Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
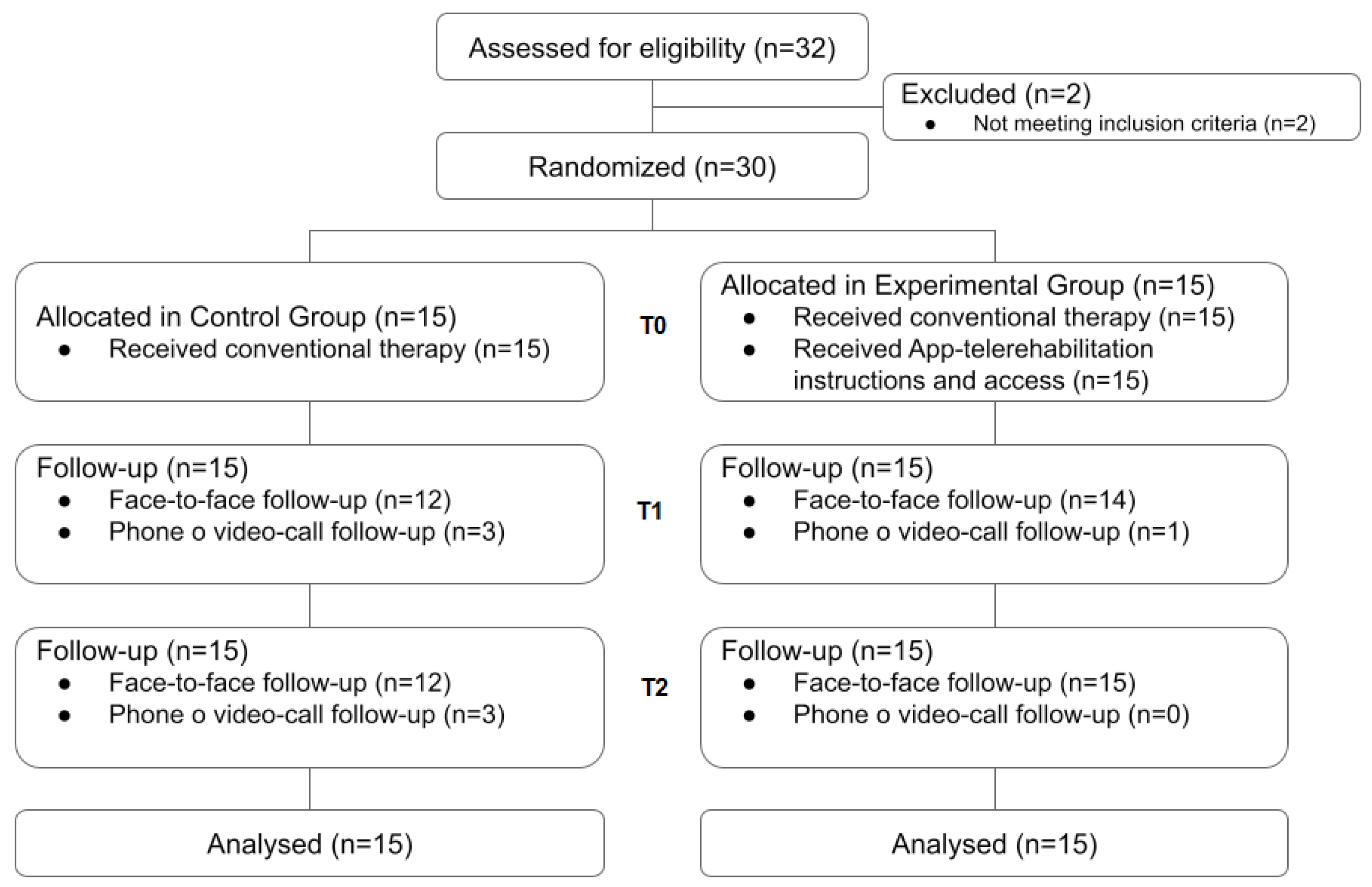
2.1. Study Design, Ethics and Sample Characteristics
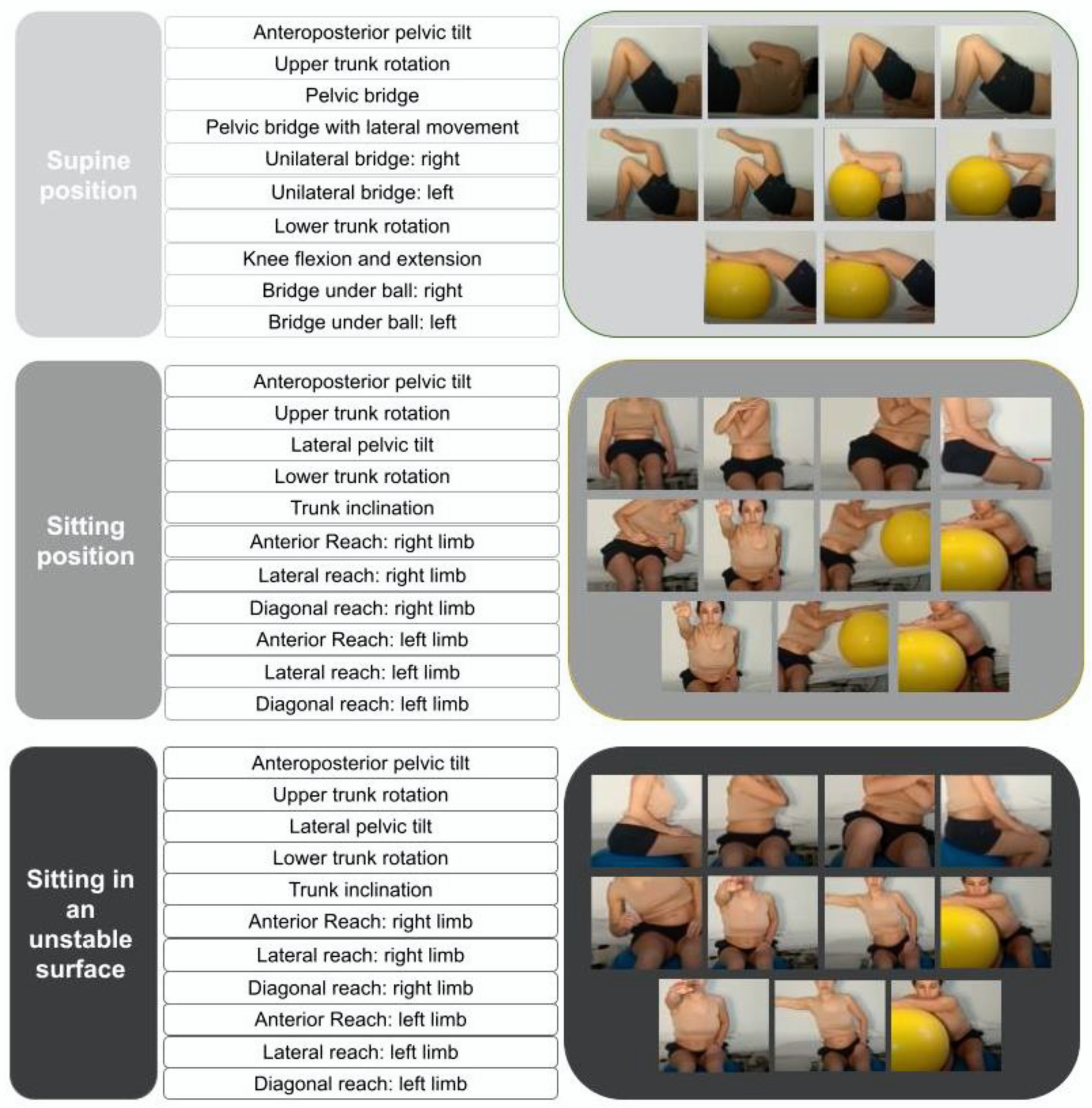
2.2. Interventions
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Seitz, R.J.; Donnan, G.A. Recovery potential after acute stroke. Front. Neurol. 2015, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture. 2017, 54, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Krishnan, R. Truncal impairment after stroke: Clinical correlates, outcome and impact on ambulatory and functional outcomes after rehabilitation. Singapore Med. J. 2021, 62, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.D.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke. 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e0219781. [Google Scholar] [CrossRef]
- Karthikbabu, S.; Chakrapani, M.; Ganesan, S.; Ellajosyula, R.; Solomon, J.M. Efficacy of Trunk Regimes on Balance, Mobility, Physical Function, and Community Reintegration in Chronic Stroke: A Parallel-Group Randomized Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 1003–1011. [Google Scholar] [CrossRef]
- Souza, D.; de Sales Santos, M.; da Silva Ribeiro, N.; Maldonado, I. Inpatient trunk exercises after recent stroke: An update meta-analysis of randomized controlled trials. NeuroRehabilitation 2019, 44, 369–377. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Cuchi, G.U.; Bagur-Calafat, C. Trunk training exercises approaches for improving trunk performance and functional sitting balance in patients with stroke: A systematic review. NeuroRehabilitation 2013, 33, 575–592. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; Hernández-Valiño, M.; Urrútia Cuchí, G. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaur, J. Effect of core strengthening with pelvic proprioceptive neuromuscular facilitation on trunk, balance, gait, and function in chronic stroke. J. Exerc. Rehabil. 2017, 13, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of Core Stability Training on Trunk Function, Standing Balance, and Mobility in Stroke Patients. Neurorehabilit. Neural Repair. 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Verheyden, G.; Vereeck, L.; Truijen, S.; Troch, M.; Herregodts, I.; Lafosse, C.; Nieuwboer, A.; De Weerdt, W. Trunk performance after stroke and the relationship with balance, gait and functional ability. Clin. Rehabil. 2006, 20, 451–458. [Google Scholar] [CrossRef]
