Effect of Walking Steps Measured by a Wearable Activity Tracker on Improving Components of Metabolic Syndrome: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
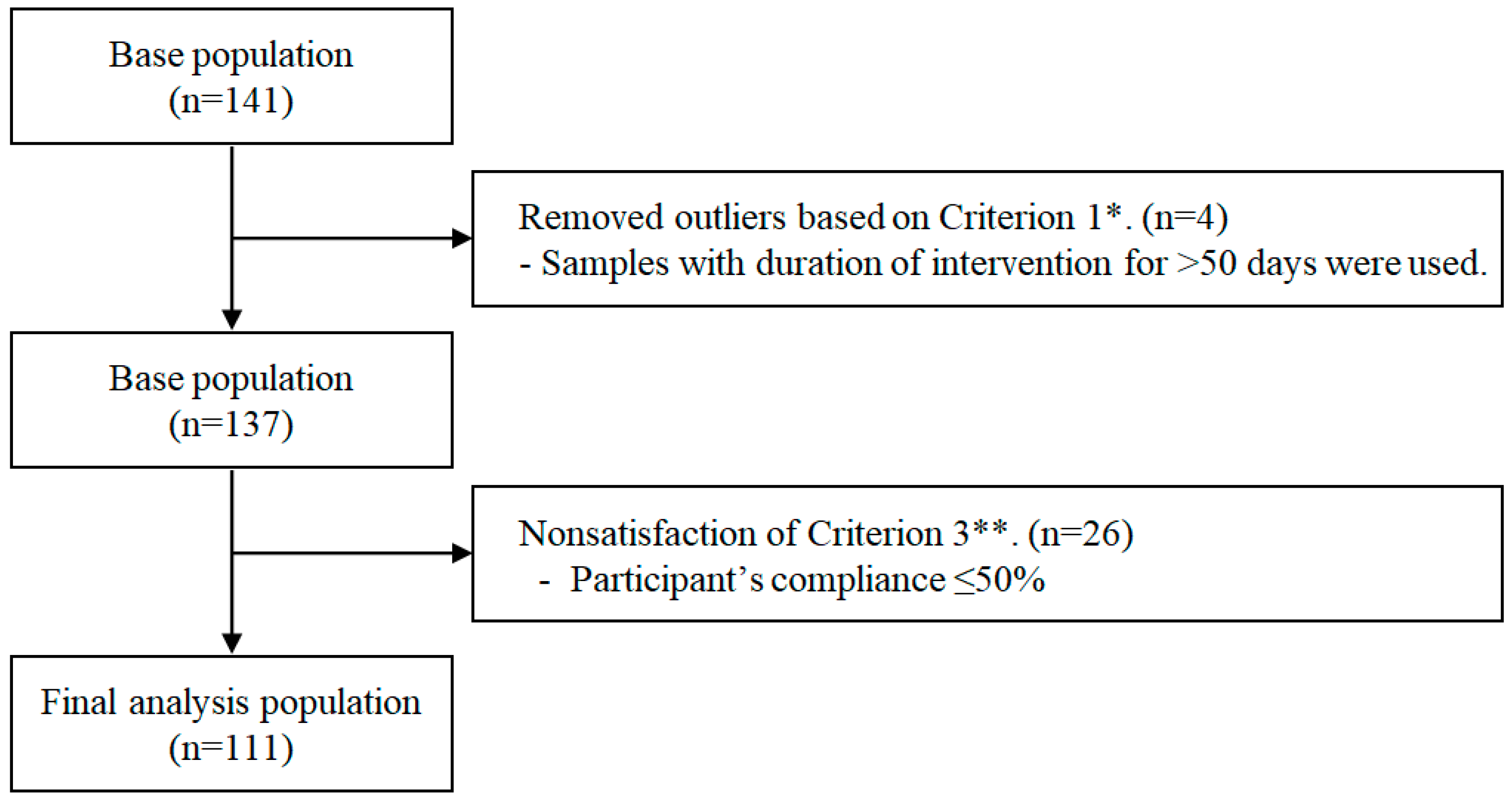
2.1. Study Design and Participants
2.2. Data Preprocessing
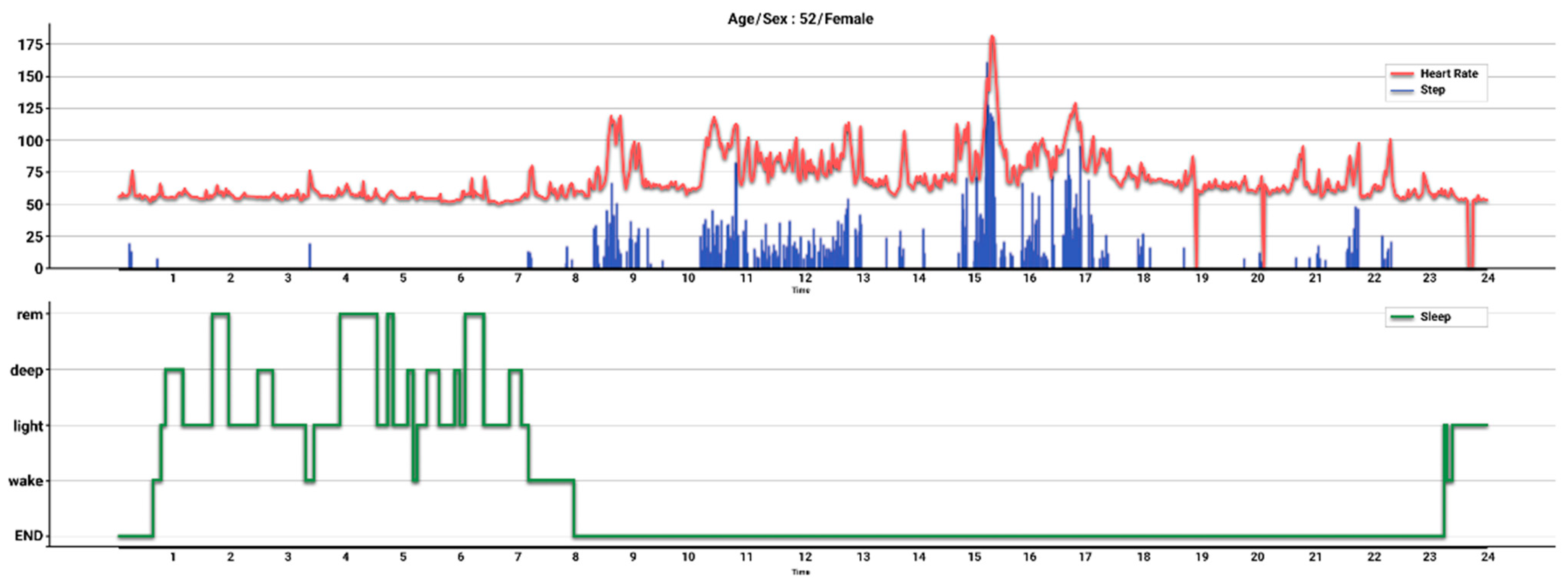
2.3. Measurement of Daily Step Count
2.4. Measurement of Anthropometric and Laboratory Data
2.5. Definition of Terms
2.6. Statistical Analysis
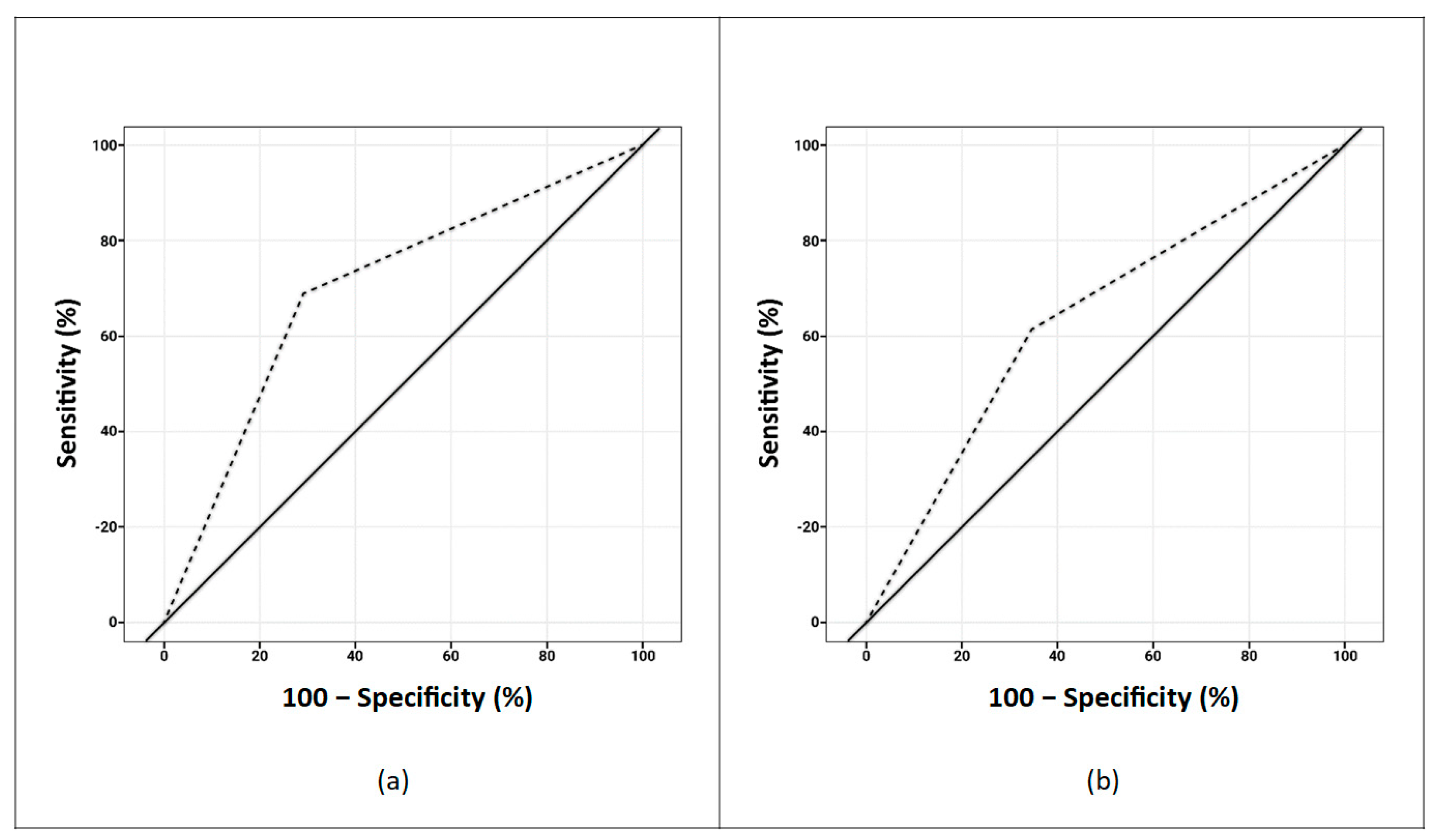
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H. Metabolic syndrome and COVID-19. Cardiol. Res. 2020, 11, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Boleti, A.P.d.A.; Almeida, J.A.; Migliolo, L. Impact of the metabolic syndrome on the evolution of neurodegenerative diseases. Neural Regen. Res. 2021, 16, 688–689. [Google Scholar]
- Li, W.; Song, F.; Wang, X.; Wang, D.; Chen, D.; Yue, W.; Zhang, H.; Wang, Z.; Lu, Z. Relationship between metabolic syndrome and its components and cardiovascular disease in middle-aged and elderly Chinese population: A national cross-sectional survey. BMJ Open 2019, 9, e027545. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Wang, Z.; Shen, K.; Chen, X. Metabolic syndrome and breast cancer: Prevalence, treatment response, and prognosis. Front. Oncol. 2021, 11, 629666. [Google Scholar] [CrossRef]
- Jimba, T.; Kaneko, H.; Yano, Y.; Itoh, H.; Yotsumoto, H.; Seki, H.; Morita, K.; Kiriyama, H.; Kamon, T.; Fujiu, K.; et al. Relation of the metabolic syndrome to incident colorectal cancer in young adults aged 20 to 49 years. Am. J. Cardiol. 2021, 158, 132–138. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Zhang, X.F.; Ouyang, Y.F.; Zhang, B.; Wang, Z.H.; Du, S.F.; Wang, H.J. Associations of sedentary time and physical activity with metabolic syndrome among chinese adults: Results from the china health and nutrition survey. Biomed. Environ. Sci. 2021, 34, 963–975. [Google Scholar]
- Chen, Y.; Yang, J.; Su, J.; Qin, Y.; Shen, C.; Li, Y.; Lu, S.; Pan, E.; Gao, Y.; Miao, D.; et al. Physical activity, sedentary time and their associations with clustered metabolic risk among people with type 2 diabetes in jiangsu province: A cross-sectional study. BMJ Open 2019, 9, e027906. [Google Scholar] [CrossRef]
- Thompson, D.L.; Rakow, J.; Perdue, S.M. Relationship between accumulated walking and body composition in middle-aged women. Med. Sci. Sports Exerc. 2004, 36, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.W.; Borup, P.; Tucker, L.; LeCheminant, J.; Allen, M.; Hebbert, W. Steps measured by pedometry and the relationship to adiposity in college women. J. Phys. Act. Health 2014, 11, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Krumm, E.M.; Dessieux, O.L.; Andrews, P.; Thompson, D.L. The relationship between daily steps and body composition in postmenopausal women. J. Womens Health 2006, 15, 202–210. [Google Scholar] [CrossRef]
- Sisson, S.B.; Camhi, S.M.; Church, T.S.; Tudor-Locke, C.; Johnson, W.D.; Katzmarzyk, P.T. Accelerometer-determined steps/day and metabolic syndrome. Am. J. Prev. Med. 2010, 38, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Duarte-Mendes, P.; Rusenhack, M.C.; Furmann, M.; Nobre, P.R.; Fachada, M.; Soares, C.M.; Teixeira, A.; Ferreira, J.P. Objectively measured sedentary behavior and physical fitness in adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 8860. [Google Scholar] [CrossRef]
