Abstract
The Chinese community-acquired pneumonia (CAP) Diagnosis and Treatment Guideline 2020 recommends quinolone antibiotics as the initial empirical treatment options for CAP. However, patients with pulmonary tuberculosis (PTB) are often misdiagnosed with CAP because of the similarity of symptoms. Moxifloxacin and levofloxacin have inhibitory effects on mycobacterium tuberculosis as compared with nemonoxacin, resulting in delayed diagnosis of PTB. Hence, the aim of this study is to compare the cost-effectiveness of nemonoxacin, moxifloxacin and levofloxacin in the treatment of CAP and to determine the value of these treatments in the differential diagnosis of PTB. Primary efficacy data were collected from phase II-III randomized, double-blind, multi-center clinical trials comparing nemonoxacin to moxifloxacin (CTR20130195) and nemonoxacin to levofloxacin (CTR20140439) for the treatment of Chinese CAP patients. A decision tree was constructed to compare the cost-utility among three groups under the perspective of healthcare system. The threshold for willingness to pay (WTP) is 1–3 times GDP per capita ($11,174–33,521). Scenarios including efficacy and cost for CAP patients with a total of 6% undifferentiated PTB. Sensitivity and scenario analyses were performed to test the robustness of basic analysis. The costs of nemonoxacin, moxifloxacin, and levofloxacin were $903.72, $1053.59, and $1212.06 and the outcomes were 188.7, 188.8, and 188.5 quality-adjusted life days (QALD), respectively. Nemonoxacin and moxifloxacin were dominant compared with levofloxacin, and the ICER of moxifloxacin compared with nemonoxacin was $551,643, which was much greater than WTP; therefore, nemonoxacin was the most cost-effective option. Regarding patients with PTB who were misdiagnosed with CAP, taking nemonoxacin could save $290.76 and $205.51 when compared with moxifloxacin and levofloxacin and resulted in a gain of 2.83 QALDs. Our findings demonstrate that nemonoxacin is the more economical compared with moxifloxacin and levofloxacin, and non-fluoroquinolone antibiotics are cost-saving and utility-increasing compared to fluoroquinolones in the differential diagnosis of PTB, which can help healthcare system in making optimal policies and help clinicians in the medication of patients.
1. Introduction
Community-acquired pneumonia (CAP) refers to inflammation or infection of the lung parenchyma that is contracted outside the hospital, including pneumonia that has a clear incubation period and pathogen infection that develops during the incubation period after admission [1]. CAP is a common disease of the respiratory system; it has a high incidence worldwide and brings a considerable economic burden to society [2,3,4,5,6]. The incidence of CAP in China is higher than that in European and American countries; owing to the combination of urban air pollution and low vaccination rates of residents [7,8], China is still facing the challenges of preventing and treating CAP and the associated burdens of the disease.
The clinical manifestations of CAP and pulmonary tuberculosis (PTB) are similar, and the imaging examination results of the two diseases are diverse. The positive rate for pathogen culture detection is low and the process is time-consuming, which makes it difficult to distinguish between the two diseases [9,10]. Among the patients with CAP in China, approximately 6% of this population comprises patients with PTB, as shown by expert investigation, which is higher than the Asian average of 3.30% [11]. The main treatment for CAP is antibiotic therapy. Levofloxacin and moxifloxacin are typical fluoroquinolone drugs with strong, broad-spectrum antibacterial effects [7,8]. However, studies have found that the use of fluoroquinolone antibiotics in the diagnosis and treatment of patients with CAP with unclear symptoms increases the risk of delaying the diagnosis of PTB [12]. Hence, some experts do not recommend the use of fluoroquinolone antibiotics as first-line treatment for CAP [13]. Nemonoxacin is a new type of non-fluoroquinolone drug that maintains strong antibacterial activity against common CAP-causing pathogens, such as Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Moraxella catarrhalis [14]. In addition to its clinically satisfactory cure rate and bacterial clearance rate, nemonoxacin has almost no antibacterial effect on Mycobacterium tuberculosis, so it can be used as an early treatment plan for patients with suspected PTB or CAP.
To our knowledge, there are currently no studies in the literature discussing the value of antibiotics in the differential diagnosis of CAP and PTB; furthermore, there is a lack of evidence of the cost-effectiveness of nemonoxacin for the treatment of CAP. Hence, the aim of this study is to compare the cost-effectiveness of nemonoxacin, moxifloxacin, and levofloxacin in the treatment of mild to moderate CAP and to determine the value of these treatments in the differential diagnosis of PTB, and to provide evidence for Chinese governments, clinicians and other researchers in policy making and clinical medication.
