Non-Invasive Prenatal Testing (NIPT) Implementation in Japan: A Comparison with the United Kingdom, Germany, Italy, Sweden, and Taiwan
Abstract
1. Introduction
2. Methods
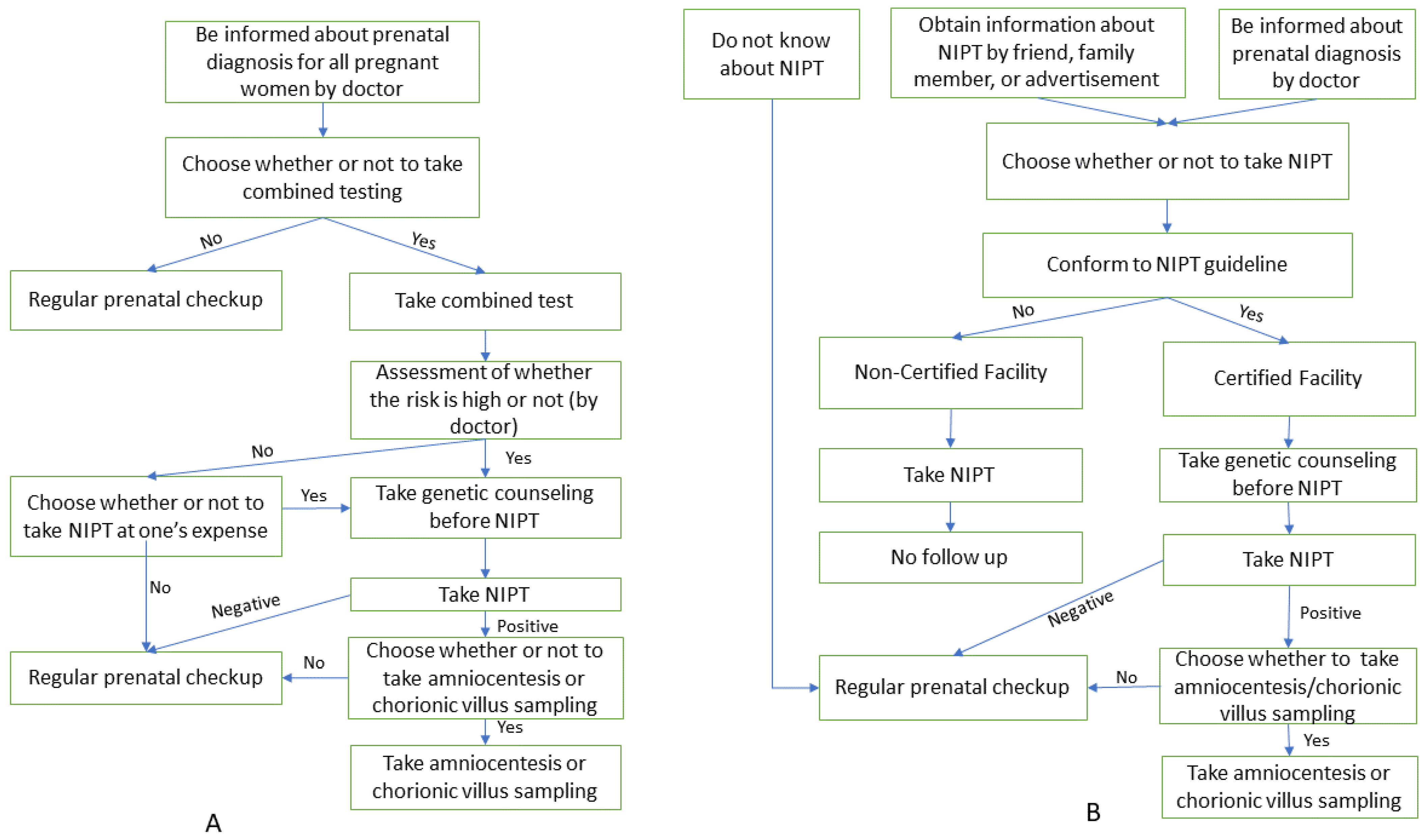
3. Results
3.1. Policy on Prenatal Screening
- (a)
- Provided information on prenatal diagnosis for all pregnant women
- (b)
- Provided primary prenatal screening for all pregnant women
3.2. Abortion Law: Allowed Abortion for Fetal Chromosomal Abnormality by Law
3.3. NIPT
- (a)
- Issued guidelines on NIPT
- (b)
- NIPT fee was covered by public insurance
- (c)
- Most expensive NIPT expenditure
3.4. Managing Organizations/Consortiums
4. Discussion
4.1. Policy on Prenatal Screening
4.2. Abortion Law
4.3. NIPT
- (a)
- Guideline
- (b)
- Public insurance and expenditure
- (c)
- Managing organizations/consortium
4.4. Limitation and Imlementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Allyse, M.; Minear, M.A.; Berson, E.; Sridhar, S.; Rote, M.; Hung, A.; Chandrasekharan, S. Non-invasive prenatal testing: A review of international implementation and challenges. Int. J. Womens Health 2015, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Non-invasive Prenatal Testing for Trisomies 21, 18, and 13, Sex Chromosome Aneuploidies, and Microdeletions: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2019, 19, 1–166. [Google Scholar]
- Gregg, A.R.; Gross, S.J.; Best, R.G.; Monaghan, K.G.; Bajaj, K.; Skotko, B.G.; Thompson, B.H.; Watson, M.S. ACMG statement on non-invasive prenatal screening for fetal aneuploidy. Genet. Med. 2013, 15, 395–398. [Google Scholar] [CrossRef]