- Amirfaiz, S.; Shahril, M.R. Objectively measured physical activity, sedentary behavior, and metabolic syndrome in adults: Systematic review of observational evidence. Metab. Syndr. Relat. Disord. 2019, 17, 1–21. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide survey of fitness trends for 2022. ACSM’s Health Fit. J. 2022, 26, 11–20. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide survey of fitness trends for 2020. ACSM’s Health Fit. J. 2019, 23, 10–18. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide survey of fitness trends for 2019. ACSM’s Health Fit. J. 2018, 22, 10–17. [Google Scholar] [CrossRef]
- Jo, A.; Coronel, B.D.; Coakes, C.E.; Mainous, A.G., 3rd. Is there a benefit to patients using wearable devices such as fitbit or health apps on mobiles? A systematic review. Am. J. Med. 2019, 132, 1394–1400.e1391. [Google Scholar]
- Diaz, K.M.; Krupka, D.J.; Chang, M.J.; Peacock, J.; Ma, Y.; Goldsmith, J.; Schwartz, J.E.; Davidson, K.W. Fitbit®: An accurate and reliable device for wireless physical activity tracking. Int. J. Cardiol. 2015, 185, 138–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How Does My Fitbit Device Calculate My Daily Activity? Available online: https://help.fitbit.com/articles/en_US/Help_article/1141.htm (accessed on 26 February 2022).
- Cole, C.L.; Vasalos, K.; Nicandri, G.; Apt, C.; Osterling, E.; Ferrara, Z.; Maloney, M.D.; Schwarz, E.M.; Rizzone, K. Use of Fitbit data to evaluate the effects of an athletic performance and injury prevention training program on daily physical levels in underrepresented minority female high school athletes: A prospective study. Orthop. Sports Med. 2020, 4, 370–376. [Google Scholar] [PubMed]