2. Materials and Methods
2.1. Source of Parameters
Primary efficacy data were collected from phase II-III randomized, double-blind, multi-center clinical trials comparing nemonoxacin to moxifloxacin (CTR20130195) and nemonoxacin to levofloxacin (CTR20140439) for treatment of Chinese CAP patients. More clinical trial information is provided in the Supplementary Materials Table S1.
For parameters that were unavailable from the literature, we conducted physician surveys. In order to make our data reliable and able to represent the overall level of China, we conducted surveys among authoritative hospitals in the eastern, middle, and western regions of China and collected information by distributing electronic questionnaires to doctors with extensive clinical experience. Finally, 31 doctors from nationwide authoritative hospitals, which were located in Beijing, Shanghai, Wuhan, and other cities, were questioned. The mean years of the clinical treatment experience of these doctors was 20 ± 8 years and their average age was 45 ± 8 years old.
2.2. Model Structure
Microsoft Excel 2019 was used to build a decision tree model (Figure 1). The research was conducted from the perspective of the Chinese healthcare system with only direct medical costs considered. The model simulated the end of treatment for 99.9% of patients. The study period was 194 days. The model structure was determined in accordance with disease-specific and expert advice.
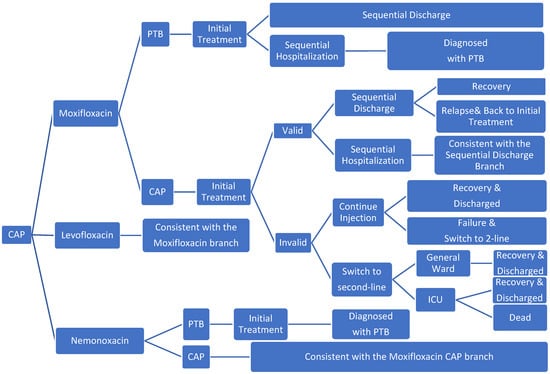
Figure 1.
Model structure (PTB, pulmonary tuberculosis).
The study population consisted of patients with mild and moderate CAP requiring treatment, which included an estimated 6% of patients with PTB who were misdiagnosed with CAP; all patients received one of the three groups of CAP drugs. After the initial CAP injections treatment, those who responded were to receive sequential outpatient or inpatient treatment; during sequential treatment, patients would receive oral therapies (more details are provided in the Supplementary Materials Table S2). Nemonoxacin has no inhibitory effect on tuberculosis, whereas levofloxacin and moxifloxacin inhibit M. tuberculosis and mask the symptoms of PTB [12]. Therefore, after the initial treatment of patients with PTB who were misdiagnosed with CAP, patients treated with nemonoxacin were to receive the standard hospitalization treatment for PTB, and the patients in the levofloxacin and moxifloxacin group received sequential CAP treatment. Although levofloxacin and moxifloxacin have been shown to have good anti-tuberculosis effects [15,16], intensive treatment is needed in the early stage of PTB. Therefore, after the short-term CAP-related treatment, patients with PTB would again show symptoms [12]. Considering that the effect may be delayed, patients who did not respond to the initial treatment received second-line treatment or to continue to take the original drug treatment. In the second-line treatment, depending on whether the patient’s condition had deteriorated, it was determined whether the patients should be admitted to general wards or ICU wards. After ICU treatment improved, patients were transferred to the general ward for second-line treatment. Patients who continued the original drug treatment entered the second-line treatment if the course of treatment was not effective. In addition, given the likelihood of the recurrence of CAP [17,18], patients who were cured initially may have been admitted to the hospital again for treatment.
2.3. Model Assumptions
To construct the model, the following necessary assumptions were made:
- That the success of treatment had occurred by the last day of the state.
- That the recurrence of CAP would have occurred by the 7th day after the initial treatment was effective.
- That the spread of tuberculosis and delayed treatment (9 days) were not considered to affect the treatment of patients with PTB.
- That the effect of minor adverse reactions on the utility value and the adverse reactions of the second-line treatment were not considered.
- That the effective rate of the second-line treatment of patients with recurrent CAP and the effective rate of PTB treatment was 100%.
- That the proportion of continued injection therapy after the initial treatment failure was the same as the rate of delay in the initial treatment failure.
- That relapsed patients with CAP were admitted to the hospital for second-line treatment.
- That patients with PTB relapsed after the CAP sequential treatment ended.