- Benn, P.; Borrell, A.; Crossley, J.; Cuckle, H.; Dugoff, L.; Gross, S.; Johnson, J.A.; Maymon, R.; Odibo, A.; Schielen, P.; et al. Position statement from the Aneuploidy Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat. Diagn. 2013, 33, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, M.; Goh, E.S.; Ungar, W.J.; Little, J.; Carroll, J.C.; Okun, N.; Huang, T.; Rousseau, F.; Dougan, S.D.; et al. Non-invasive Prenatal Testing for Trisomies 21, 18, and 13, Sex Chromosome Aneuploidies, and Microdeletions in Average-Risk Pregnancies: A Cost-Effectiveness Analysis. J. Obstet. Gynaecol. Can. 2020, 42, 740–749.e12. [Google Scholar] [CrossRef] [PubMed]
- Samura, O.; Sekizawa, A.; Suzumori, N.; Sasaki, A.; Wada, S.; Hamanoue, H.; Hirahara, F.; Sawai, H.; Nakamura, H.; Yamada, T.; et al. Current status of non-invasive prenatal testing in Japan. J. Obstet. Gynaecol. Res. 2017, 43, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Suzumori, N.; Ebara, T.; Kumagai, K.; Goto, S.; Yamada, Y.; Kamijima, M.; Sugiura-Ogasawara, M. Non-specific psychological distress in women undergoing non-invasive prenatal testing because of advanced maternal age. Prenat. Diagn. 2014, 34, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, A. NIPT: Non-Invasive Prenatal Testing Japan: Ministry of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/11908000/000559098.pdf (accessed on 8 March 2020).
- Guidelines for New Prenatal Genetic Testing Using Maternal Blood: Ethical Committee on the Japanese Society of Obstetrics and Gynecology. 2019. Available online: https://www.jsog.or.jp/modules/news_m/index.php?content_id=843 (accessed on 4 May 2020).
- Fetal Anomaly Screening Programme Handbook England: Public Health England. 2015. Available online: https://www.gov.uk/government/publications/fetal-anomaly-screening-programme-handbook (accessed on 3 March 2020).
- Gynecology NFoSoOa. Analysis of Foetal DNA in the Woman’s Blood: Non-Invasive Prenatal Testing (NIPT) for Trisomy 13, 18 and 21. 2016. Available online: http://www.nfog.org/files/guidelines/NIPT%202016%2006%2005%20.pdf (accessed on 4 May 2020).
