Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins
Abstract
1. Introduction
2. Biomass
3. Bio-Based Compounds in the Flame Retardant Industry
3.1. Saccharide-Based Products
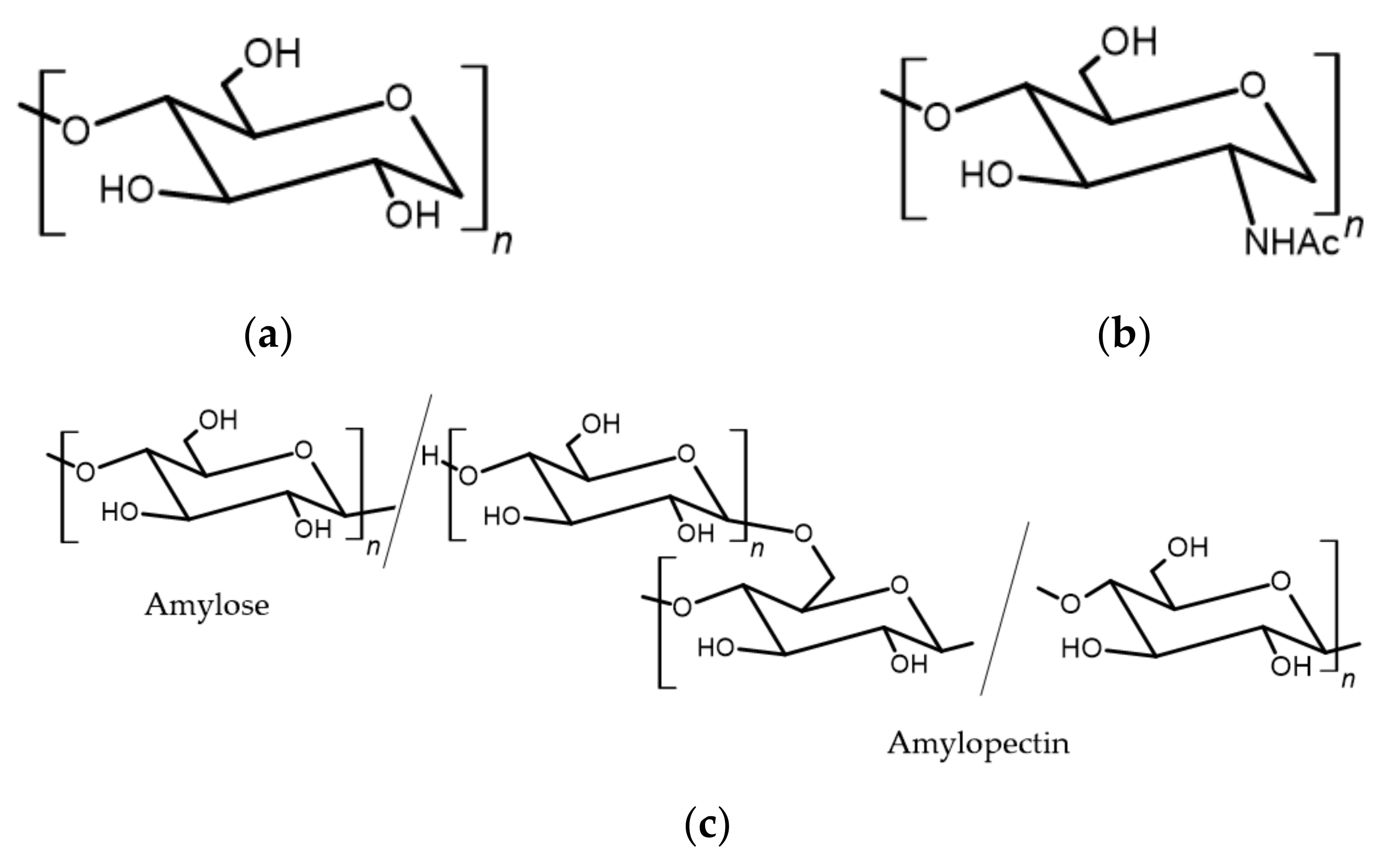
3.1.1. Cellulose
3.1.2. Starch and Its Derivatives
3.1.3. Chitosan
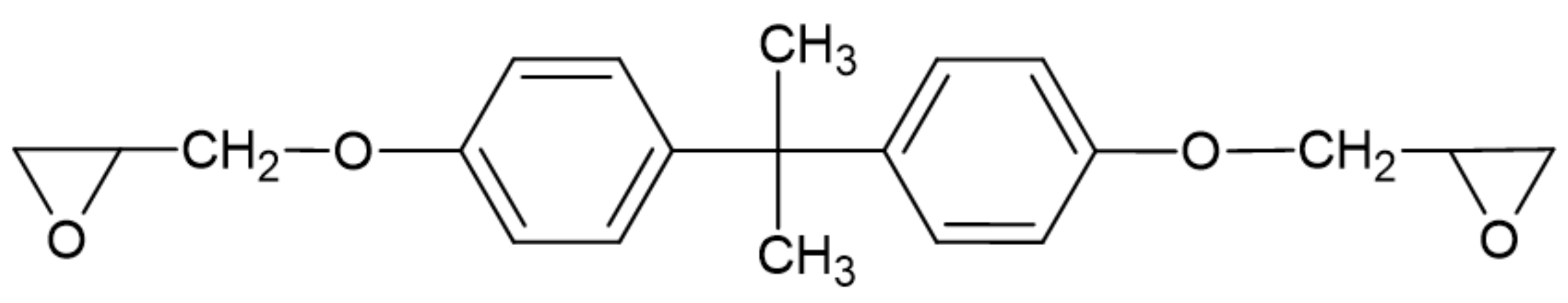
3.2. Bio-Based Aromatic Compounds
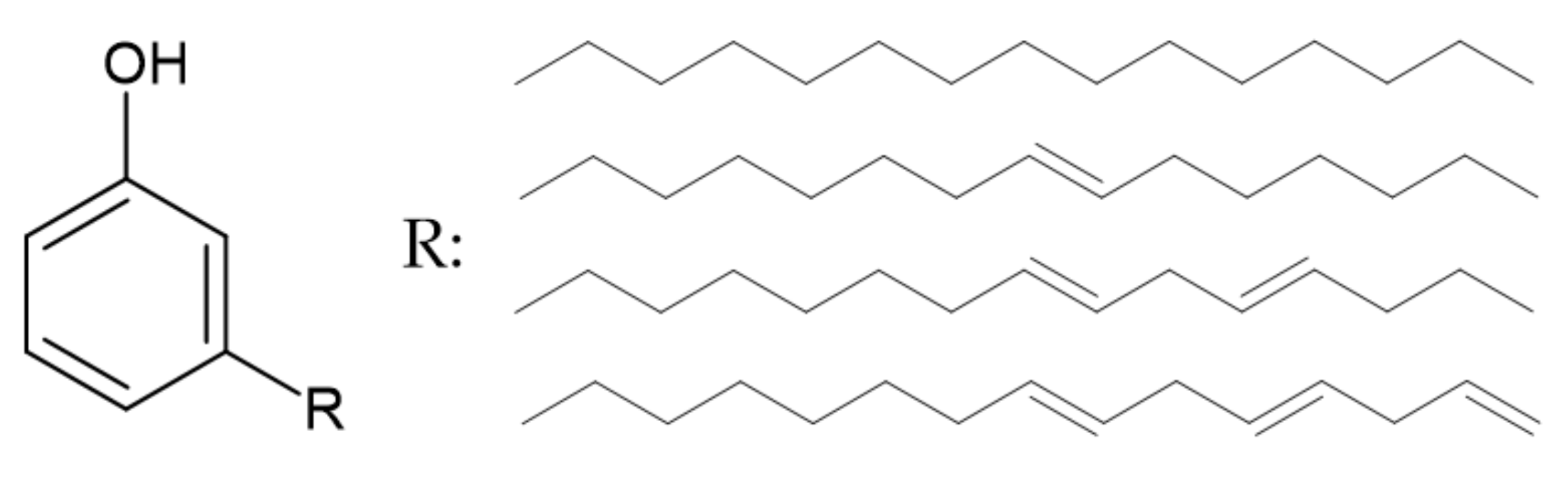
3.2.1. Lignin
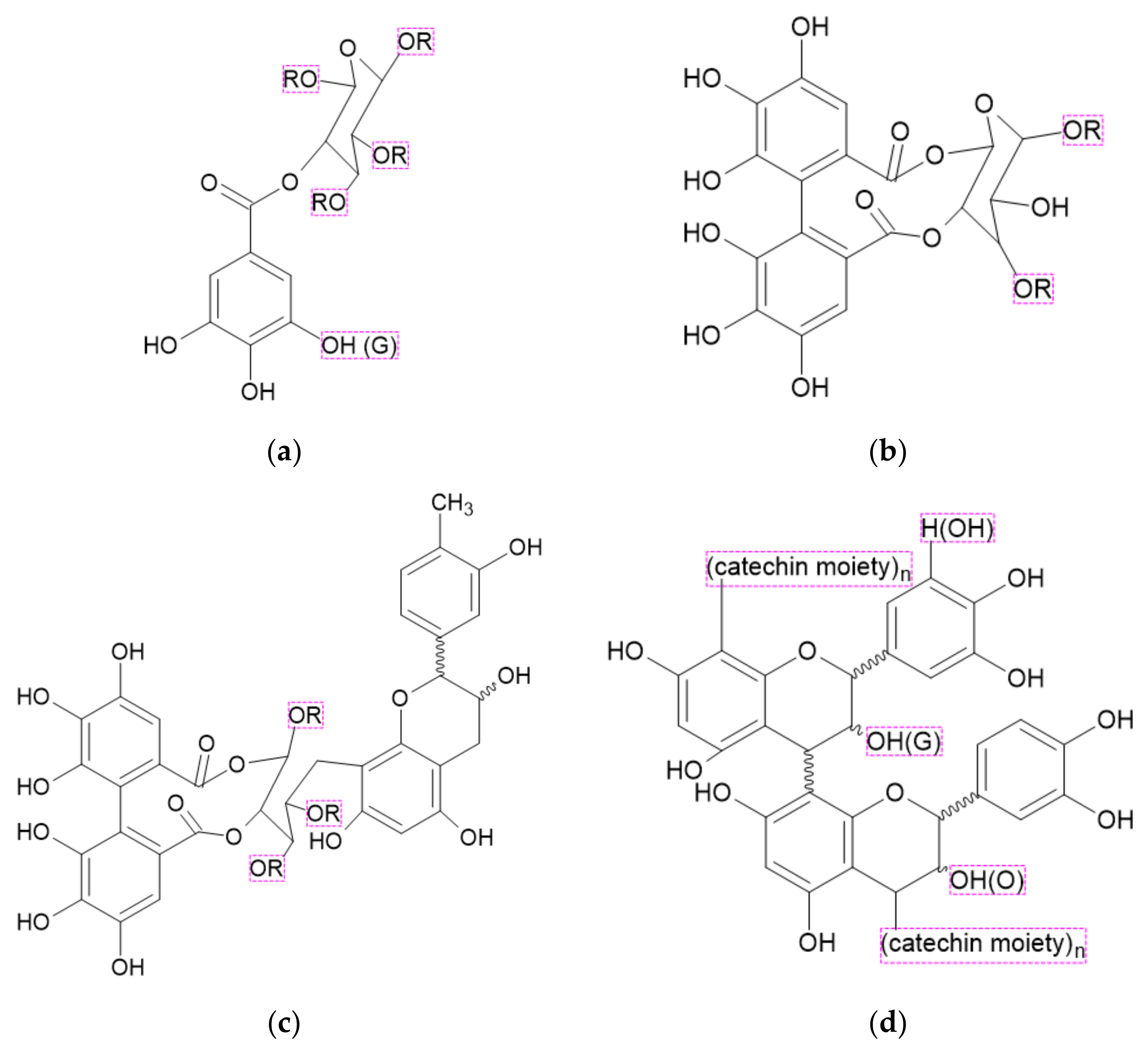
3.2.2. Tannins
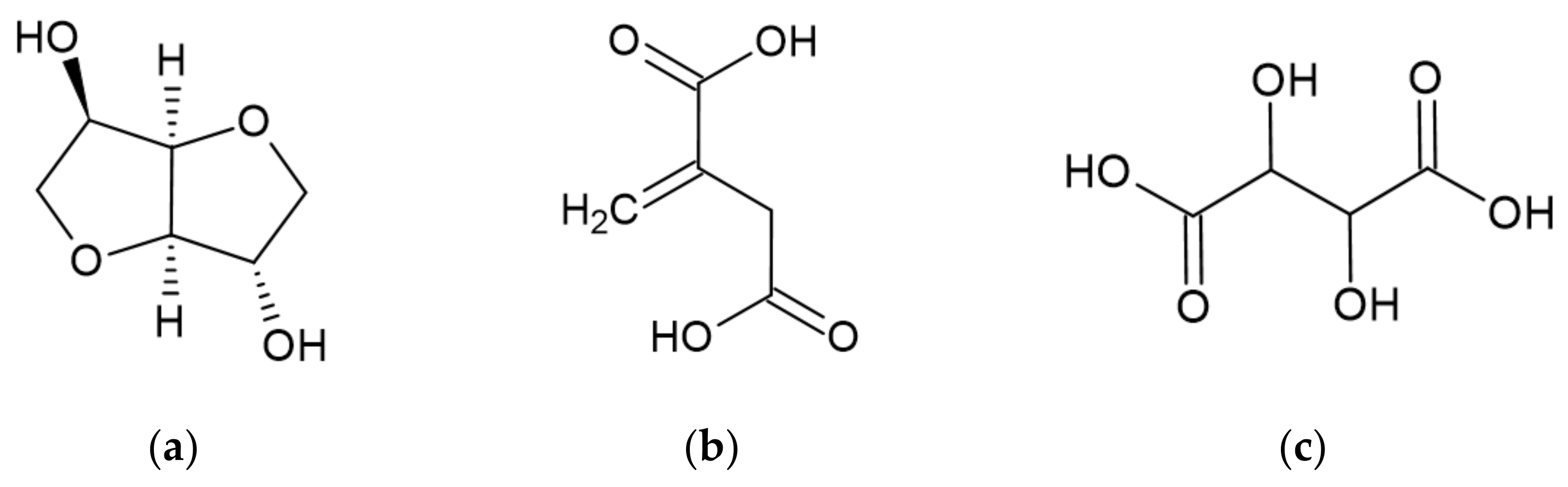
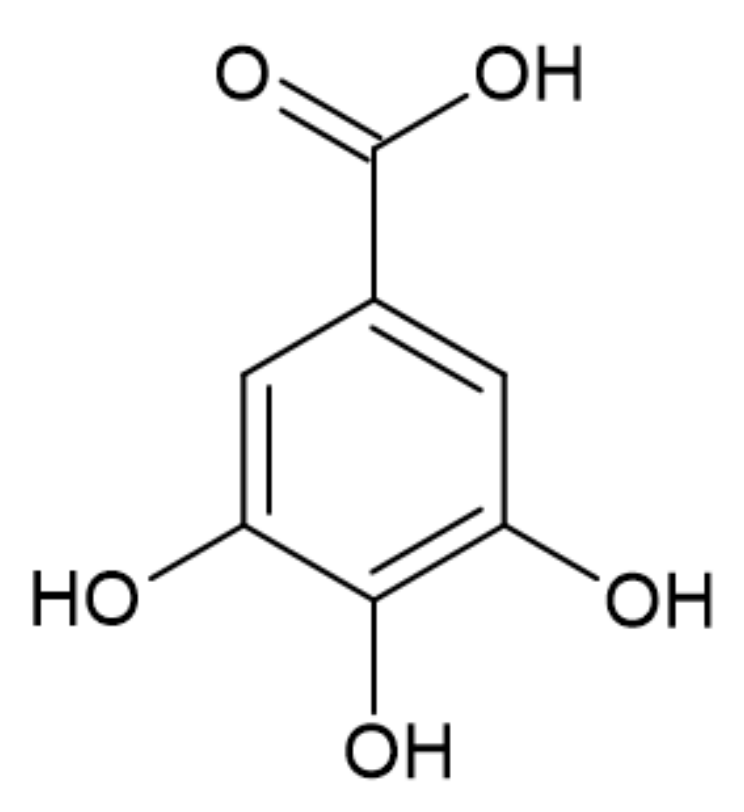
3.2.3. Gallic Acid