2.4. Transition Probability
The initial curative effects of the three groups of drugs were in keeping with the efficacy of Visit 2 (3–5 days of treatment). The data for nemonoxacin, levofloxacin, and moxifloxacin were all derived from phase II–III clinical trials. For patients whose initial treatment failed, 91% and 86% of patients in the moxifloxacin and levofloxacin groups, respectively, chose to continue treatment with the original drug owing to delayed efficacy, which were extracted from similar cost-effectiveness analysis [19]. The delayed rate of efficacy in the nemonoxacin group was determined to be 93% through expert consultation. The recurrence rate after successful initial treatment was calculated from the following equation:
In Equation (1), and represent the effective rates of initial treatment and complete treatment (1–2 days after the end of treatment), respectively, and R represents the rate of delayed efficacy in the population with initial treatment failure.
More details about clinical efficacy, transition probability, treatment time are provided in Table 1.

Table 1.
Input parameters used in the analysis.
2.5. Cost
From the perspective of the healthcare system, our research only focused on the direct medical costs, which mainly comprised the costs of antibiotics, examinations, auxiliary medication, bed days, nursing, and adverse reaction (AE) treatment. In terms of drug costs, the prices of nemonoxacin, moxifloxacin, and levofloxacin oral and injection solutions and other drugs were determined regarding the national median bid price in 2021. AE was considered to include gastrointestinal disorders (nausea, diarrhea, vomiting, and abdominal discomfort), skin and subcutaneous tissue disorders (rash), neurological disorders (dizziness, headache), and abnormal detection (increased transaminases, decreased WBC, blood bilirubin increase); related treatment costs were from expert consultation. In addition, the second-line treatment drugs were determined through expert consultation, and the dosage of each drug was determined in accordance with the prescriber’s instructions. The value and source of each cost parameter are presented in Table 1; dosing regimens are presented in the Supplementary Materials Table S2. The costs in this research are in keeping with 2021 values of CNY (1 CNY = 0.15699 USD).
2.6. Utility
The range of utility was 0–1; the utility for the death state was 0 and that for the healthy population was 1. Utilities for initial injection therapy in patients with CAP were 0.56, 0.88, and 0.82 for CAP outpatients or inpatients with sequential treatment; for CAP patients in second-line or ICU general wards were 0.53 and 0.30, respectively. For PTB patients, utilities of no treatment, hospital discharge, and hospitalization were 0.68, 0.83, and 0.59, respectively. All utilities were from the published literature. More details are shown in Table 1.
2.7. Cost-Effectiveness Analysis
A cost-utility analysis (CUA) was adopted in our study. The effect index used during this study was the quality-adjusted life day (QALD), and the incremental cost-effectiveness ratio (ICER) and the incremental net monetary benefit (INMB) were used to compare the cost-effectiveness of each plan; the calculation method of INMB is shown in Equation (2). According to WHO recommendations, willingness to pay (WTP) was set at 1–3 times GDP per capita (CNY 72,447–217,341). When the ICER was below CNY 217,341, there was a certain cost-effective advantage [32]. Furthermore, we used 1-time GDP as WTP when calculating INMB, and INMB over 0 means economical. Owing to the short research period, we did not consider discounts.
2.8. Sensitivity Analysis
Taking the uncertainty of the value of each parameter into account, sensitivity analysis was conducted. One-way sensitivity analysis was conducted to explore the economics of each program when the parameters were changed between the upper and lower limits; INMB was used as the economic measure, and the 10 parameters that had the greatest impact on INMB were selected to be plotted as a cyclone graph. Probability sensitivity analysis was conducted using Monte Carlo simulation with 10,000 iterations, a scatter diagram was drawn, and the cost-effective acceptability chart (CEAC) curve was used to analyze the economic situation under different values of WTP.
2.9. Scenario Analysis
Similar economic evaluation research tended to ignore the proportion of patients with CAP who are misdiagnosed with PTB [18,21,23,30,33,34]; therefore, to explore the reliability of the conclusions, and to further verify the value of the differential diagnosis of PTB, a scenario analysis in which PTB was not considered was performed.
3. Results
3.1. Basic Analysis Results
The basic analysis results of the three interventions are provided in Table 2. The cumulative costs of nemonoxacin, moxifloxacin, and levofloxacin were CNY 5859 (USD 904), CNY 6831 (USD 1054), and CNY 7858 (USD 1212), and the cumulative utilities were 188.7, 188.8, and 188.5 QALDs, respectively. In terms of the effectiveness, the cumulative utilities of the three drugs were almost the same. In terms of cost-effectiveness, nemonoxacin and moxifloxacin were dominant compared with levofloxacin, and the ICER of moxifloxacin, compared with nemonoxacin, was CNY 3576,742 (USD 551,643), which was much greater than WTP; therefore, nemonoxacin was the most cost-effective option.

Table 2.
Basic analysis and scenario analysis results.