- Gamble, K.; Chiu, A.; Peiris, C. Core Stability Exercises in Addition to Usual Care Physiotherapy Improve Stability and Balance After Stroke: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 762–775. [Google Scholar] [CrossRef]
- Chen, Y.; Abel, K.; Janecek, J.; Chen, Y.; Zheng, K.; Cramer, S. Home-based technologies for stroke rehabilitation: A systematic review. Int. J. Med. Inform. 2019, 123, 11–22. [Google Scholar] [CrossRef]
- Leochico, C.F.D.; Espiritu, A.I.; Ignacio, S.D.; Mojica, J.A.P. Challenges to the Emergence of Telerehabilitation in a Developing Country: A Systematic Review. Front. Neurol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Tchero, H.; Tabue Teguo, M.; Lannuzel, A.; Rusch, E. Telerehabilitation for Stroke Survivors: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2018, 20, e10867. [Google Scholar] [CrossRef]
- Ostrowska, P.M.; Śliwiński, M.; Studnicki, R.; Hansdorfer-Korzon, R. Telerehabilitation of post-stroke patients as a therapeutic solution in the era of the covid-19 pandemic. Healthcare 2021, 9, 654. [Google Scholar] [CrossRef]
- Turolla, A.; Rossettini, G.; Viceconti, A.; Palese, A.; Geri, T. Musculoskeletal Physical Therapy During the COVID-19 Pandemic: Is Telerehabilitation the Answer? Phys. Ther. 2020, 100, 1260–1264. [Google Scholar] [CrossRef]
- Øra, H.P.; Kirmess, M.; Brady, M.C.; Partee, I.; Hognestad, R.B.; Johannessen, B.B.; Thommessen, B.; Becker, F. The effect of augmented speech-language therapy delivered by telerehabilitation on poststroke aphasia—a pilot randomized controlled trial. Clin. Rehabil. 2020, 34, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Rodríguez, M.T.; Collado Vázquez, S.; Martín Casas, P.; Cano de la Cuerda, R. Apps en neurorrehabilitación. Una revisión sistemática de aplicaciones móviles. Neurología. 2018, 33, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Moral-Munoz, J.A.; Zhang, W.; Cobo, M.J.; Kaber, D.B. Smartphone-based systems for physical rehabilitation applications: A systematic review. Assist. Technol. 2019, 33, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, M.; Zhou, L. Use of mobile applications in post-stroke rehabilitation: A systematic review. Top. Stroke Rehabil. 2018, 13. [Google Scholar] [CrossRef]