- de Zambotti, M.; Goldstone, A.; Claudatos, S.; Colrain, I.M.; Baker, F.C. A validation study of Fitbit Charge 2™ compared with polysomnography in adults. Chronobiol. Int. 2018, 35, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Herzig, K.H.; Ahola, R.; Leppäluoto, J.; Jokelainen, J.; Jämsä, T.; Keinänen-Kiukaanniemi, S. Light physical activity determined by a motion sensor decreases insulin resistance, improves lipid homeostasis and reduces visceral fat in high-risk subjects: PreDiabEx study RCT. Int. J. Obes. 2014, 38, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Bhopal, R.S.; Douglas, A.; Wallia, S.; Forbes, J.F.; Lean, M.E.; Gill, J.M.; McKnight, J.A.; Sattar, N.; Sheikh, A.; Wild, S.H.; et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: A family-cluster randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 218–227. [Google Scholar] [CrossRef]
- Singer, J.D. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J. Educ. Behav. Stat. 1998, 23, 323–355. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Yu, J.; Chen, R.; Gao, J.; Ding, R.; Fu, Y.; Zhang, L.; Hu, D. Socioeconomic status and metabolic syndrome in the general population of China: A cross-sectional study. BMC Public Health 2012, 12, 921. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kang, H.T.; Nam, C.M.; Park, B.J.; Linton, J.A.; Lee, Y.J. Sex differences in the relationship between socioeconomic status and metabolic syndrome: The Korean National Health and Nutrition Examination Survey. Diabetes Res. Clin. Pract. 2012, 96, 400–406. [Google Scholar] [CrossRef]