In the value of differential diagnosis of PTB, PTB patients who were misdiagnosed as CAP treated with nemonoxacin, moxifloxacin and levofloxacin would cost CNY 9375 (USD 1472), CNY 11,227 (USD 1763) and CNY 10,684 (USD 1677), respectively. Compared with moxifloxacin and levofloxacin, nemonoxacin saved CNY 1852 (USD 291) and CNY 1309 (USD 205), accounting for approximately 32.7% and 23.1% of the treatment cost of PTB (CNY 5658) [24]. Moreover, treatment with nemonoxacin resulted in an increase of 2.83 QALDs compared to the other two drugs. Further details are presented in Table 2. Furthermore, we decomposed the cumulative costs of these three drugs and the corresponding cost-breakdown table is presented in Table 3.

Table 3.
Breakdown for basic analysis costs (CNY).
3.2. Results of the Sensitivity Analysis
3.2.1. One-Way Sensitivity Analysis
As shown in Figure 2, the recurrence rate, price of injections, and the number of initial treatment days were the most consequential factors affecting the economy of each drug. From the Figure 2B,C, we can see that nemonoxacin had more NMB than the other drugs when the upper and lower limits of each variable were changed; therefore, nemonoxacin can be considered a cost-effective option compared with moxifloxacin or levofloxacin. Moxifloxacin is more cost-effective compared with moxifloxacin or levofloxacin, as Figure 2A shows.
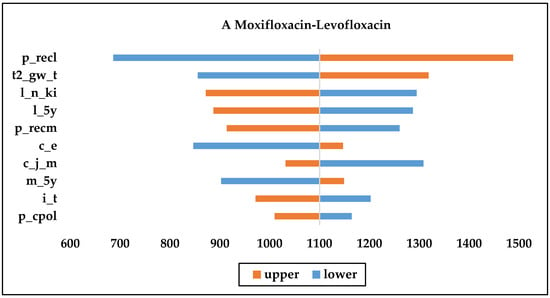
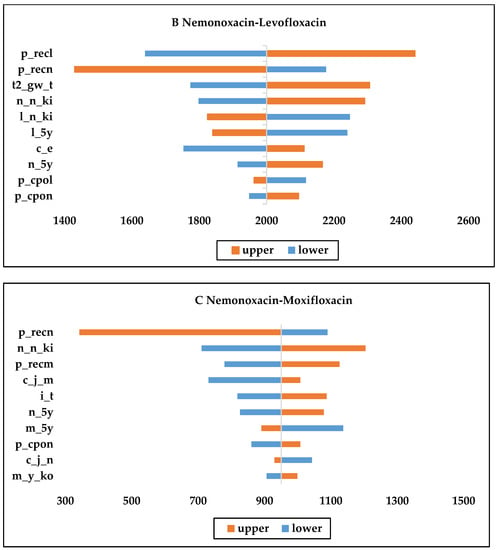
Figure 2.
One-way sensitivity analysis chart. (c_j_m: daily cost of moxifloxacin injection; c_j_n: daily cost of nemonoxacin injection; c_e: daily cost of second-line therapy; m_5y: Moxifloxacin initial treatment effective rate; l_5y: Levofloxacin initial treatment effective rate; n_5y: Nemonoxacin initial treatment effective rates; n_ki: proportion of original drug treatment after initial failure of levofloxacin; n_n_ki: proportion of original drug treatment after initial failure of nemonoxacin; i_t: initial treatment day; t2_gw_t: second-line treatment days in general; p_recm: recurrence rate for moxifloxacin after effective initial treatment; p_recl: recurrence rate for levofloxacin after effective initial treatment; p_recn: recurrence rate for nemonoxacin after effective initial treatment; p_cpol: initial treatment effect delay rate for levofloxacin; p_cpon; initial treatment effect delay rate for nemonoxacin; m_y_ko: sequential proportion of hospitalizations after successful initial treatment with moxifloxacin).
3.2.2. Probabilistic Sensitivity Analysis
In the scatter plot (Figure 3A), the horizontal axis represents the incremental utilities of nemonoxacin compared with moxifloxacin and levofloxacin and the vertical axis represents the corresponding incremental cost. The dotted lines indicate the values of the two WTPs, respectively, one time and three times per capita GDP. As can be seen from the figure, for moxifloxacin and levofloxacin, 5.28% and 0.03% of all 10,000 points, respectively, at the lower right of the WTP of the three times GDP. That is, compared with moxifloxacin and levofloxacin, nemonoxacin is very cost-effective.