- Rodríguez, M.T.S.; Vázquez, S.C.; Casas, P.M.; De Cuerda, R.C. Neurorehabilitation and apps: A systematic review of mobile applications. Neurología 2018, 33, 313–326. [Google Scholar] [CrossRef]
- Nussbaum, R.; Kelly, C.; Quinby, E.; Mac, A.; Parmanto, B.; Dicianno, B.E. Systematic Review of Mobile Health Applications in Rehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, 115–127. [Google Scholar] [CrossRef]
- Chan, B.K.S.; Ng, S.S.M.; Ng, G.Y.F. A Home-Based Program of Transcutaneous Electrical Nerve Stimulation and Task-Related Trunk Training Improves Trunk Control in Patients with Stroke: A Randomized Controlled Clinical Trial. Neurorehabil. Neural Repair. 2015, 29, 70–79. [Google Scholar] [CrossRef]
- Verheyden, G.; Kersten, P. Investigating the internal validity of the Trunk Impairment Scale (TIS) using Rasch analysis: The TIS 2.0. Disabil. Rehabil. 2010, 32, 2127–2137. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Urrútia, G.; Bagur-Calafat, C.; Caballero-Gómez, F.M.; Germán-Romero, A.; Girabent-Farrés, M. Validation of the Spanish version of the Trunk Impairment Scale Version 2.0 (TIS 2.0) to assess dynamic sitting balance and coordination in post-stroke adult patients. Top. Stroke Rehabil. 2016, 23, 225–232. [Google Scholar] [CrossRef]
- Health Quality Ontario, Ministry of Health and Long-Term Care. Quality-Based Procedures: Clinical Handbook for Stroke (Acute and Postacute) [Internet]; Queen’s Printer for Ontario: Toronto, ON, Canada, 2016. Available online: http://health.gov.on.ca/en/pro/programs/ecfa/docs/qbp_stroke.pdf (accessed on 10 March 2022).
- Salgueiro, C.; Urrùtia, G.; Cabanas-Valdés, R. Available apps for stroke telerehabilitation during corona virus disease 2019 confinement in Spain. Disabil. Rehabil. Assist. Technol. 2021, 12. [Google Scholar] [CrossRef]
