-00228-0 (accessed on 8 January 2021). [CrossRef] [PubMed]
- Vogler, S.; Paris, V.; Ferrario, A.; Wirtz, V.J.; de Joncheere, K.; Schneider, P.; Pedersen, H.; Dedet, G.; Babar, Z. How can pricing and reimbursement policies improve affordable access to medicines? Lessons learned from European countries. Appl. Health Econ. Health Policy 2017, 15, 307–321. [Google Scholar] [CrossRef]
- Ferrario, A.; Kanavos, P. Dealing with uncertainty and high prices of new medicines: A comparative analysis of the use of managed entry agreements in Belgium, England, the Netherlands and Sweden. Soc. Sci. Med. 2015, 124, 39–47. [Google Scholar] [CrossRef]
- Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009-13. BMJ 2017, 359, j4530. [Google Scholar] [CrossRef]
- Wenzl, M.; Chapman, S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states: How they work and possible improvements going forward. OECD Health Work. Pap. 2019, 115. [Google Scholar] [CrossRef]
- Morel, T.; Arickx, F.; Befrits, G.; Siviero, P.; Van Der Meijden, C.; Xoxi, E.; Simoens, S. Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: A comparative study of managed entry agreements across seven European countries. Orphanet J. Rare Dis. 2013, 8, 198. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). WHO Guideline on Country Pharmaceutical Pricing Policies (Web Annex B). 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/335705/9789240011908-eng.pdf (accessed on 20 January 2021).
- Eichler, H.; Trusheim, M.; Schwarzer-Daum, B.; Larholt, K.; Zeitlinger, M.; Brunninger, M.; Sherman, M.; Strutton, D.; Hirsch, G. Precision Reimbursement for Precision Medicine: Using Real-World Evidence to Evolve from Trial-and-Project to Track-and-Pay to Learn-and-Predict. Clin. Pharmacol. Ther. 2021, 111, 52–62. Available online: https://pubmed.ncbi.nlm.nih.gov/34716918/ (accessed on 29 November 2021). [CrossRef]
- Whittal, A.; Jommi, C.; de Pouvourville, G.; Taylor, D.; Annemans, L.; Schoonaert, L.; Vermeersch, S.; Hutchings, A.; Patris, J. Faciliating More Efficient Negotiations for Innovative Therapies: A Value-Based Negotiation Framework. Int. J. Technol. Assess Health Care 2022, 38, e23. Available online: https://pubmed.ncbi.nlm.nih.gov/35274602/ (accessed on 20 March 2022). [CrossRef]
- Toumi, M.; Jarosławski, S.; Sawada, T.; Kornfeld, Å. The Use of Surrogate and Patient-Relevant Endpoints in Outcomes-Based Market Access Agreements: Current Debate. Appl. Health Econ. Health Policy 2017, 15, 5–11. Available online: https://pubmed.ncbi.nlm.nih.gov/27581118/ (accessed on 19 March 2022). [CrossRef] [PubMed]
- Cole, A.; Cubi-Molla, P.; Pollard, J.; Sim, D.; Sullivan, R.; Sussex, J.; Lorgelly, P. Making Outcome-Based Payment a Reality in the NHS. 2019. Available online: http://www.cancerresearchuk.org/ (accessed on 17 March 2022).