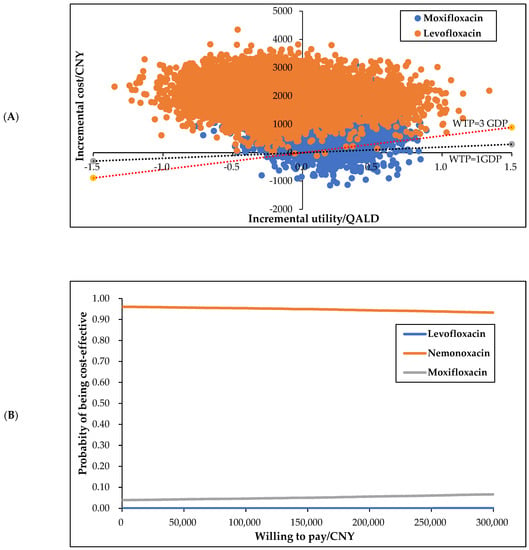
Figure 3.
Scatter plot (A) and cost-effective acceptability plot (B).
The CEAC (Figure 3B) shows the possibility of being the most cost-effective for each option under different WTPs. It can be seen from the figure that when the WTP fluctuated between CNY 0–300,000, nemonoxacin was always the most economical option. The probabilistic sensitivity analysis results are highly consistent with the basic analysis results, confirming that the basic conclusions are highly reliable; that is, compared with moxifloxacin and levofloxacin, nemonoxacin is the most cost-effective option.
3.2.3. Scenario Analysis
Assuming that the proportion of misdiagnosed PTB in CAP patients is 0, the effectiveness and cost-effectiveness of the results were similar to the basic analysis; that is, nemonoxacin was the most cost-effective solution. Further details are presented in Table 2.
4. Discussion
4.1. Literature Review
To our knowledge, there are few articles at present that have focused on the economic evaluation of nemonoxacin in the treatment of CAP and there is no literature discussing the value of antibiotics in the differential diagnosis of CAP and PTB. Xiwen [23] discussed the economics of moxifloxacin and levofloxacin in the initial treatment of mild to moderate CAP in accordance with the perspective of Chinese healthcare system. The clinical efficacy of each drug was obtained from a meta-analysis of previous studies, and a CUA model was constructed as per these findings. They found that moxifloxacin showed better effectiveness and cost-effectiveness when compared with levofloxacin. However, the meta-analysis of the literature used in the study is of low quality, and there were large biases in the blinding and randomization. Lloyd [33] used the MOTIV trial that compared the cost-effectiveness of moxifloxacin and levofloxacin combined with ceftriaxone in the treatment of CAP in the German population using cost minimum analysis from the payer perspective, which could not fully measure the comprehensive value of each drug. Cornelis [35] adopted a crossover trial and used a combination of cost minimum analysis and CUA to explore the economic differences between lactam monotherapy, lactam and macrolide combined therapy, and quinolone monotherapy. Quinolone antibiotics were found to be similar to the other two types with no remarkable differences in cost-effectiveness.
4.2. The Value of Nemonoxacin in CAP and in the Differential Diagnosis of PTB
The value of nemonoxacin in the differential diagnosis of CAP and PTB is reflected in the fact that nemonoxacin is not effective against M. tuberculosis and can therefore isolate potential patients with PTB from people with suspected CAP in the initial treatment stage. Consequently, patients with PTB can be diagnosed and treated in a timely manner, which prevents the delayed diagnosis arising from the masking effect of quinolone drugs on M. tuberculosis and thereby avoids the disease deterioration, utility and cost losses caused by the delayed diagnosis. In addition, the trial results showed that the recurrence rate after nemonoxacin treatment was lower than levofloxacin and moxifloxacin, thus the overall effectiveness for the treatment of CAP is more reliable.
Basic analysis results showed that there is little difference in the effectiveness of the three drugs. Compared with moxifloxacin and levofloxacin, treatment with nemonoxacin resulted in savings of CNY 972 (USD 153) and CNY 1999 (USD 314), respectively. The results of the scenario analysis showed that when misdiagnosed PTB was not considered, the economic advantage of nemonoxacin over the other two groups was still obvious, which verified that nemonoxacin was a cost-effective option compared with levofloxacin and moxifloxacin in the treatment of CAP.
PTB patients misdiagnosed with CAP treated with nemonoxacin, moxifloxacin and levofloxacin were not only cost-saving, but also utility-increasing. For value-in-money, nemonoxacin saved CNY 1852 (USD 291) and CNY 1309 (USD 206) compared with moxifloxacin and levofloxacin, respectively, accounting for approximately 32.7% and 23.1% of the total cost of PTB treatment. Simultaneously, for patients with PTB who were misdiagnosed with CAP, the administration of nemonoxacin, compared with moxifloxacin and levofloxacin, caused an increase of 2.83 QALDs. These findings indicated that non-fluoroquinolone antibiotics were of remarkable value in the differential diagnosis of PTB compared with other fluoroquinolone antibiotics.