- Requena, M.; Montiel, E.; Baladas, M.; Muchada, M.; Boned, S.; López, R.; Rodríguez-Villatoro, N.; Juega, J.; García-Tornel, Á.; Rodríguez-Luna, D.; et al. Farmalarm. Stroke 2019, 50, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.L.; Radtka, S.; Melnick, M.E.; Abrams, G.M.; Byl, N.N. Development and validation of the Function In Sitting Test in adults with acute stroke. J. Neurol. Phys. Ther. 2010, 34, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.L.; Rivera, M.; McCarthy, L. Reliability of the Function in Sitting Test (FIST). Rehabil Res. Pract. 2014, 2014, 593280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Caballero-Gómez, F.M.; Cervera-Cuenca, C.; Moya-Valdés, R.; Rodríguez-Rubio, P.R.; Urrútia, G. Validation and reliability of the Spanish version of the Function in Sitting test (S-FIST) to assess sitting balance in subacute post-stroke adult patients. Top. Stroke Rehabil. 2017, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Benaim, C.; Pérennou, D.A.; Villy, J.; Rousseaux, M.; Pelissier, J.Y. Validation of a standardized assessment of postural control in stroke patients: The Postural Assessment Scale for Stroke Patients (PASS). Stroke 1999, 30, 1862–1868. [Google Scholar] [CrossRef]
- Cabanas Valdés, R.M.; Girabent Farrés, M.; Cánovas Vergé, D.; Caballero Gómez, F.M.; Germán Romero, A.; Bagur Calafat, C. Traducción y validación al español de la Postural Assessment Scale for Stroke Patients (PASS) para la valoración del equilibrio y del control postural en pacientes postictus. Rev. Neurol. 2015, 60, 151. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Al-Eisa, E.S.; Anwer, S.; Sarkar, B. Reliability, validity, and responsiveness of three scales for measuring balance in patients with chronic stroke. BMC Neurol. 2018, 18, 141. [Google Scholar] [CrossRef]
- BTS Bioengineering. G-WALK: Sistema Inercial Inalámbrico Para la Evaluación Funcional de Movimiento. Available online: https://www.btsbioengineering.com/es/products/g-walk-inertial-motion-system/ (accessed on 16 January 2022).
- Zhang, W.; Smuck, M.; Legault, C.; Ith, M.A.; Muaremi, A.; Aminian, K. Gait Symmetry Assessment with a Low Back 3D Accelerometer in Post-Stroke Patients. Sensors 2018, 18, 3322. [Google Scholar] [CrossRef]