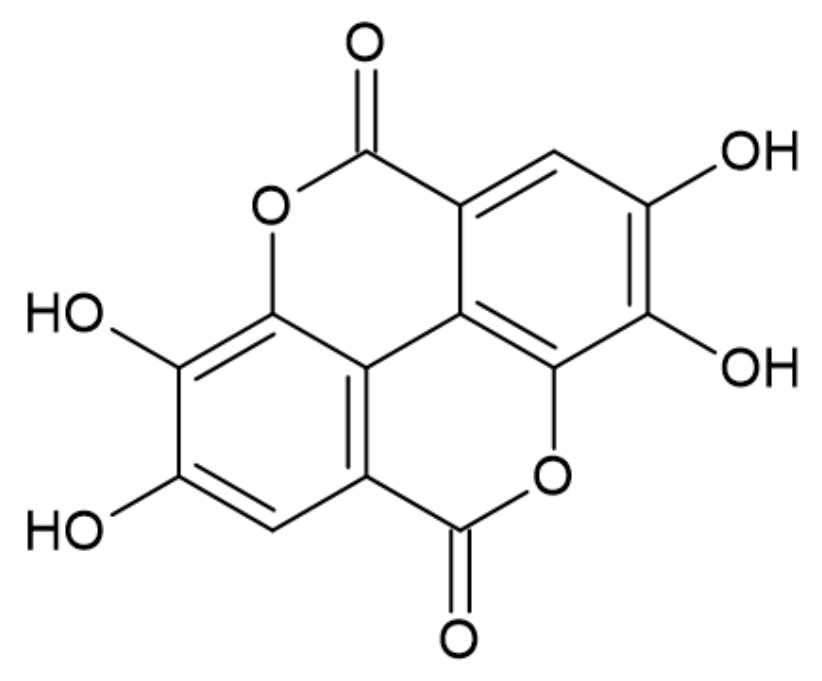
3.2.4. Ellagic Acid
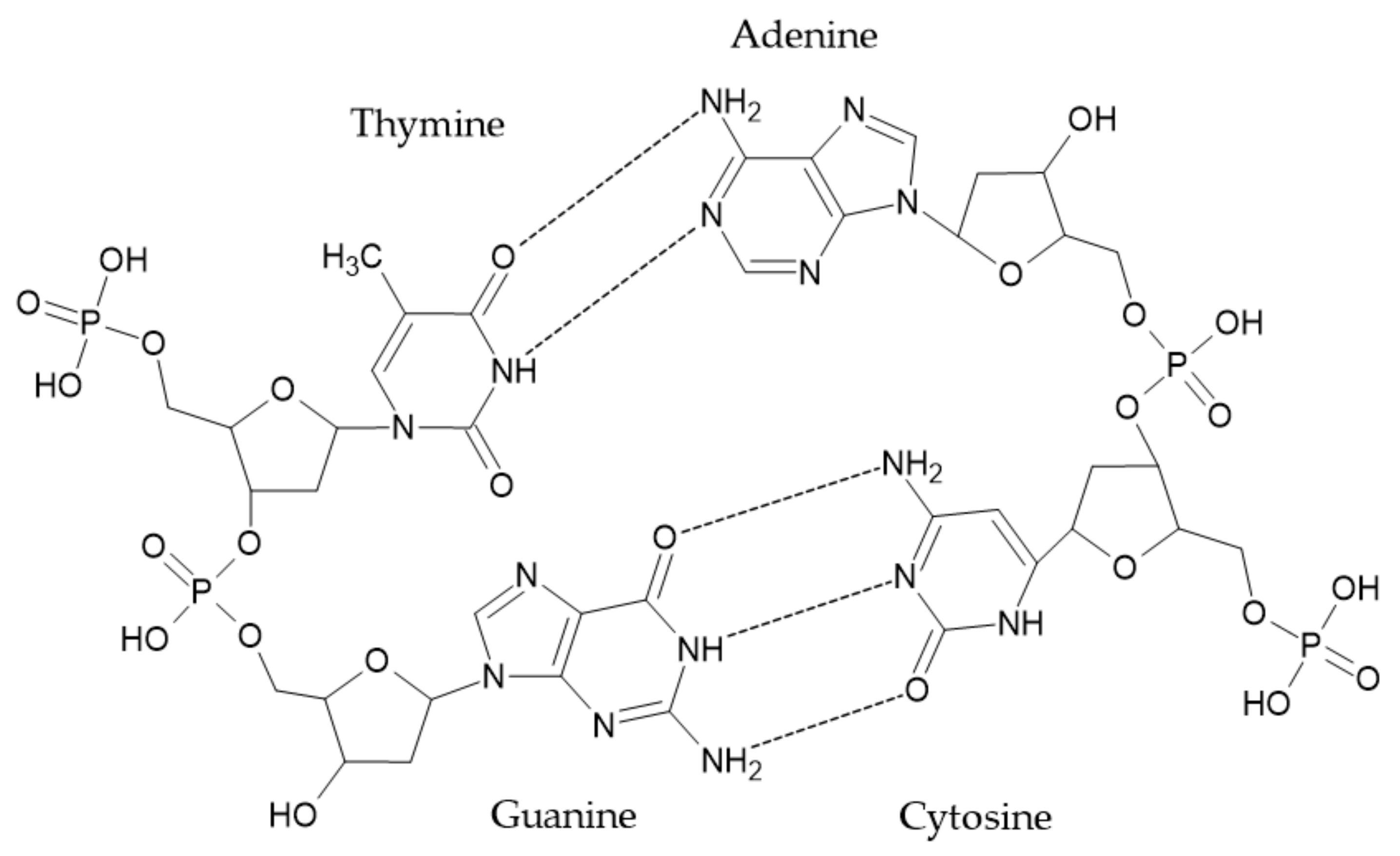
3.3. DNA
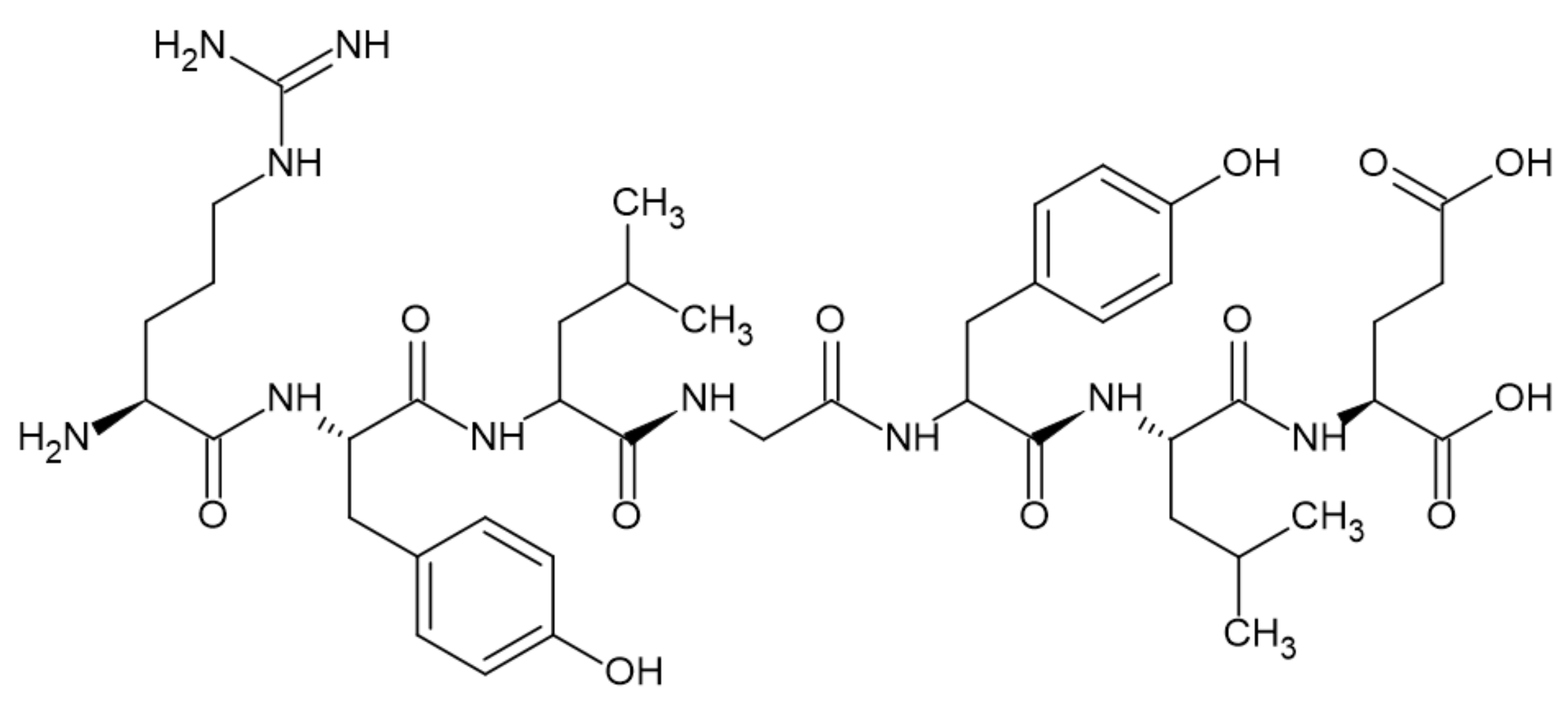
3.4. Proteins
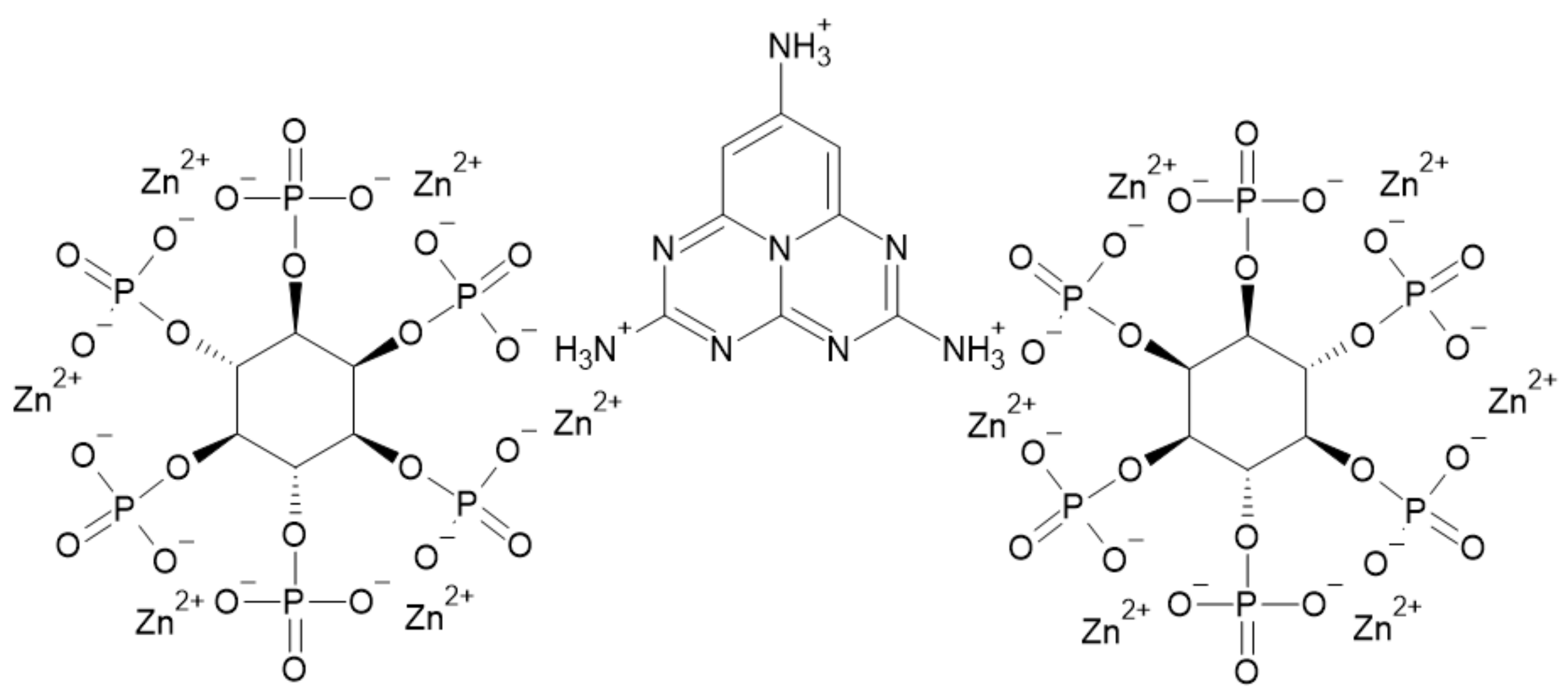
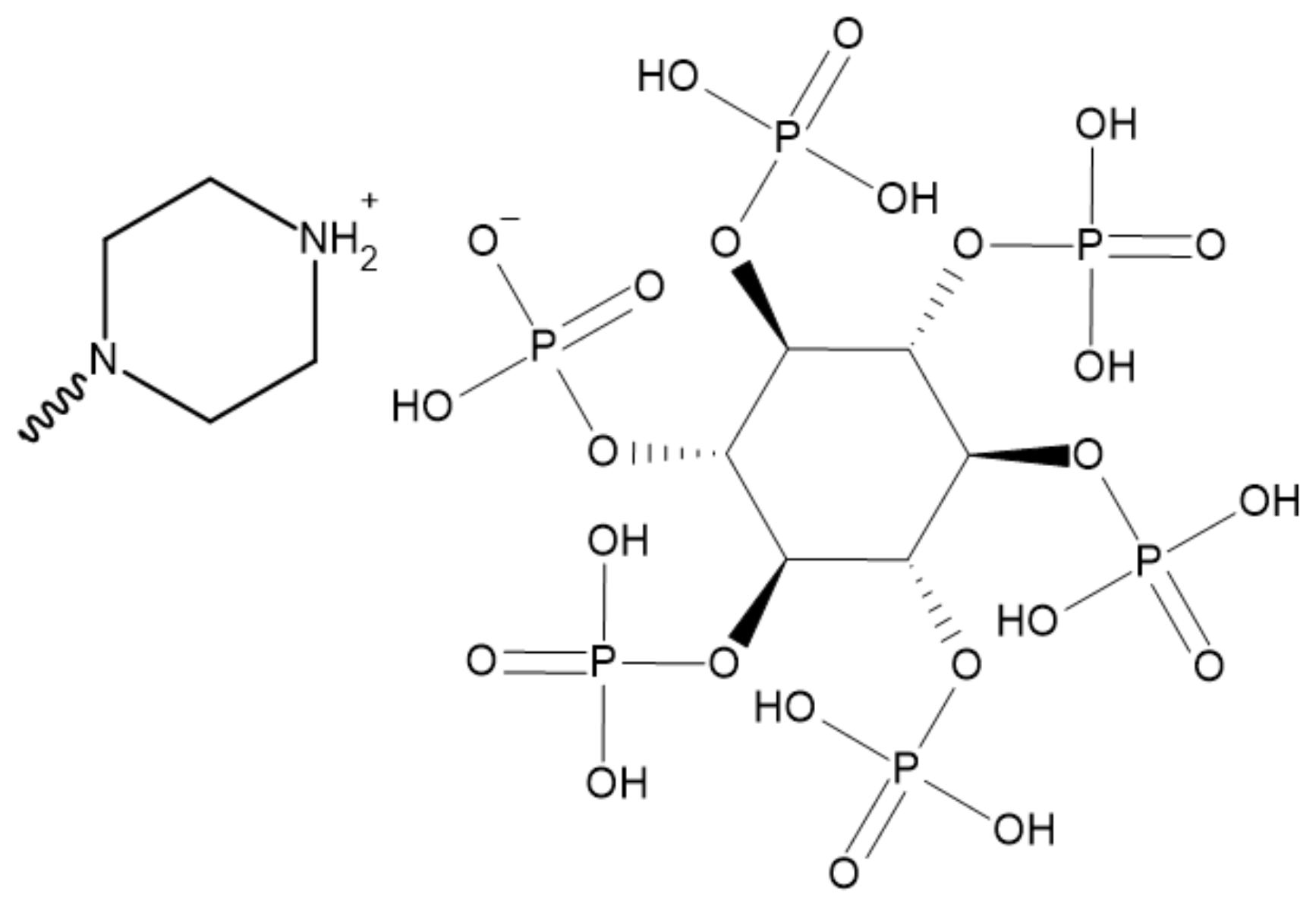
3.5. Phytic Acid
4. The Toxicity of Bio-Based Compounds
5. Bio-Based Flame Retardants for Thermosetting Resins
5.1. Bio-Based FRs for Epoxy Resin
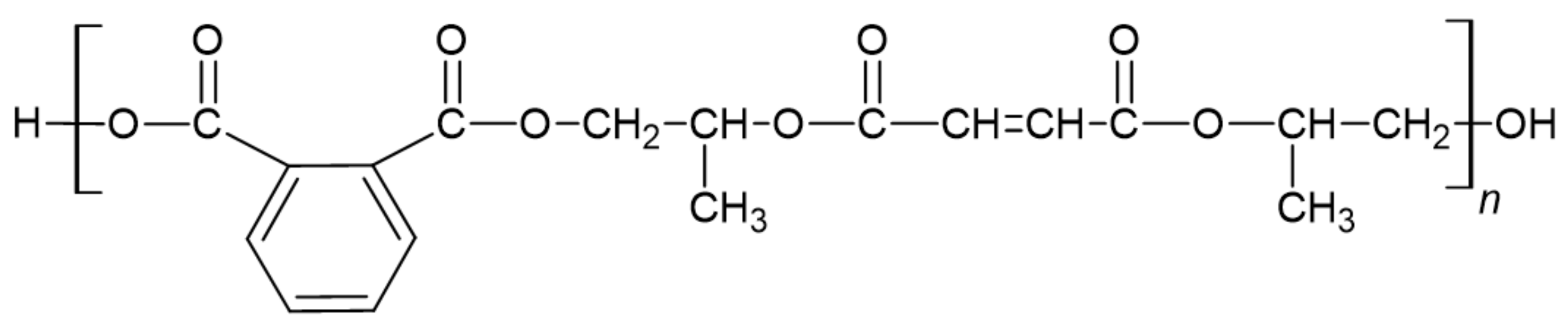
5.2. Bio-Based FRs for Unsaturated Polyester Resin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Analiza Produkcji, Zapotrzebowania Oraz Odzysku Tworzyw Sztucznych w Europie; Plastics Europe: Warsaw, Poland, 2022; p. 34.
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.; Darabi, A. Advancements in traditional and nanosized flame retardants for polymers—A review. J. Appl. Polym. Sci. 2021, 138, 50050. [Google Scholar] [CrossRef]
- Półka, M.; Woliński, M.; Salamonowicz, Z. Analysis of susceptibility to ignition of dust layer and dust cloud of selected hardened unsaturated polyester resins. Polimery 2014, 59, 805–810. [Google Scholar] [CrossRef]
- Półka, M.; Kukfisz, B.; Auguścik, M.; Leszczyńska, M.; Ryszkowska, J. Impact of flame retardants on the smoke generating properties of flexible and semi-rigid polyurethane foams. Przemysł Chem. 2017, 96, 1472–1476. [Google Scholar] [CrossRef]
- Dowbysz, A.; Samsonowicz, M.; Kukfisz, B. Modification of Glass/Polyester Laminates with Flame Retardants. Materials 2021, 14, 7901. [Google Scholar] [CrossRef]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- The Flame Retardants Market. Available online: https://www.flameretardants-online.com/flame-retardants/market (accessed on 10 February 2022).