- Yeung, K.; Suh, K.; Basu, A.; Bansal, A.; Garrison, L.P.; Carlson, J.J. Paying for Cures: How Can We Afford It? Managed Care Pharmacy Stakeholder Perceptions of Policy Options to Address Affordability of Prescription Drugs. J. Manag. Care Spéc. Pharm. 2017, 23, 1084–1090. Available online: https://pubmed.ncbi.nlm.nih.gov/28944726/ (accessed on 17 March 2022). [CrossRef] [PubMed]
- Jørgensen, J.; Kefalas, P. Reimbursement of licensed cell and gene therapies across the major European healthcare markets. J. Mark Access Health Policy 2015, 3, 29321. Available online: https://pubmed.ncbi.nlm.nih.gov/27123175/ (accessed on 17 March 2022). [CrossRef] [PubMed]
- Eichler, H.-G.; Adams, R.; Andreassen, E.; Arlett, P.; van de Casteele, M.; Chapman, S.J.; Goettsch, W.G.; Martinsson, J.L.; Llinares-Garcia, J.; Nachtnebel, A.; et al. Exploring the opportunities for alignment of regulatory postauthorization requirements and data required for performance-based managed entry agreements. Int. J. Technol. Assess. Health Care 2021, 37, e83. Available online: https://pubmed.ncbi.nlm.nih.gov/34424152/ (accessed on 1 March 2022). [CrossRef] [PubMed]
- Dunlop, W.C.; Staufer, A.; Levy, P.; Edwards, G.J. Innovative pharmaceutical pricing agreements in five European markets: A survey of stakeholder attitudes and experience. Health Policy 2018, 122, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.; Toumi, M.; Dussart, C.; Borissov, B.; Dabbous, O.; Badora, K.; Auquier, P. Funding breakthrough therapies: A systematic review and recommendation. Health Policy 2018, 122, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.J.; Sullivan, S.D.; Garrison, L.P.; Neumann, P.J.; Veenstra, D.L. Linking payment to health outcomes: A taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy 2010, 96, 179–190. [Google Scholar] [CrossRef]
- Garrison, L.P.; Towse, A.; Briggs, A.; de Pouvourville, G.; Grueger, J.; Mohr, P.E.; Severens, J.; Siviero, P.; Sleeper, M. Performance-based risk-sharing arrangements—Good practices for design, implementation, and evaluation: Report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health 2013, 16, 703–719. Available online: https://pubmed.ncbi.nlm.nih.gov/23947963/ (accessed on 23 November 2020). [CrossRef]
- Michelsen, S.; Nachi, S.; Van Dyck, W.; Simoens, S.; Huys, I. Barriers and Opportunities for Implementation of Outcome-Based Spread Payments for High-Cost, One-Shot Curative Therapies. Front. Pharmacol. 2020, 11, 1. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2020.594446/full (accessed on 10 December 2020). [CrossRef]
- Bohm, N.; Bermingham, S.; Jones, F.G.; Gonçalves-Bradley, D.C.; Diamantopoulos, A.; Burton, J.R.; Laing, H. The Challenges of Outcomes-Based Contract Implementation for Medicines in Europe. Pharmacoeconomics 2021, 40, 13–29. Available online: https://link.springer.com/article/10.1007/s40273-021-01070-1 (accessed on 22 November 2021). [CrossRef]
- Vreman, R.; Broekhoff, T.; Leufkens, H.; Mantel-Teeuwisse, A.; Goettsch, W. Application of Managed Entry Agreements for Innovative Therapies in Different Settings and Combinations: A Feasibility Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8309. Available online: https://www.mdpi.com/1660-4601/17/22/8309 (accessed on 16 November 2020). [CrossRef] [PubMed]
- Facey, K.M.; Espin, J.; Kent, E.; Link, A.; Nicod, E.; O’Leary, A.; Xoxi, E.; van de Vijver, I.; Zaremba, A.; Benisheva, T.; et al. Implementing Outcomes-Based Managed Entry Agreements for Rare Disease Treatments: Nusinersen and Tisagenlecleucel. Pharmacoeconomics 2021, 39, 1021–1044. Available online: https://link.springer.com/article/10.1007/s40273-021-01050-5 (accessed on 29 July 2021). [CrossRef] [PubMed]
- Makady, A.; van Acker, S.; Nijmeijer, H.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. Conditional Financing of Drugs in the Netherlands: Past, Present, and Future—Results from Stakeholder Interviews. Value Health 2019, 22, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.J.; Chambers, J.D.; Simon, F.; Meckley, L.M. Risk-sharing arrangements that link payment for drugs to health outcomes are proving hard to implement. Health Aff. 2011, 30, 2329–2337. Available online: https://pubmed.ncbi.nlm.nih.gov/22147861/ (accessed on 19 March 2022). [CrossRef] [PubMed]