4.3. Strengths and Limitations
First, the study found that the use of fluoroquinolone antibiotics in the diagnosis and treatment of CAP patients with unclear symptoms increases the risk of delaying the diagnosis of tuberculosis [12]. Second, in the previous articles studying the economics of CAP drugs, the misdiagnosis of tuberculosis patients had not been considered [18,21,23,31,33,34]. This study considered that a certain proportion of patients with CAP were misdiagnosed with tuberculosis and performed a CUA analysis on this basis. In addition, the clinical path of our research is more comprehensive, and the research results are more in line with the “real world” situation. For example, previous studies have ignored recurrence in patients with successful initial treatment [23]; however, the clinical trial results showed that the effective rate of each drug at Visit 3 was substantially lower than that at Visit 2. Consequently, we believe that in addition to the factors of the change of trial personnel, patients for whom the drug is effective at the initial stage of treatment are likely to relapse in the later stage of treatment or after the end of treatment [34]. Finally, the clinical efficacy data of this study were derived from a multicenter, randomized, double-blind, parallel-controlled randomized controlled trial, which has high reliability and is very suitable for CAP-related evaluations based on the Chinese population.
This study also has certain limitations. First, the relevant costs, such as hospitalization fees, examination fees, and nursing fees, lack a national representative data set; the costs are instead derived from the survey results of experts from different regions with a limited sample size and may not be representative of the whole country. Therefore, the relevant parameters in the model are slightly biased. However, considering that this bias is the same among all groups, it should not affect the analysis results. Second, owing to the lack of utility data for hospitalized patients with CAP, this study adopted the utility value used in hospital-acquired pneumonia-related studies. Considering that the antibiotic treatment plan used and the patients’ conditions are similar [23], we believe that the utilities of these studies are the best source of data. It should be noted that the abovementioned factors in this study have been subjected to sensitivity analyses and the results of these analyses showed that these assumptions should not affect the research conclusions. Third, the indirect costs ignored due to our choice of the healthcare system perspective and inaccessibility of relevant data, may lead to bias in cost estimates. However, due to the short duration of the study, the indirect costs such as loss of work, transportation, and nutrition were roughly the same across groups, so it would not have had an impact on our conclusion.
5. Conclusions
Our study reveals that nemonoxacin is more economical than moxifloxacin and levofloxacin in the treatment of mild to moderate CAP, and we also confirmed the values in money and utility of non-fluoroquinolone antibiotics in the differential diagnosis of pulmonary tuberculosis compared with fluoroquinolone antibiotics. Due to the reduced treatment cost, compared with moxifloxacin and levofloxacin, nemonoxacin is the most cost-effective option in the initial treatment of CAP under the current WTP. For PTB patients misdiagnosed as CAP, treatment with nemonoxacin caused an average saving of CNY 1600 (USD 251.2) compared with fluoroquinolone antibiotics, about 28.3% of total treatment cost of PTB, as well as an increase of 2.83 QALDs in utility. These results may help healthcare system make relevant policies and help clinicians in choosing optimal drugs in treating CAP. Further research based on patient or society perspective with a more precise calculation of both direct and indirect cost is needed to provide additional information about more non-fluoroquinolone and fluoroquinolone antibiotics in the treatment of CAP or misdiagnosed PTB.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19084816/s1. Table S1 Basic information of clinical research. Table S2 Dosing regimen.