| Participants with Metabolic Syndrome (N = 43) | Participants without Metabolic Syndrome (N = 68) | p-Value | |
|---|---|---|---|
| Intervention period (days) | 59.6 ± 5.6 | 59.6 ± 5.7 | 0.982 |
| Total step counts per day | 11681 ± 3198 | 11651 ± 4227 | 0.968 |
| Compliance (%) | 85.8 ± 13.4 | 81.6 ± 15.3 | 0.140 |
| Variables | Participants with Metabolic Syndrome | p-Value | Participants without Metabolic Syndrome | p-Value | ||
|---|---|---|---|---|---|---|
| (N = 43) | (N = 68) | |||||
| Baseline | Post-Intervention | Baseline | Post-Intervention | |||
| Age, years | 59.4 ± 10.0 | 47.7 ± 14.1 | ||||
| Male sex | 8 (18.6) | 12 (17.7) | ||||
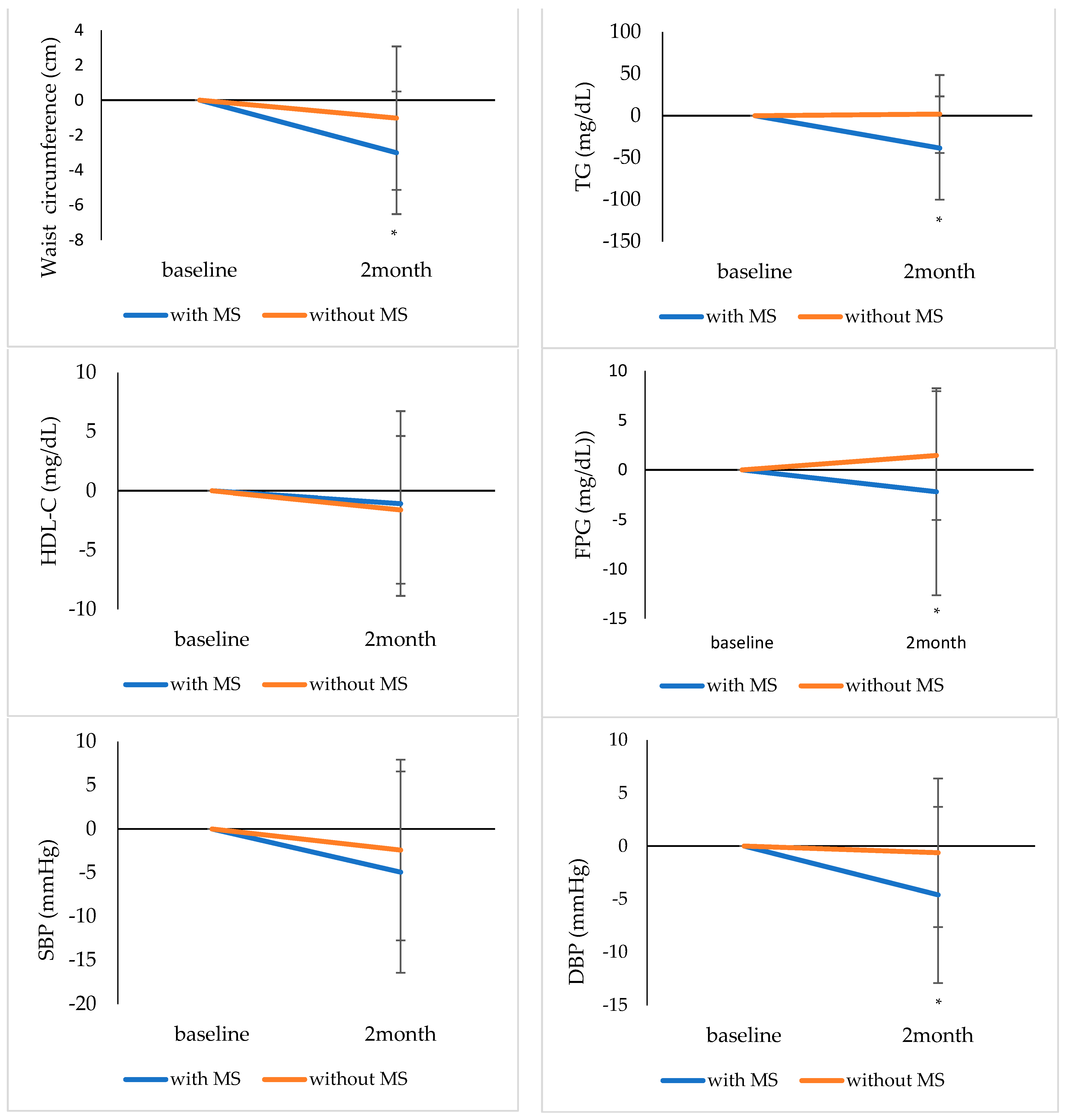
| BMI, kg/m2 | 26.9 ± 2.1 | 26.3 ± 2.1 | <0.001 | 23.8 ± 2.8 | 23.4 ± 2.5 | <0.001 |
| Waist circumference, cm | 89.4 ± 4.9 | 86.3 ± 6.5 | <0.001 | 80.4 ± 7.3 | 79.3 ± 6.7 | 0.043 |
| TG, mg/dL | 147.0 (106.0–185.0) | 116.0 (84.0–147.0) | <0.001 | 82.5 (64.5–120.5) | 81.5 (59.0–119.5) | 0.807 |
| HDL-C, mg/dL | 54.4 ± 11.2 | 53.4 ± 9.4 | 0.376 | 63.2 ± 11.6 | 61.6 ± 10.2 | 0.038 |
| FPG, mg/dL | 107.4 ± 14.7 | 105.2 ± 12.6 | 0.175 | 94.7 ± 6.9 | 96.1 ± 8.1 | 0.070 |
| SBP, mmHg | 129.7 ± 10.2 | 124.8 ± 9.4 | 0.008 | 115.4 ± 10.8 | 113.1 ± 11.2 | 0.062 |
| DBP, mmHg | 84.4 ± 9.0 | 79.8 ± 8.0 | 0.001 | 72.3 ± 8.3 | 71.5 ± 8.3 | 0.469 |
| Medication for hypertension | 21 (48.8) | 2 (2.9) | ||||
| Medication for diabetes | 6 (14.0) | 2 (2.9) | ||||
| Smoking | 7 (16.3) | 2 (2.9) | ||||
| Alcohol consumption | 10 (23.3) | 20 (29.4) | ||||