- Terui, Y.; Suto, E.; Konno, Y.; Kubota, K.; Iwakura, M.; Satou, M.; Nitta, S.; Hasegawa, K.; Satake, M.; Shioya, T. Evaluation of gait symmetry using a tri-axial accelerometer in stroke patients. NeuroRehabilitation 2018, 42, 173–180. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 10 March 2022).
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef]
- Cabrera-Martos, I.; Ortiz-Rubio, A.; Torres-Sánchez, I.; López-López, L.; Jarrar, M.; Valenza, M.C. The Effectiveness of Core Exercising for Postural Control in Patients with Stroke: A Systematic Review and Meta-Analysis. PM R 2020, 12, 1157–1168. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Ammendolia, A. Why is telerehabilitation necessary? A pre-post COVID-19 comparative study of ICF activity and participation. J. Enabling Technol. 2021, 15, 117–121. [Google Scholar] [CrossRef]
- Salgueiro, C.; Urrútia, G.; Cabanas-Valdés, R. Telerehabilitation for balance rehabilitation in the subacute stage of stroke: A pilot controlled trial. NeuroRehabilitation 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Howes, S.; Stephenson, A.; Murphy, P.; Deutsch, J.; Stokes, M.; Pedlow, K.; McDonough, S. Factors influencing the delivery of telerehabilitation for stroke: A systematic review. Physiotherapy 2021, 114, e71–e244. [Google Scholar] [CrossRef]
- Knepley, K.D.; Mao, J.Z.; Wieczorek, P.; Okoye, F.O.; Jain, A.P.; Harel, N.Y. Impact of Telerehabilitation for Stroke-Related Deficits. Telemed. e-Health 2020, 27, 239–246. [Google Scholar] [CrossRef]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Saywell, N.L.; Vandal, A.C.; Mudge, S.; Hale, L.; Brown, P.; Feigin, V.; Hanger, C.; Taylor, D. Telerehabilitation After Stroke Using Readily Available Technology: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2021, 35, 88–97. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Zafonte, R.; Hefner, J.; Iaccarino, M.A.; Silver, J.; Paganoni, S. Evidence-Based Physiatry: Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke. Am. J. Phys. Med. Rehabil. 2020, 99, 764–765. [Google Scholar] [CrossRef]
- National Library of Medicine. “Telerehabilitation and Stroke” Research Results. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=telerehabilitation+stroke&filter=years.2018-2021&timeline=expanded (accessed on 16 January 2022).