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A review of sustainable and environment-friendly flame retardants used in plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame retardant polymer materials: An update and the future for 3D printing developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. (Eds.) Bio-Based Flame Retardants. In Towards Bio-Based Flame Retardant Polymers; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–72. [Google Scholar]
- Zörb, C.; Lewandowski, I.; Kindervater, R.; Göttert, U.; Patzelt, D. Bio-based Resources and Value Chains. In Bioeconomy: Shaping the Transition to a Sustainable, Bio-Based Economy; Lewandowski, I., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 75–95. [Google Scholar]
- Lehr, J.K.; Keeley, J.; Kingery, T.B. (Eds.) Biomass: Renewable Energy from Plants and Animals. In Alternative Energy and Shale Gas Encyclopedia; Wiley Series of Energy; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 657–662. [Google Scholar]
- Ciechanska, D.; Wietecha, J.; Kucharska, M.; Wrześniewska-Tosik, K.; Kopania, E. Biomasa jako źródło funkcjonalnych materiałów polimerowych. Polimery 2014, 59, 383–392. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; De Oliveira Matias, J.C.; Da Silva Catalão, J.P. (Eds.) Introduction. In Torrefaction of Biomass for Energy Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 1–43. [Google Scholar]
- Ioelovich, M. Plant Biomass as a Renewable Source of Biofuels and Biochemicals; Lambert Academic Publishing: Saarbrücken, Germany, 2013. [Google Scholar]
- Takada, A.; Kadokawa, J. Fabrication and characterization of polysaccharide ion gels with ionic liquids and their further conversion into value-added sustainable materials. Biomolecules 2015, 5, 244–262. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef]
- Singh, N.; Sahu, O. Sustainable cyclodextrin in textile applications. In The Impact and Prospects of Green Chemistry for Textile Technology; Shahid ul, I., Butola, B.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 83–105. [Google Scholar]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stab. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Fuessl, A.; Yamamoto, M.; Schneller, A. Opportunities in Bio-Based Building Blocks for Thermoplastic Polymers. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Kumar, P.; Dubey, K.K. Citric Acid Cycle Regulation: Back Bone for Secondary Metabolite Production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–181. [Google Scholar]
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 421–442. [Google Scholar]
- Battegazzore, D.; Bocchini, S.; Nicola, G.; Martini, E.; Frache, A. Isosorbide, a green plasticizer for thermoplastic starch that does not retrogradate. Carbohydr. Polym. 2015, 119, 78–84. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.; Lu, X.; Li, Y.; Li, X.; Li, J.-J. Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus. Microb. Cell Factories 2014, 13, 108. [Google Scholar] [CrossRef]
- Cao, M. Synthesis and Properties of Poly(Itaconic Acid). Master’s Thesis, University of New Hampshire, Durham, NH, USA, 2005. [Google Scholar]
- Gurtler, J.B.; Mai, T.L. Traditional Preservatives—Organic Acids. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 119–130. [Google Scholar]
- Fukami, T.; Tahara, S.; Yasuda, C.; Nakasone, K. Structural Refinements and Thermal Properties of L(+)-Tartaric, D(−)-Tartaric, and Monohydrate Racemic Tartaric Acid. Int. J. Chem. 2016, 8, 9–21. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Sain, M. Lignin-Reinforced Rubber Composites. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 195–206. [Google Scholar]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Patachia, S.; Croitoru, C. 14—Biopolymers for wood preservation. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 305–332. [Google Scholar]
- Athiappan, M.; Srinivasan, S.; Anandan, R.; Rajaram, J. Novel process of ellagic acid synthesis from waste generated from mango pulp processing industries. In Emerging Technologies in Environmental Bioremediation; Shah, M.P., Rodriguez-Couto, S., Şengör, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–454. [Google Scholar]
- Sebestyén, Z.; Jakab, E.; Badea, E.; Barta-Rajnai, E.; Şendrea, C.; Czégény, Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J. Anal. Appl. Pyrolysis 2019, 138, 178–187. [Google Scholar] [CrossRef]
- Lopes, A.; Carneiro, E.; Rios, M.; Hiluy Filho, J.; Carioca, J.; Barros, J.; Mazzetto, S. Study of antioxidant property of a thiosphorated compound derived from cashew nut shell liquid in hydrogenated naphthenics oils. Braz. J. Chem. Eng. 2008, 25, 119–127. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, F.; Souza, J.; França, F.; Ricardo, N.; Feitosa, J. Thermal Oligomerisation of Cardanol. e-Polymers 2006, 6. [Google Scholar] [CrossRef]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-Review of Analytical Methods used in Quantification of Ellagic Acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Omar, S.H. Biophenols: Impacts and Prospects in Anti-Alzheimer Drug Discovery. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–148. [Google Scholar]