| Analysis | Participants with Metabolic Syndrome, OR (95% CI) | Participants without Metabolic Syndrome, OR (95% CI) |
|---|---|---|
| Unadjusted (per 1000-steps/day) | 1.30 (1.04–1.61) | 1.13 (0.93–1.37) |
| Multivariable-adjusted * (per 1000-steps/day) | 1.24 (1.01–1.53) | 1.14 (0.93–1.40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Choi, J.-E.; Lee, H.S.; Jeon, S.; Lee, J.-W.; Hong, K.-W. Effect of Walking Steps Measured by a Wearable Activity Tracker on Improving Components of Metabolic Syndrome: A Prospective Study. Int. J. Environ. Res. Public Health 2022, 19, 5433. https://doi.org/10.3390/ijerph19095433
Park J-M, Choi J-E, Lee HS, Jeon S, Lee J-W, Hong K-W. Effect of Walking Steps Measured by a Wearable Activity Tracker on Improving Components of Metabolic Syndrome: A Prospective Study. International Journal of Environmental Research and Public Health. 2022; 19(9):5433. https://doi.org/10.3390/ijerph19095433
Chicago/Turabian StylePark, Jae-Min, Ja-Eun Choi, Hye Sun Lee, Soyoung Jeon, Ji-Won Lee, and Kyung-Won Hong. 2022. "Effect of Walking Steps Measured by a Wearable Activity Tracker on Improving Components of Metabolic Syndrome: A Prospective Study" International Journal of Environmental Research and Public Health 19, no. 9: 5433. https://doi.org/10.3390/ijerph19095433
APA StylePark, J.-M., Choi, J.-E., Lee, H. S., Jeon, S., Lee, J.-W., & Hong, K.-W. (2022). Effect of Walking Steps Measured by a Wearable Activity Tracker on Improving Components of Metabolic Syndrome: A Prospective Study. International Journal of Environmental Research and Public Health, 19(9), 5433. https://doi.org/10.3390/ijerph19095433