- Laver, K.E.; Adey-Wakeling, Z.; Crotty, M.; Lannin, N.A.; George, S.; Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020, 1, CD010255. [Google Scholar] [CrossRef]
| Variables | Pre-Intervention | |
|---|---|---|
| Control Group (n = 15) | Experimental Group (n = 15) | |
| Characteristics | ||
| Age (years), mean (SD) | 64.53 (9.40) | 57.27 (14.35) |
| Weight (Kilograms), mean (SD) | 75.83 (12.76) | 80.83 (11.05) |
| Height (centimeters), mean (SD) | 164.40 (10.18) | 169.13 (9.49) |
| Body Mass Index, mean (SD) | 27.73 (3.54) | 26.15 (3.61) |
| Sex (male/female) | 10/5 | 10/5 |
| Stroke risk factors (yes/no) | 8/7 | 4/11 |
| Predominant risk factor | High Blood Pressure | High Blood Pressure |
| Active lifestyle before stroke (yes/no) | 7/8 | 8/7 |
| Stroke (hemorrhagic/ischaemic) | 5/10 | 5/10 |
| Predominant stroke localization | Middle Cerebral Artery | Middle Cerebral Artery |
| Hemiplegia (right/left) | 7/8 | 10/5 |
| Time post-stroke (years), mean (SD) | 4.06 (4.43) | 4.61 (3.38) |
| Smartphone user (yes/no) | 14/1 | 15/0 |
| Coventional Physiotherapy | ||
| Times/week (frequency) | ||
| >4 | 1 | 0 |
| 3–4 | 0 | 2 |
| 1–2 | 13 | 12 |
| no therapy | 1 | 1 |
| Scales | ||
| Sitting balance, mean (SD) | ||
| S-TIS 2.0 (balance) | 4.27 (1.62) | 4.73 (2.12) |
| S-TIS 2.0 (coordination) | 3.07 (1.33) | 2.87 (1.13) |
| S-TIS 2.0 (total) | 7.33 (2.38) | 7.60 (2.77) |
| S-FIST (total) | 56.86 (3.40) | 52.6 (9.58) |
| Standing balance, mean (SD) | ||
| S-PASS (mobility) | 18.67 (2.85) | 18.93 (2.89) |
| S-PASS (balance) | 10.47 (2.61) | 9.46 (2.23) |
| S-PASS (total) | 29.13 (5.00) | 28.4 (4.90) |
| BBS | 41.27 (15.42) | 43.2 (12.73) |
| Falls | 0 | 0 |
| Gait—G-Walk, mean (SD) | ||
| Stance phase % (affected limb) | 5.18 (4.36) | 6.13 (4.70) |
| Stance phase % (less affected limb) | 5.38 (5) | 5.13 (3.72) |
| Swing phase % (affected limb) | 8.11 (8.03) | 10.05 (9.10) |
| Swing phase % (less affected limb) | 7.13 (6.71) | 7.54 (8.20) |
| Double Support % (affected limb) | 4.56 (5.28) | 4.51 (5.84) |
| Double Support % (less affected limb) | 4.50 (4.89) | 1.57 (1.33) |
| Single Support % (affected limb) | 5.68 (5.18) | 6.20 (4.39) |
| Single Support % (less affected limb) | 5.86 (4.75) | 4.85 (4.18) |
| Cadence (steps/minute) | 30.47 (20.01) | 22.49 (23.07) |
| Speed (meters/seconds) | 0.39 (0.38) | 0.36 (0.35) |
| Stride (meters), (affected limb) | 0.33 (0.28) | 0.35 (0.40) |
| Stride (meters), (less affected limb) | 0.34 (0.28) | 0.36 (0.40) |
| Sitting Balance | Control Group | Experimental Group | |||||
|---|---|---|---|---|---|---|---|