- Alberti, A.; Granato, D.; Nogueira, A.; Mafra, L.I.; Colman, T.A.D.; Schnitzler, R. Modelling the thermal decomposition of 3,4,5-trihydroxybenzoic acid using ordinary least square regression. Int. Food Res. J. 2016, 23, 30–33. [Google Scholar]
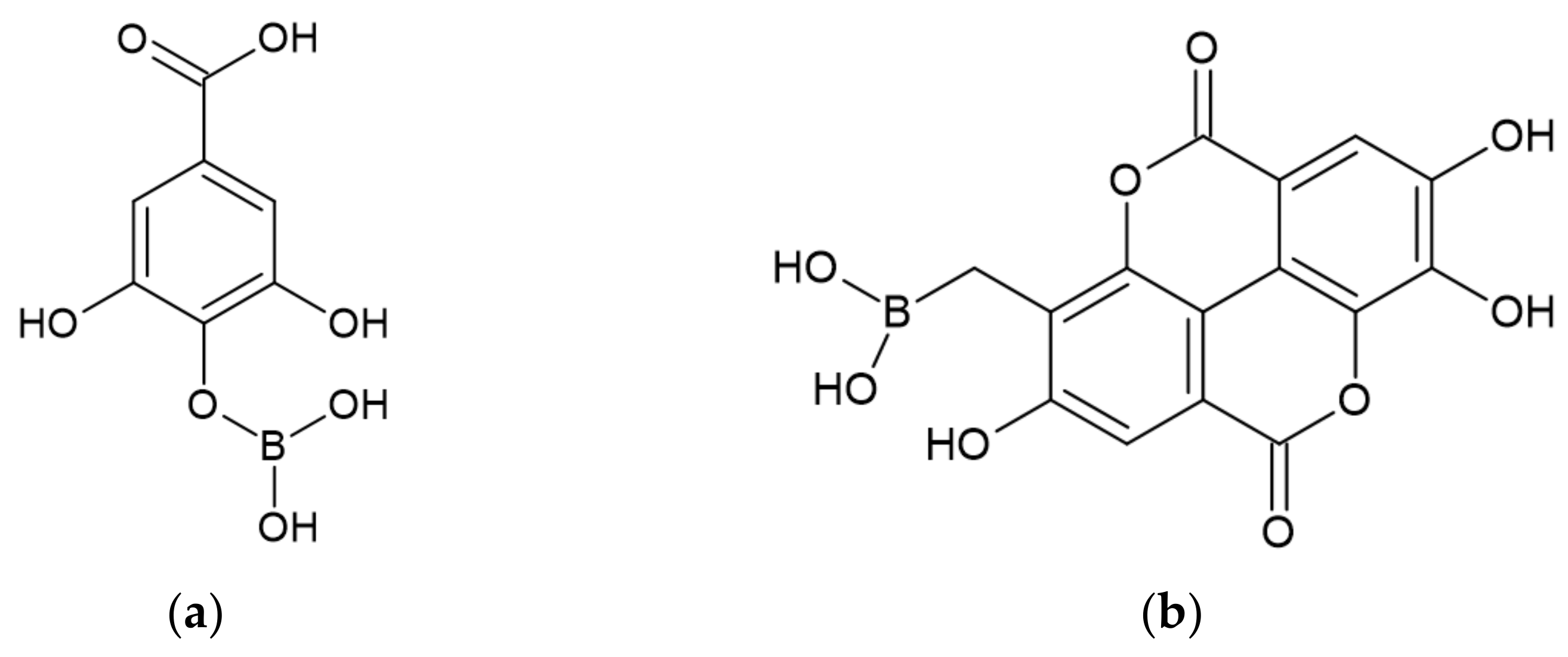
- Karaseva, V.; Bergeret, A.; Lacoste, C.; Fulcrand, H.; Ferry, L. New Biosourced Flame Retardant Agents Based on Gallic and Ellagic Acids for Epoxy Resins. Molecules 2019, 24, 4305. [Google Scholar] [CrossRef]
- Brat, P. Rapid analysis of phytochemicals in fruit and vegetables. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Tomás-Barberán, F.A., Gil, M.I., Eds.; Woodhead Publishing: Sawston, UK, 2008; pp. 248–278. [Google Scholar]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Kendall, C.; Eriksen, A.M.H.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef]
- Alongi, J.; Di Blasio, A.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B.; Camino, G. Thermal degradation of DNA, an all-in-one natural intumescent flame retardant. Polym. Degrad. Stab. 2015, 113, 110–118. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. (Eds.) Proteins. In Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2017; pp. 21–71. [Google Scholar]
- Reddy, A.B.; Manjula, B.; Sudhakar, K.; Sivanjineyulu, V.; Jayaramudu, T.; Sadiku, E.R. Polyethylene/Other Biomaterials-based Biocomposites and Bionanocomposites. In Polyethylene-Based Biocomposites and Bionanocomposites; Wiley-Scrivener: Salem, MA, USA, 2016; pp. 279–314. [Google Scholar]
- Mocanu, A.M.; Moldoveanu, C.; Odochian, L.; Paius, C.M.; Apostolescu, N.; Neculau, R. Study on the thermal behavior of casein under nitrogen and air atmosphere by means of the TG-FTIR technique. Thermochim. Acta 2012, 546, 120–126. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Odochian, L.; Paius, C.; Lorela, I.; Baiceanu, A. Study on the thermal behavior of casein in air. Acta Chem. IASI 2013, 21, 31–46. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Tyminski, L.; Znajewska, Z.; Dąbrowska, G. Characteristics and functions of hydrophobins and their use in manifold industries. Postępy Mikrobiol. 2018, 57, 374–384. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crops Prod. 2021, 164, 113349. [Google Scholar] [CrossRef]
- Daneluti, A.; Matos, J. Study of thermal behavior of phytic acid. Braz. J. Pharm. Sci. 2013, 49, 275–283. [Google Scholar] [CrossRef]
- Malucelli, G.; Bosco, F.; Alongi, J.; Carosio, F.; Di Blasio, A.; Mollea, C.; Cuttica, F.; Casale, A. Biomacromolecules as novel green flame retardant systems for textiles: An overview. RSC Adv. 2014, 4, 46024–46039. [Google Scholar] [CrossRef]
- Kangas, H.; Pitkanen, M. Environmental, Health, and Safety (EHS) Aspects of Cellulose Nanomaterials. In Lignocellulosics; Filpponen, I., Peresin, M.S., Nypelo, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–374. [Google Scholar]
- El-Naggar, M.E.; El-Rafie, M.H.; El-sheikh, M.A.; El-Feky, G.S.; Hebeish, A. Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. Int. J. Biol. Macromol. 2015, 81, 718–729. [Google Scholar] [CrossRef]
- Yu, M.; Ji, N.; Wang, Y.; Dai, L.; Xiong, L.; Sun, Q. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100. [Google Scholar] [CrossRef]
- Kurniasih, M.; Purwati; Dewi, R.S. Toxicity tests, antioxidant activity, and antimicrobial activity of chitosan. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 12037. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Matica, A.; Menghiu, G.; Ostafe, V. Toxicity of chitosan based products. New Front. Chem. 2017, 26, 65–74. [Google Scholar]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and Lignin Based Materials for the Removal of Heavy Metals from Waste Water-An Overview. Z. Für Phys. Chem. 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Sudip, K.S.; Smita, R.; Mahendra, G.; Sangeeta, R. Biodegradation of Lignin from Pulp and Paper Mill Effluent: Optimization and Toxicity Evaluation. J. Hazard. Toxic Radioact. Waste 2020, 24, 4020032. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review: Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S. A review on occurrence, measurement, toxicity and tannin removal processes from wastewaters. Environ. Eng. Manag. J. 2019, 18, 109–123. [Google Scholar] [CrossRef]
- Malucelli, G. Biomacromolecules and Bio-Sourced Products for the Design of Flame Retarded Fabrics: Current State of the Art and Future Perspectives. Molecules 2019, 24, 3774. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame retardant bio-based epoxy thermosets: A review. Compos. Part B Eng. 2019, 179, 107487. [Google Scholar] [CrossRef]
- Speight, J.G. (Ed.) Sources and Types of Organic Pollutants. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; pp. 153–201. [Google Scholar]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K. (Eds.) Element and Processing. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 18, pp. 431–499. [Google Scholar]
- Gibson, G. Epoxy Resins. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 773–797. [Google Scholar]