Author Contributions
Conceptualization, M.Z. and Z.C.; methodology, M.Z. and Z.C.; software, M.Z.; validation, M.Z., Z.C. and W.T.; formal analysis, M.Z.; investigation, M.Z.; resources, M.Z. and X.P.; data curation, M.Z., Y.Y. and X.P.; writing—original draft preparation, M.Z.; writing—review and editing, M.Z., Z.C. and W.T.; supervision, W.T.; funding acquisition, W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: General Program of National Natural Science Foundation of China (72174207).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. This is a model-based analysis. No formal ethical approval and informed consent are needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Respiratory Medicine Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of community-acquired pneumonia in Chinese adults. Chin. J. Tuberc. Respir. Med. 2016, 39, 253–279. [Google Scholar]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M.; Nakama, T.; Ishida, M.; Morimoto, H.; Nagasaki, Y.; Shiramizu, R.; Hamashige, N.; Chikamori, M.; Yoshida, L.; Ariyoshi, K.; et al. High Incidence of Community-Acquired Pneumonia among Rapidly Aging Population in Japan: A Prospective Hospital-Based Surveillance. Jpn. J. Infect. Dis. 2014, 67, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Pei, Z.; Wang, S.; Feng, J.; Xu, L.; Gao, P.; Cao, B.; Zhan, S. Incidence of community-acquired pneumonia in urban China: A national population-based study. Vaccine 2020, 38, 8362–8370. [Google Scholar] [CrossRef]
- Radovanovic, D.; Sotgiu, G.; Jankovic, M.; Mahesh, P.A.; Marcos, P.J.; Abdalla, M.I.; Di Pasquale, M.F.; Gramegna, A.; Terraneo, S.; Blasi, F.; et al. An international perspective on hospitalized patients with viral community-acquired pneumonia. Eur. J. Intern. Med. 2019, 60, 54–70. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Yunjing, Z.; Zeshuai, W.; Xinwei, D. Value of combined detection of PCT, WBC, and Th1/Th2 in the differential diagnosis of early community—Acquired pneumonia and tuberculosis. J. Clin. Exp. Med. 2021, 20, 1178–1181. [Google Scholar]
- Walker, T.M.; Miotto, P.; Köser, C.U.; Fowler, P.W.; Knaggs, J.; Iqbal, Z.; Hunt, M.; Chindelevitch, L.; Farhat, M.R.; Cirillo, D.M.; et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: A genotypic analysis. Lancet Microbe 2022, 3, e265–e273. [Google Scholar] [CrossRef]
- Song, J.-H.; Oh, W.S.; Kang, C.-I.; Chung, D.R.; Peck, K.R.; Ko, K.S.; Yeom, J.S.; Kim, C.K.; Kim, S.W.; Chang, H.-H.; et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: A prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents 2008, 31, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, L.; Xu, H.; Huang, W. Effect of fluoroquinolone treatment on the diagnosis of pulmonary tuberculosis:clinical analysis of 103cases of tuberculosis misdiagnosed as community-acquired pneumonia. Chin. J. Infect. Chemother. 2013, 13, 261–265. [Google Scholar]
- Wenjie, H.; Li, L. Should fluoroquanolones be the first-line empirical antibiotics in the treatment of community-acquired pneumonia in China: Right or not? Chin. J. Lung Dis. 2010, 3, 155–157. [Google Scholar]
- Min, H.; Xiaohua, Q. Nemonoxacin: A novel nonfluorinated quinolone antibiotic. Chin. J. Infect. Chemother. 2018, 18, 663–671. [Google Scholar]
- Tweed, C.D.; Wills, G.H.; Crook, A.M.; Amukoye, E.; Balanag, V.; Ban, A.Y.; Bateson, A.L.; Betteridge, M.C.; Brumskine, W.; Caoili, J.; et al. A partially randomised trial of pretomanid, moxifloxacin and pyrazinamide for pulmonary TB. Int. J. Tuberc. Lung Dis. 2021, 25, 305–314. [Google Scholar] [CrossRef]
- Laohapojanart, N.; Ratanajamit, C.; Kawkitinarong, K.; Srichana, T. Efficacy and safety of combined isoniazid-rifampicin-pyrazinamide-levofloxacin dry powder inhaler in treatment of pulmonary tuberculosis: A randomized controlled trial. Pulm. Pharmacol. Ther. 2021, 70, 102056. [Google Scholar] [CrossRef]
- Anzueto, A.; Niederman, M.S.; Pearle, J.; Restrepo, M.I.; Heyder, A.; Choudhri, S.H. Community-Acquired Pneumonia Recovery in the Elderly (CAPRIE): Efficacy and Safety of Moxifloxacin Therapy versus That of Levofloxacin Therapy. Clin. Infect. Dis. 2006, 42, 73–81. [Google Scholar] [CrossRef]
- Pliakos, E.E.; Andreatos, N.; Tansarli, G.S.; Ziakas, P.D.; Mylonakis, E. The Cost-Effectiveness of Corticosteroids for the Treatment of Community-Acquired Pneumonia. Chest 2019, 155, 787–794. [Google Scholar] [CrossRef]