| Middle Intervention | Post-Intervention | ΔT0–T2 | Middle Intervention | Post-Intervention | ΔT0–T2 | p-Value (Intergroup *) | |
| S-TIS 2.0 (balance) | 4.15 (2.54) | 4.31 (1.84) | 0.23 (1.30) | 6.15 (2.12) | 6.71 (2.33) | 1.86 (1.56) | 0.007 |
| p-value (intragroup *) | 0.534 | 0.001 | |||||
| S-TIS 2.0 (coordination) | 3.00 (1.91) | 3.15 (1.91) | 0.08 (1.44) | 3.15 (1.72) | 3.64 (1.15) | 0.71 (0.91) | 0.424 |
| p-value (intragroup *) | 0.851 | 0.012 | |||||
| S-TIS 2.0 (total) | 7.15 (4.20) | 7.46 (3.57) | 0.31 (2.10) | 9.31 (3.5) | 10.36 (3.03) | 2.57 (1.87) | 0.032 |
| p-value (intragroup *) | 0.606 | 0.000 | |||||
| S-FIST (total) | 54.15 (3.87) | 53.54 (6.84) | −1.15 (4.36) | 52.69 (10.26) | 54.71 (3.47) | 2.36 (6.66) | 0.574 |
| p-value (intragroup *) | 0.358 | 0.208 | |||||
| Standing Balance | Control Group | Experimental Group | |||||
|---|---|---|---|---|---|---|---|
| Middle Intervention | Post-Intervention | ΔT0–T2 | Middle Intervention | Post-intervention | ΔT0–T2 | p-Value (Intergroup *) | |
| S-PASS (mobility) | 18.85 (3.31) | 18.69 (3.50) | 0.15 (1.52) | 19.54 (3.13) | 20.43 (3.48) | 1.43 (2.28) | 0.208 |
| p-value (intragroup *) | 0.721 | 0.035 | |||||
| S-PASS (balance) | 10.46 (2.70) | 10.46 (3.01) | −0.08 (1.04) | 9.69 (2.39) | 9.79 (2.52) | 0.29 (0.91) | 0.532 |
| p-value (intragroup *) | 0.794 | 0.263 | |||||
| S-PASS (total) | 29.31 (5.54) | 29.15 (6.04) | 0.08 (1.93) | 28.54 (5.43) | 30.21 (5.35) | 1.71 (2.43) | 0.633 |
| p-value (intragroup *) | 0.888 | 0.02 | |||||
| BBS | 40.69 (15.69) | 42.54 (14.49) | 2.46 (2.85) | 44 (13.22) | 44.93 (12.29) | 1.93 (2.95) | 0.647 |
| p-value (intragroup *) | 0.009 | 0.029 | |||||
| Falls | 0.47 (0.88) | 0.15 (0.38) | 0.15 | 0.15 (0.38) | 0.14 (0.36) | 0.14 | 0.999 |
| p-value (intragroup *) | 0.165 | 0.165 | |||||
| Gait | Control Group | Experimental Group | |||||
|---|---|---|---|---|---|---|---|
| Middle Intervention | Post-Intervention | ΔT0–T2 | Middle Intervention | Post-Intervention | ΔT0–T2 | p-Value (Intergroup *) | |
| Phase | |||||||
| Stance phase % ** (affected limb) | 4.53 (2.99) | 4.01 (2.21) | 1.74 (5.14) | 5.25 (3.25) | 6.43 (4.21) | 0.16 (6.13) | 0.568 |
| p-value (intragroup *) | 0.541 | 0.833 | |||||
| Stance phase % ** (less affected limb) | 4.07 (3.03) | 4.95 (2.99) | 1.14 (5.78) | 5.19 (4.97) | 6.53 (4.63) | −0.93 (5.23) | 0.395 |
| p-value (intragroup *) | 0.334 | 0.334 | |||||
| Swing phase % ** (affected limb) | 5.00 (5.22) | 6.13 (4.60) | 1.98 (8.36) | 5.87 (6.54) | 8.33 (8.19) | 1.71 (9.85) | 0.909 |