- Lee, S.-H.; Oh, S.-W.; Lee, Y.-H.; Kim, I.-J.; Lee, D.-J.; Lim, J.-C.; Park, C.-C.; Kim, H.-D. Preparation and properties of flame-retardant epoxy resins containing reactive phosphorus flame retardant. J. Eng. Fibers Fabr. 2020, 15, 1558925020901323. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J.; Qu, H. Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin. Polymers 2020, 12, 212. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of chicken eggshell on the flame-retardant and smoke suppression properties of an epoxy-based traditional APP-PER-MEL system. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
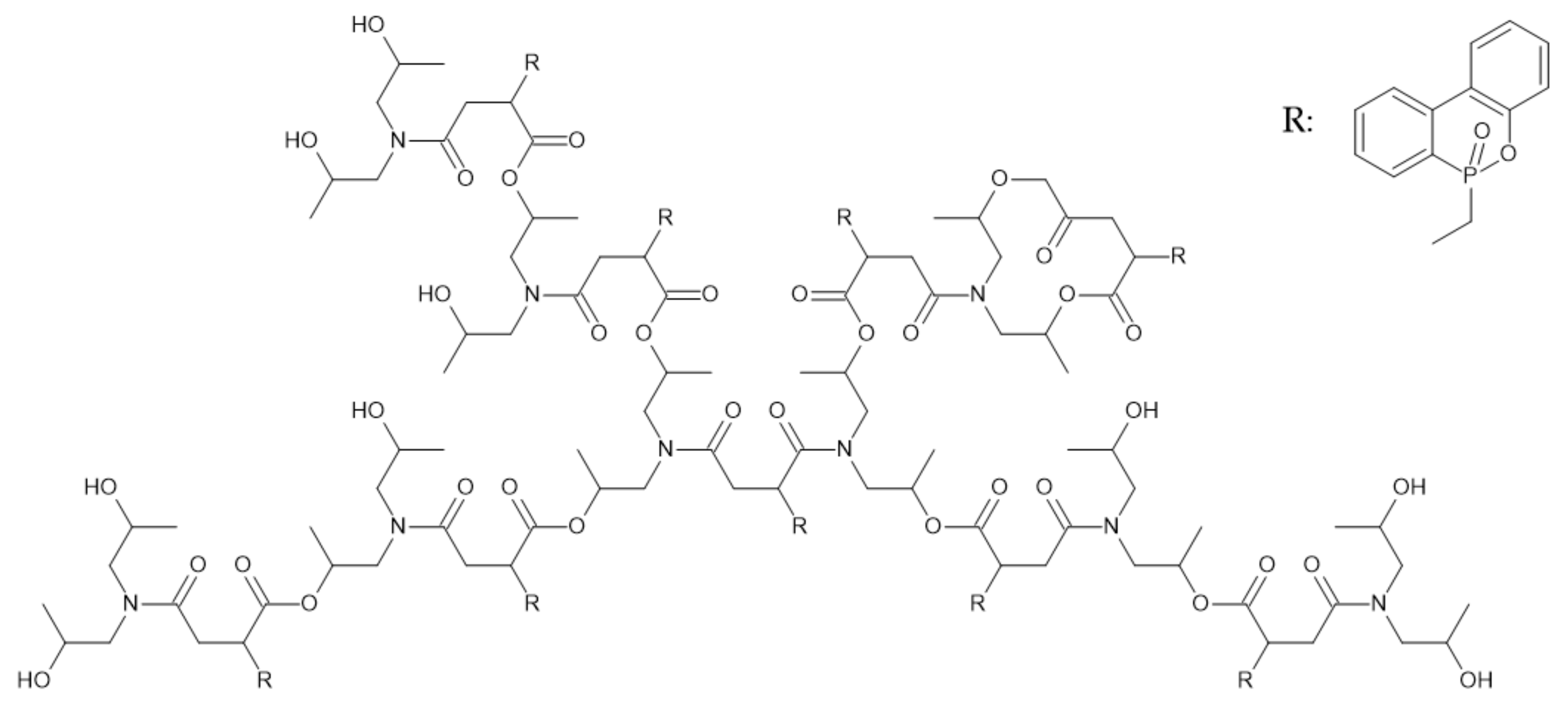
- Liu, C.; Tao, Y.; Xu, Y.-J.; Liu, Y.; Zhu, P.; Wang, Y.-Z. Effect of Bio-Based Cobalt Alginate on the Fire Safety and Mechanical Properties for Epoxy Resin. Macromol. Mater. Eng. 2021, 306, 2100466. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z. Synthesis of a bio-based piperazine phytate flame retardant for epoxy resin with improved flame retardancy and smoke suppression. Polym. Adv. Technol. 2021, 32, 4282–4295. [Google Scholar] [CrossRef]
- Anh, N.T.; Tung, N.Q.; Canh, N.X.; Hoan, N.V. Eco-friendly flame retardant additives for epoxy resin nanocomposites. EurAsian J. BioSci. 2019, 13, 323–332. [Google Scholar]
- Zhang, J.; Mi, X.; Chen, S.; Xu, Z.; Zhang, Z.; Miao, M.; Wang, J. A bio-based hyperbranched flame retardant for epoxy resins. Chem. Eng. J. 2019, 381, 122719. [Google Scholar] [CrossRef]
- Nguyen, T.A. Biocomposites Developed with Litchi Peel Based on Epoxy Resin: Mechanical Properties and Flame Retardant. J. Chem. 2021, 2021, 3287733. [Google Scholar] [CrossRef]
- Jones, F.R. Unsaturated Polyester Resins. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 743–772. [Google Scholar]
- Pang, Y.; Cho, D.; Han, S.O.; Park, W.H. Interfacial Shear Strength and Thermal Properties of Electron Beam-Treated Henequen Fibers Reinforced Unsaturated Polyester Composites. Macromol. Res. 2005, 13, 453–459. [Google Scholar] [CrossRef]
- Dhoakiya, B. Unsaturated Polyester Resin for Specialty Applications. In Polyester; IntechOpen: London, UK, 2012; p. 402. [Google Scholar]
- Athawale, A.A.; Pandit, J.A. Unsaturated Polyester Resins, Blends, Interpenetrating Polymer Networks, Composites, and Nanocomposites: State of the Art and New Challenges. In Unsaturated Polyester Resins; Thomas, S., Hosur, M., Chirayil, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–42. [Google Scholar]
- Sałasińska, K.; Celiński, M.; Mizera, K.; Barczewski, M.; Kozikowski, P.; Leszczyński, M.K.; Domańska, A. Moisture Resistance, Thermal Stability and Fire Behavior of Unsaturated Polyester Resin Modified with L-histidinium Dihydrogen Phosphate-Phosphoric Acid. Molecules 2021, 26, 932. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Zhang, Q.; Chen, Z.; Chen, T.; Jiang, J. Preparation of phosphorylated chitosan-coated carbon microspheres as flame retardant and its application in unsaturated polyester resin. Polym. Adv. Technol. 2019, 30, 1933–1942. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, J.; Yu, Y.; Zhang, Q.; Chen, T.; Ni, L. Layer-by-layer assembled diatomite based on chitosan and ammonium polyphosphate to increase the fire safety of unsaturated polyester resins. Powder Technol. 2020, 364, 36–48. [Google Scholar] [CrossRef]
- Farishi, S.; Rifathin, A.; Ramadhoni, B.F. Phosphorus/Nitrogen Grafted Lignin as a Bio-based Flame Retardant for Unsaturated Polyester Resin. In Proceedings of the International Manufacturing Engineering Conference & the Asia Pacific Conference on Manufacturing Systems, Putrayaha, Malaysia, 21–22 August 2019; Springer: Singapore, 2020; pp. 429–434. [Google Scholar]
- Zhang, J.; Zhang, H.; Wang, X.; Zhang, M. Modified hemp fiber combined with melamine cyanurate for enhancing fire safety and mechanical properties of unsaturated polyester resins for automobile parts. AIP Adv. 2021, 11, 125329. [Google Scholar] [CrossRef]

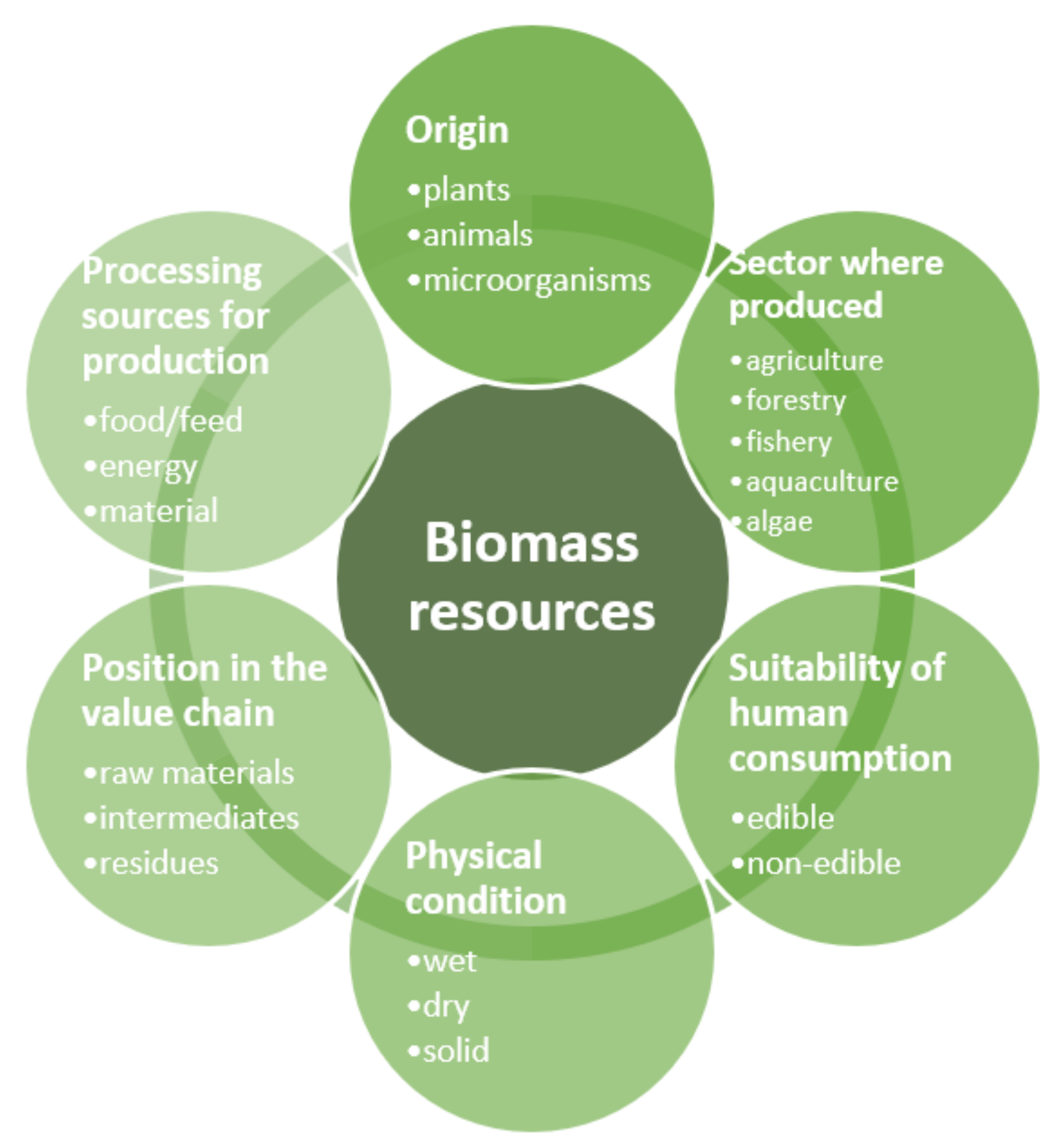
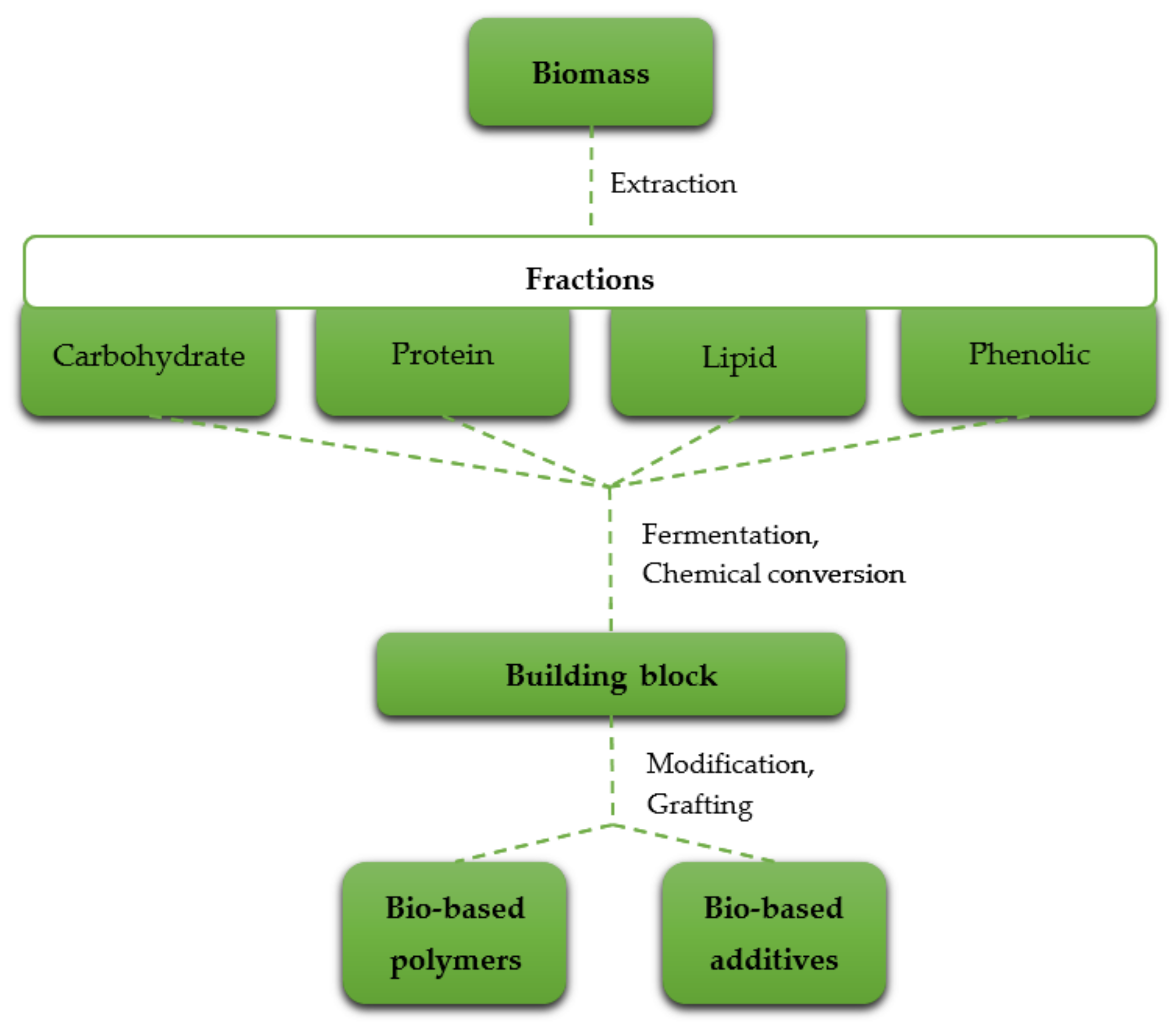




| Group of Compounds | Subgroup | Example | Molecular Formula |
|---|---|---|---|
| Carbohydrates | Monosaccharides | Glucose | C6H12O6 |
| Polysaccharides | Starch | (C6H10O5)n | |
| Cellulose | (C6H10O5)n | ||
| Xylose | C5H10O5 | ||
| Phenolic compounds | Lignin | Coniferyl, coumaryl, and sinapyl alcohols | C9H10O2, C10H12O3, C11H14O4 |
| Oils | Triglycerides | Oleic acid | C18H34O2 |
| Proteins | Amino acids | Alanine | C3H7NO2 |
| Biomass | Cellulose [wt%] | Hemicelluloses [wt%] | Lignin [%] |
|---|---|---|---|
| White cotton | 94–96 | 1–2 | <1 |
| Brown cotton | 85–88 | 2–3 | 5–7 |
| Flax | 85–88 | 5–6 | 3–5 |
| Softwood | 46–48 | 20–23 | 27–28 |
| Hardwood | 44–46 | 25–27 | 22–25 |
| Bagasse | 37–39 | 23–25 | 19–21 |
| Corn stalks | 35–37 | 24–26 | 18–20 |
| Corn cobs | 34–36 | 36–38 | 9–11 |
| Corn stover | 35–37 | 28–30 | 18–20 |
| Wheat straw | 34–36 | 28–30 | 15–17 |
| Rice straw | 34–36 | 25–27 | 7–9 |
| Switchgrass | 36–38 | 26–28 | 17–19 |
| Waste of textile | 97–98 | 1–2 | <1 |
| Cotton linter | 95–96 | 1–2 | <1 |
| Used office paper | 60–62 | 4–6 | 1–2 |
| Used newspaper | 38–40 | 18–20 | 20–22 |
| Used cardboard | 58–60 | 14–15 | 10–12 |
| Olive pomace | 23–25 | 22–24 | 32–34 |