- Callenbach, M.H.; Ádám, L.; Vreman, R.A.; Németh, B.; Kaló, Z.; Goettsch, W.G. Reimbursement and payment models in Central and Eastern European as well as Middle Eastern countries: A survey of their current use and future outlook. Drug Discov. Today 2022, 28, 103433. Available online: https://pubmed.ncbi.nlm.nih.gov/36372328/ (accessed on 14 November 2022). [CrossRef]
- Ádám, I.; Callenbach, M.; Németh, B.; Vreman, R.A.; Tollin, C.; Pontén, J.; Daouw, D.; Elvidge, J.; Crabb, N.; van Waalwijk, D. Outcome-based reimbursement in Central-Eastern Europe and Middle-East. Front. Med. 2022, 9, 940886. Available online: https://pubmed.ncbi.nlm.nih.gov/36213666/ (accessed on 14 November 2022). [CrossRef]
- Goodman, C.; Villarivera, C.; Gregor, K.; Van Bavel, J. Regulatory, Policy, and Operational Considerations for Outcomes-Based Risk-Sharing Agreements in the U.S. Market: Opportunities for Reform. J. Manag. Care Spec. Pharm. 2019, 25, 1174–1181. [Google Scholar] [CrossRef]
- Garrison, L.P.; Carlson, J.J.; Bajaj, P.S.; Towse, A.; Neumann, P.J.; Sullivan, S.D.; Westrich, K.; Dubois, R.W. Private Sector Risk-Sharing Agreements in the United States: Trends, barriers, and prospects. Am. J. Manag. Care 2015, 21, 632–640. [Google Scholar]
- Neyt, M.; Gerkens, S.; Miguel, L.S.; Vinck, I.; Thiry, N.; Cleemput, I. An evaluation of managed entry agreements in Belgium: A system with threats and (high) potential if properly applied. Health Policy 2020, 124, 959–964. [Google Scholar] [CrossRef]
- Dabbous, M.; Chachoua, L.; Caban, A.; Toumi, M. Managed Entry Agreements: Policy Analysis from the European Perspective. Value Health 2020, 23, 425–433. Available online: https://pubmed.ncbi.nlm.nih.gov/32327159/ (accessed on 28 January 2022). [CrossRef]
- Makady, A.; van Veelen, A.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. Implementing managed entry agreements in practice: The Dutch reality check. Health Policy 2019, 123, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.; Kefalas, P. The use of innovative payment mechanisms for gene therapies in Europe and the USA. Regen. Med. 2021, 16, 405–421. Available online: www.futuremedicine.com (accessed on 29 November 2021). [CrossRef] [PubMed]
- Wettstein, D.J.; Boes, S. The impact of reimbursement negotiations on cost and availability of new pharmaceuticals: Evidence from an online experiment. Health Econ. Rev. 2020, 10, 13. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32440753 (accessed on 23 November 2020). [CrossRef] [PubMed]
- Nazareth, T.; Ko, J.J.; Sasane, R.; Frois, C.; Carpenter, S.; Demean, S.; Vegesna, A.; Wu, E.; Navarro, R.P. Outcomes-based contracting experience: Research findings from U.S. and European stakeholders. J. Manag. Care Spec. Pharm. 2017, 23, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Arāja, D.; Bochenek, T.; Čatić, T.; Dankó, D.; Dimitrova, M.; Fürst, J.; Greičiūtė-Kuprijanov, I.; Hoxha, I.; Jakupi, A.; et al. The Implementation of Managed Entry Agreements in Central and Eastern Europe: Findings and Implications. Pharmacoeconomics 2017, 35, 1271–1285. Available online: https://pubmed.ncbi.nlm.nih.gov/28836222/ (accessed on 20 January 2021). [CrossRef] [PubMed]
- Vogler, S.; Zimmermann, N.; Habimana, K. Study of the Policy Mix for the Reimbursement of Medicinal Products. Proposal for a Best Practice-Based Approach Based on Stakeholder Assessment; European Comission: Vienna, Austria, 2014. [Google Scholar]
- Morgan, S.G.; Vogler, S.; Wagner, A.K. Payers’ experiences with confidential pharmaceutical price discounts: A survey of public and statutory health systems in North America, Europe, and Australasia. Health Policy 2017, 121, 354–362. [Google Scholar] [CrossRef]