- Friedman, H.; Song, X.; Crespi, S.; Navaratnam, P. Comparative Analysis of Length of Stay, Total Costs, and Treatment Success between Intravenous Moxifloxacin 400 mg and Levofloxacin 750 mg among Hospitalized Patients with Community-Acquired Pneumonia. Value Health 2009, 12, 1135–1143. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Zhou, F.; Li, H.; Xing, X.; Han, X.; Wang, Y.; Zhang, C.; Suo, L.; Wang, J.; Yu, G.; et al. Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: A retrospective multicentre survey. BMJ Open 2018, 8, e018709. [Google Scholar] [CrossRef]
- Torres, A.; Garau, J.; Arvis, P.; Carlet, J.; Choudhri, S.; Kureishi, A.; Le Berre, M.; Lode, H.; Winter, J.; Read, R.C.; et al. Moxifloxacin Monotherapy Is Effective in Hospitalized Patients with Community-Acquired Pneumonia: The MOTIV Study—A Randomized Clinical Trial. Clin. Infect. Dis. 2008, 46, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, Y.; Yang, Z.; Wang, H.; Jin, S.; Jiao, Y.; Metersky, M.; Huang, Y. Initial empiric antibiotic therapy for community-acquired pneumonia in Chinese hospitals. Clin. Microbiol. Infect. 2018, 24, 658.e1–658.e6. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Han, Y.; Jian, Y.; Chen, L.; Xuan, J. Clinical Benefits and Cost-Effectiveness of Moxifloxacin as Initial Treatment for Community-Acquired Pneumonia: A Meta-Analysis and Economic Evaluation. Clin. Ther. 2021, 43, 1894–1909.e1. [Google Scholar] [CrossRef] [PubMed]
- Lapointe-Shaw, L.; Voruganti, T.; Kohler, P.; Thein, H.-H.; Sander, B.; McGeer, A. Cost-effectiveness analysis of universal screening for carbapenemase-producing Enterobacteriaceae in hospital inpatients. Eur. J. Clin. Microbiol. 2017, 36, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, G.; Jiang, X.; Yao, J.; You, J.H. Cost-effectiveness of video-observed therapy for ambulatory management of active tuberculosis during the COVID-19 pandemic in a high-income country. Int. J. Infect. Dis. 2021, 113, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chabala, C.; Turkova, A.; Thomason, M.J.; Wobudeya, E.; Hissar, S.; Mave, V.; van der Zalm, M.; Palmer, M.; Kapasa, M.; Bhavani, P.K.; et al. Shorter treatment for minimal tuberculosis (TB) in children (SHINE): A study protocol for a randomised controlled trial. Trials 2018, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Chinese Medical Association. Guidelines for primary care of pulmonary tuberculosis (2018). Chin. J. Gen. Pract. 2019, 18, 709–717. [Google Scholar]
- Machlaurin, A.; Dolk, F.C.K.; Setiawan, D.; Van Der Werf, T.S.; Postma, M.J. Cost-Effectiveness Analysis of BCG Vaccination against Tuberculosis in Indonesia: A Model-Based Study. Vaccines 2020, 8, 707. [Google Scholar] [CrossRef]
- Hamel, M.B.; Phillips, R.S.; Davis, R.B.; Teno, J.; Connors, A.F.; Desbiens, N.; Lynn, J.; Dawson, N.V.; Fulkerson, W.; Tsevat, J. Outcomes and cost-effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am. J. Med. 2000, 109, 614–620. [Google Scholar] [CrossRef]
- Egger, M.E.; Myers, J.A.; Arnold, F.W.; Pass, L.A.; Ramirez, J.A.; Brock, G.N. Cost effectiveness of adherence to IDSA/ATS guidelines in elderly patients hospitalized for Community-Aquired Pneumonia. BMC Med. Inform. Decis. Mak. 2016, 16, 34. [Google Scholar] [CrossRef]
- Lim, V.W.; Wee, H.L.; Lee, P.; Lin, Y.; Tan, Y.R.; Tan, M.X.; Lin, L.W.; Yap, P.; Chee, C.B.; Barkham, T.; et al. Cross-sectional study of prevalence and risk factors, and a cost-effectiveness evaluation of screening and preventive treatment strategies for latent tuberculosis among migrants in Singapore. BMJ Open 2021, 11, e050629. [Google Scholar] [CrossRef] [PubMed]
- Guoen, L. Chinese Guidelines for Pharmacoeconomics Evaluation 2020; China Market Press: Beijing, China, 2020. [Google Scholar]
- Lloyd, A.; Holman, A.; Evers, T. A cost-minimisation analysis comparing moxifloxacin with levofloxacin plus ceftriaxone for the treatment of patients with community-acquired pneumonia in Germany: Results from the MOTIV trial. Curr. Med. Res. Opin. 2008, 24, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Pliakos, E.E.; Ziakas, P.D.; Mylonakis, E. The Cost-effectiveness of Cefazolin Compared with Antistaphylococcal Penicillins for the Treatment of Methicillin-Sensitive Staphylococcus aureus Bacteremia. Open Forum Infect. Dis. 2021, 8, ofab476. [Google Scholar] [CrossRef] [PubMed]
- van Werkhoven, C.H.; Postma, D.F.; Mangen, M.J.; Oosterheert, J.J.; Bonten, M.J. Cost-effectiveness of antibiotic treatment strategies for community-acquired pneumonia: Results from a cluster randomized cross-over trial. BMC Infect. Dis. 2017, 17, 52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).