| Flame Retardant | Type of Compound | Mode of Action | Effects | The Optimum Amount of Additive | Reference |
|---|---|---|---|---|---|
| Flame retardants for epoxy resin | |||||
| Core-shell graphitic carbon nitride/zinc phytate (PIPT) | Derivative of phytic acid | Forming stable, dense char layer and limiting heat transfer by labyrinth effect | Increase LOI Reduce PHRR rate and THR Prolong TTI Reduce FGI Reduce PSPR and TSP Reduce effective heat of combustion Increase residue mass Reduce tensile strength, elongation at , and impact strength | 5 phr | [75] |
| Intumescent flame retardant system (APP/PER/MEL/eggshell) | Biowaste | Forming thermally stable char layer blocking mass and heat transfer between gas and condensed phases | Increase LOI Reduce PHR and THR Reduce TSR Reduce smoke density rating Increase residue mass | 40 wt% (wt% of eggshell) | [76] |
| Cobalt alginate | Derivative of alginate | Forming a thin char layer and diluting combustible gases | Increase LOI Remain unchanged TTI Reduce PHRR Slightly increase THR Reduce FIGRA Reduce TSP Reduce the smoke density Increase char residue Reduce tensile strength, tensile modulus, and flexural strength are lower Improve the impact strength | 3 wt% | [77] |
| Piperazine phytate | Derivative of phytic acid | Degradation of ammonium compounds resulting in NH3 release, diluting flammable gases, and promoting char formation | Increase LOI Reduce PHRR and THR Reduce PSPR and TSP Increase residue mass Reduce FIGRA Reduce tensile strength and elongation at | 15 wt% | [78] |
| Fly ash (FA)/modified with HCl/modified with NaOH | Biowaste | Not mentioned | Decrease burning rate Increase LOI Insignificantly reduces flexural, tensile, and impact strength Increase compressive strength | 20 wt% | [79] |
| ITA-HBP | Derivative of itaconic acid | Forming of the char layer, blocking heat and oxygen permeation. Releasing free radicals promotes pyrolysis. Releasing volatile compounds diluting flammable gases. | Increase LOI Make EP self-extinguishing Reduce PHRR and THR Shorten TTI Reduce TSR Improve impact and flexural strength, and toughness | 20 phr | [80] |
| Lychee peel | Biowaste | Forming of the rich-carbon char layer, blocking fuel transport. Absorbing free radicals. | Slightly increase LOI Decrease the burning rate Increase tensile strength, compressive strength, and impact strength Decrease flexural strength | 20 wt% | [81] |
| Gallic acid, Ellagic acid, Derivatives of acids (GA, EA, GAD, EAD) | Derivatives of gallic and ellagic acids | Forming of the protective layer slowing pyrolysis. | Shorten TTI Reduce PHRR and THR Reduce burning rate | 10 wt% | [41] |
| Flame retardants for unsaturated polyester resin | |||||
| L-histidinium dihydrogen phosphate–phosphoric acid (LHP) | Derivative of aminoacid | Forming of the char layer blocks heat transport and slows the burning rate. Probably also a heat sink effect and diluting oxygen concentration due to decomposition of propionic acid. | Makes UPR self-extinguishing Reduces PHRR and THR Reduces TSR Reduces smoke density | 30 wt% | [86] |
| Phosphorylated chitosan-coated carbon microspheres (PCCCM | Derivative of chitosan | Forming of the tortuous char layer blocks heat and pyrolysis products transport. Dilution of combustible gases. | Slightly increases LOI value Increases residue mass Shortens TTI Reduces PHRR Slightly increases THR Reduces PSPR and TSP | 3 wt% | [87] |
| LbL assembled diatomite based on chitosan and APP (DCHAPP) | Derivative of chitosan | Forming of compact and colloidal char layer blocking heat transport and preventing the transmission of pyrolysis compounds. | Increases LOI Increases char residue Reduces PHRR and THR Prolongs TTI Reduces SPR and TSR Slightly improves flexural and tensile strength | 25 phr (9 bilayers) | [88] |
| PN-lignin | Derivative of lignin | Forming of the char layer blocks heat and gas transfer. | Decreases the burning rate Slightly improves thermal stability | 12,5 wt% | [89] |
| Modified hemp fiber combined with melamine cyanurate | Biowaste | Forming of the expandable char layer blocks heat transfer and isolation from the air. | Increase LOI Decreases PHRR and THR Decreases TSR Increases residue mass | 30 wt% (hemp fiber 3 wt%) | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowbysz, A.; Samsonowicz, M.; Kukfisz, B. Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins. Int. J. Environ. Res. Public Health 2022, 19, 4828. https://doi.org/10.3390/ijerph19084828
Dowbysz A, Samsonowicz M, Kukfisz B. Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins. International Journal of Environmental Research and Public Health. 2022; 19(8):4828. https://doi.org/10.3390/ijerph19084828
Chicago/Turabian StyleDowbysz, Adriana, Mariola Samsonowicz, and Bożena Kukfisz. 2022. "Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins" International Journal of Environmental Research and Public Health 19, no. 8: 4828. https://doi.org/10.3390/ijerph19084828
APA StyleDowbysz, A., Samsonowicz, M., & Kukfisz, B. (2022). Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins. International Journal of Environmental Research and Public Health, 19(8), 4828. https://doi.org/10.3390/ijerph19084828