- Holtorf, A.-P.; Gialama, F.; Wijaya, K.E.; Kaló, Z. External Reference Pricing for Pharmaceuticals—A Survey and Literature Review to Describe Best Practices for Countries with Expanding Healthcare Coverage. Value Health Reg. Issues 2019, 19, 122–131. Available online: https://pubmed.ncbi.nlm.nih.gov/31416014/ (accessed on 20 January 2021). [CrossRef]
- Pauwels, K.; Huys, I.; Vogler, S.; Casteels, M.; Simoens, S. Managed entry agreements for oncology drugs: Lessons from the European experience to inform the future. Front. Pharmacol. 2017, 8, 171. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2017.00171/full (accessed on 20 January 2021). [CrossRef]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015, 42, 533–544. Available online: https://link.springer.com/article/10.1007/s10488-013-0528-y (accessed on 11 January 2021). [CrossRef]
- Validity and Reliability of Questionnaires. Available online: https://www.slideshare.net/Venkitachalam/validity-and-reliability-of-questionnaires (accessed on 30 July 2021).
- Bolarinwa, O.A. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger. Postgrad. Med. J. 2015, 22, 195. Available online: https://www.npmj.org/article.asp?issn=1117-1936;year=2015;volume=22;issue=4;spage=195;epage=201;aulast=Bolarinwa (accessed on 20 July 2021). [CrossRef]
- Home Page—LimeSurvey—Easy Online Survey Tool. Available online: https://www.limesurvey.org/ (accessed on 20 January 2022).
- Microsoft Excel-Spreadsheetsoftware. Microsoft 365. Available online: https://www.microsoft.com/nl-nl/microsoft-365/excel (accessed on 20 January 2022).
- Qualitative Data Analysis Software. NVivo. Available online: https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home (accessed on 8 September 2021).
- Rotar, A.M.; Preda, A.; Löblová, O.; Benkovic, V.; Zawodnik, S.; Gulacsi, L.; Niewada, M.; Boncz, I.; Petrova, G.; Dimitrova, M.; et al. Rationalizing the introduction and use of pharmaceutical products: The role of managed entry agreements in Central and Eastern European countries. Health Policy 2018, 122, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.Z.; Godman, B.; Gargano, L.P.; Azevedo, P.S.; Garcia, M.M.; Cazarim, M.S.; Pantuzza, L.L.N.; Ribeiro-Junior, N.G.; Pereira, A.L.; Borin, M.C.; et al. Integrative Review of Managed Entry Agreements: Chances and Limitations. Pharmacoeconomics 2020, 38, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Maskineh, C.; Nasser, S.C. Managed Entry Agreements for Pharmaceutical Products in Middle East and North African countries: Payer and Manufacturer Experience and Outlook. Value Health Reg Issues 2018, 16, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Antonanzas, F.; Juárez-Castelló, C.; Lorente, R.; Rodríguez-Ibeas, R. The Use of Risk-Sharing Contracts in Healthcare: Theoretical and Empirical Assessments. Pharmacoeconomics 2019, 37, 1469–1483. Available online: https://link.springer.com/article/10.1007/s40273-019-00838-w (accessed on 23 November 2020). [CrossRef]
- Andersson, E.; Svensson, J.; Persson, U.; Lindgren, P. Risk sharing in managed entry agreements—A review of the Swedish experience. Health Policy 2020, 124, 404–410. [Google Scholar] [CrossRef]
- Adamski, J.; Godman, B.; Ofierska-Sujkowska, G.; Osińska, B.; Herholz, H.; Wendykowska, K.; Laius, O.; Jan, S.; Sermet, C.; Zara, C.; et al. Risk sharing arrangements for pharmaceuticals: Potential considerations and recommendations for European payers. BMC Health Serv. Res. 2010, 10. [Google Scholar] [CrossRef]
- Ádám, I.; Callenbach, M.; Németh, B.; Vreman, R.A.; Pontén, J.; Strbad, T.; Dawoud, D.; Kostyuk, A.; Seyam, A.; Nagy, L. Delayed payment schemes in Central-Eastern Europe and Middle-East. Front Med. 2022, 12, 9. Available online: https://pubmed.ncbi.nlm.nih.gov/36035424/ (accessed on 14 November 2022). [CrossRef]
- Barlow, J.F.; Yang, M.; Teagarden, J.R. Are Payers Ready, Willing, and Able to Provide Access to New Durable Gene Therapies? Value Health 2019, 22, 642–647. [Google Scholar] [CrossRef]
- Impact HTA. Health Technology Assessment. Work Package 10. Available online: https://www.impact-hta.eu/work-package-10 (accessed on 16 May 2022).