- Rummer, A.; Sieben, W.; Mosch, C.; Assall, O.; Sauerland, S. Nicht invasive Pränataldiagnostik mittels molekulargenetischer Tests (NIPT) zur Erkennung der Trisomien 13, 18 und 21. Med. Genet. 2019, 31, 275–282. [Google Scholar] [CrossRef]
- Policy Statement on Noninvasive Testing of Fetal Aneuploidy Using Maternal Blood. 2013. Available online: https://www.jsog.or.jp/news/pdf/guidelineForNIPT_20130309.pdf (accessed on 5 May 2020).
- Japan Ministry of Health, L.W. Maternal Health Act. 1996. Available online: https://www.mhlw.go.jp/web/t_doc?dataId=80120000&dataType=0&pageNo=1. (accessed on 5 May 2020).
- The Human Fertilisation and Embryology Authority. Human Fertilisation and Embryology Act. 2009. Available online: http://www.legislation.gov.uk/ukpga/1990/37/crossheading/the-human-fertilisation-and-embryology-authority-its-functions-and-procedure (accessed on 5 May 2020).
- Karow, J. Buoyed by National Screening Committee Recommendation, UK Providers Expect Demand for NIPT to Grow. 2016. Available online: https://www.genomeweb.com/molecular-diagnostics/buoyed-national-screening-committee-recommendation-uk-providers-expect-demand (accessed on 4 May 2020).
- FMoJaCPiG. Gesetzüber genetische Untersuchungen bei Menschen. In German. 2009. Available online: https://www.gesetze-im-internet.de/gendg/ (accessed on 4 May 2020).
- Bundestag. Schwangerschaftsabbruch. In German. 1995. Available online: https://www.buzer.de/s1.htm?g=StGB&a=218-219 (accessed on 5 May 2020).
- Italy HHCo. DNA-based Non-Invasive Prenatal Testing—NIPT. 2015. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2438_allegato.pdf (accessed on 23 May 2021).
- New Birth Path, the Combined Test and the NIPT Test. 2019. Available online: https://www.toscana-notizie.it/-/nuovo-percorso-nascita-il-test-combinato-e-il-test-nipt (accessed on 8 March 2021).
- Italian Republic. Law 22 May 1978, n. 194. Available online: https://www.ieb-eib.org/en/law/early-life/unclassified/law-abortion-in-italy-215.html (accessed on 5 May 2020).
- Law (2006: 351) on Genetic Integrity etc. 2006. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-2006351-om-genetisk-integritet-mm_sfs-2006-351 (accessed on 5 May 2020).
- Socialstyrelsen. Fosterdiagnostik och Preimplantatorisk Genetisk Diagnostic. 2012. Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/foreskrifter-och-allmanna-rad/2012-12-34.pdf (accessed on 3 March 2021).
- Ministry of Social. Abortion Law (1974: 595). 1974. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/abortlag-1974595_sfs-1974-595 (accessed on 4 May 2020).
- GU. Prise. In Swedish. 2020. Available online: https://www.gynhalsan.se/priser-24731650 (accessed on 5 May 2020).
- Fox, D.; Prenatal Screening Policy in International Perspective: Lessons from Israel, Cyprus, Taiwan, China, and Singapore. Yale J. Health Policy Law Ethics. 2010. Available online: http://hdl.handle.net/20.500.13051/6104 (accessed on 4 May 2020).
- Ministry of Health and Welfare. The Genetic Health Act. 2009. Available online: https://law.moj.gov.tw/Eng/LawClass/LawAll.aspx?PCode=L0070001 (accessed on 5 May 2020).
- SG. SOFIVA NIPS. 2020. Available online: https://www.sofivagenomics.com.tw/en/Product/1/2/item/40 (accessed on 5 May 2020).
- Gemeinsamer Bundesausschuss. G-BA update No. 4/2019. Available online: https://www.g-ba.de/service/fachnews/164/ (accessed on 5 May 2020).
- Massamama. NIPT. Available online: https://vasamamma.se/nipt/ (accessed on 5 May 2020).