| p-value (intragroup *) | 0.800 | 0.648 | |||||
| Swing phase % ** (less affected limb) | 3.71 (3.99) | 4.99 (4.04) | 2.14 (7.81) | 5.35 (6.45) | 7.02 (6.30) | 0.52 (7.76) | 0.923 |
| p-value (intragroup *) | 0.881 | 0.848 | |||||
| Support | |||||||
| Double Support % ** (affected limb) | 2.65 (3.92) | 2.77 (3.47) | 1.79 (4.65) | 0.82 (0.97) | 1.27 (1.76) | 0.30 (2.51) | 0.299 |
| p-value (intragroup *) | 0.512 | 0.096 | |||||
| Double Support % ** (less affected limb) | 3.82 (9.52) | 2.68 (3.36) | 1.82 (6.38) | 2.49 (3.17) | 4.56 (3.95) | −0.05 (7.06) | 0.034 |
| p-value (intragroup *) | 0.370 | 0.022 | |||||
| Single Support % ** (affected limb) | 5.16 (6.86) | 5.3 (2.79) | 0.56 (5.98) | 4.23 (3.46) | 6.03 (4.98) | −1.18 (6.53) | 0.921 |
| p-value (intragroup) | 0.498 | 0.405 | |||||
| Single Support %** (less affected limb) | 5.16 (6.86) | 5.30 (2.79) | 0.56 (5.98) | 4.23 (3.46) | 6.03 (4.98) | −1,18 (6.53) | 0.840 |
| p-value (intragroup *) | 0.117 | 0.428 | |||||
| Others | |||||||
| Cadence (steps/minute **) | 30.88 (21.93) | 26.88 (24.97) | 7.44 (16.74) | 32.9 (22.19) | 29.49 (21.68) | −4.89 (18.57) | 0.292 |
| p-value (intragroup *) | 0.606 | 0.929 | |||||
| Speed (meters/seconds) | 0.86 (0.44) | 0.94 (0.35) | 0.01 (0.05) | 0.95 (0.34) | 0.94 (0.36) | −0.08 (0.45) | 0.951 |
| p-value (intragroup *) | 0.940 | 0.929 | |||||
| Stride (meters), (affected limb) | 1.20 (0.50) | 1.26 (0.31) | 0.01 (0.15) | 1.28 (0.70) | 1.30 (0.55) | −0.06 (0.52) | 0.984 |
| p-value (intragroup *) | 0.55 | 0.606 | |||||
| Stride (meters), (less affected limb) | 1.21 (0.50) | 1.26 (0.32) | −0.02 (0.14) | 1.30 (0.71) | 1.30 (0.56) | −0.06 (0.52) | 0.987 |
| p-value (intragroup *) | 0.547 | 0.615 | |||||
| Adherence | Exercises Performed | |||
|---|---|---|---|---|
| (%) | Exercises/Day | (%) | ||
| Mean (SD) | 8.2 (8.1) | 13.66 | 11.84 (9.41) | 37.09 |
| Min-max | 0–23 | 0–32 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgueiro, C.; Urrútia, G.; Cabanas-Valdés, R. Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 5689. https://doi.org/10.3390/ijerph19095689
Salgueiro C, Urrútia G, Cabanas-Valdés R. Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(9):5689. https://doi.org/10.3390/ijerph19095689
Chicago/Turabian StyleSalgueiro, Carina, Gerard Urrútia, and Rosa Cabanas-Valdés. 2022. "Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 9: 5689. https://doi.org/10.3390/ijerph19095689
APA StyleSalgueiro, C., Urrútia, G., & Cabanas-Valdés, R. (2022). Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(9), 5689. https://doi.org/10.3390/ijerph19095689