- Kanavos, P.; Ferrario, A.; Tafuri, G.; Siviero, P. Managing Risk and Uncertainty in Health Technology Introduction: The Role of Managed Entry Agreements. Glob. Policy 2017, 8, 84–92. [Google Scholar] [CrossRef]
- Efthymiadou, O.; Kanavos, P. Determinants of Managed Entry Agreements in the context of Health Technology Assessment: A comparative analysis of oncology therapies in four countries. Int. J. Technol. Assess. Health Care 2021, 37, e31. Available online: https://pubmed.ncbi.nlm.nih.gov/33509311/ (accessed on 16 May 2022). [CrossRef]


| Types of Models |
|---|
| Reimbursement models Financial-based reimbursement models Discounts/rebates Budget threshold/dedicated funds Price-volume agreements Outcome-based reimbursement models Value-based pricing Pay-for-outcome/outcome guarantees Conditional treatment continuation Coverage with evidence development |
| Payment models Upfront payment Delayed payment models Pay at outcomes achieved Annuity payments Health leasing/subscription |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callenbach, M.H.E.; Vreman, R.A.; Mantel-Teeuwisse, A.K.; Goettsch, W.G. When Reality Does Not Meet Expectations—Experiences and Perceived Attitudes of Dutch Stakeholders Regarding Payment and Reimbursement Models for High-Priced Hospital Drugs. Int. J. Environ. Res. Public Health 2023, 20, 340. https://doi.org/10.3390/ijerph20010340
Callenbach MHE, Vreman RA, Mantel-Teeuwisse AK, Goettsch WG. When Reality Does Not Meet Expectations—Experiences and Perceived Attitudes of Dutch Stakeholders Regarding Payment and Reimbursement Models for High-Priced Hospital Drugs. International Journal of Environmental Research and Public Health. 2023; 20(1):340. https://doi.org/10.3390/ijerph20010340
Chicago/Turabian StyleCallenbach, Marcelien H. E., Rick A. Vreman, Aukje K. Mantel-Teeuwisse, and Wim G. Goettsch. 2023. "When Reality Does Not Meet Expectations—Experiences and Perceived Attitudes of Dutch Stakeholders Regarding Payment and Reimbursement Models for High-Priced Hospital Drugs" International Journal of Environmental Research and Public Health 20, no. 1: 340. https://doi.org/10.3390/ijerph20010340
APA StyleCallenbach, M. H. E., Vreman, R. A., Mantel-Teeuwisse, A. K., & Goettsch, W. G. (2023). When Reality Does Not Meet Expectations—Experiences and Perceived Attitudes of Dutch Stakeholders Regarding Payment and Reimbursement Models for High-Priced Hospital Drugs. International Journal of Environmental Research and Public Health, 20(1), 340. https://doi.org/10.3390/ijerph20010340