- Lee, E., II. Young People’s Attitudes to Abortion for Abnormality. Fem. Psychol. 2000, 10, 396–399. [Google Scholar] [CrossRef]
- Nicolaides, K.H. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn. 2011, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yamada, T.; Tanaka, S.; Sekizawa, A.; Hirose, T.; Suzumori, N.; Kaji, T.; Kawaguchi, S.; Hasuo, Y.; Nishizawa, H.; et al. Evaluation of the clinical performance of noninvasive prenatal testing at a Japanese laboratory. J. Obstet. Gynaecol. Res. 2021, 47, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Bowman-Smart, H.; Savulescu, J.; Mand, C.; Gyngell, C.; Pertile, M.D.; Lewis, S.; Delatycki, M.B. ‘Small cost to pay for peace of mind’: Women’s experiences with non-invasive prenatal testing. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; White, K.; Shabbeer, J.; Schmid, M. Maternal age trends support uptake of non-invasive prenatal testing (NIPT) in the low-risk population. J. Matern. Fetal. Neonatal Med. 2019, 32, 4039–4042. [Google Scholar] [CrossRef] [PubMed]
- Minear, M.A.; Lewis, C.; Pradhan, S.; Chandrasekharan, S. Global perspectives on clinical adoption of NIPT. Prenat Diagn. 2015, 35, 959–967. [Google Scholar] [CrossRef] [PubMed]
| Japan [9,10,14,15] | UK [11,16,17] | Germany [13,18,19] | Italy [20,21,22] | Sweden [12,23,24,25,26] | Taiwan [27,28,29] | ||
|---|---|---|---|---|---|---|---|
| (1) Policy on prenatal screening | Provided information on prenatal diagnosis for all pregnant women | No | Yes | Yes | Yes | Yes | Yes |
| Provided primary prenatal screening for all pregnant women | No | Yes | Yes | Yes | Yes | Yes | |
| (2) Abortion law | Allowed abortion for fetal chromosomal abnormality by law | No | Yes | Yes | No | Yes | Yes |
| (3) NIPT | (1)-a. Guideline (government) | No | Yes | Yes | Yes | No (evaluated report) | No |
| (1)-b. Guideline (academic society) | Yes | No | No | No | Yes | No | |
| (2) Public health insurance for NIPT | No | Yes | Yes | Yes (partially) | Yes | No | |
| (3) Cost for NIPT (trisomy 13, 18 and 21) without insurance | Certified facilities 190,000 yen | 40,000–60,000 yen | No information | 30,000–50,000 yen | 50,000–60,000 yen | 80,000–130,000 yen | |
| Non-certified facilities 160,000 yen | |||||||
| (4) Managing organization/consortium | Formed NIPT consortium | Yes | No | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, M.; Linh, L.K.; M. Sayed, A.; Imoto, A.; Sato, M.; Dila, K.A.S.; Huy, N.T.; Moji, K. Non-Invasive Prenatal Testing (NIPT) Implementation in Japan: A Comparison with the United Kingdom, Germany, Italy, Sweden, and Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 16404. https://doi.org/10.3390/ijerph192416404
Takahashi M, Linh LK, M. Sayed A, Imoto A, Sato M, Dila KAS, Huy NT, Moji K. Non-Invasive Prenatal Testing (NIPT) Implementation in Japan: A Comparison with the United Kingdom, Germany, Italy, Sweden, and Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(24):16404. https://doi.org/10.3390/ijerph192416404
Chicago/Turabian StyleTakahashi, Mayo, Le Khac Linh, Ahmad M. Sayed, Atsuko Imoto, Miho Sato, Kadek Agus Surya Dila, Nguyen Tien Huy, and Kazuhiko Moji. 2022. "Non-Invasive Prenatal Testing (NIPT) Implementation in Japan: A Comparison with the United Kingdom, Germany, Italy, Sweden, and Taiwan" International Journal of Environmental Research and Public Health 19, no. 24: 16404. https://doi.org/10.3390/ijerph192416404
APA StyleTakahashi, M., Linh, L. K., M. Sayed, A., Imoto, A., Sato, M., Dila, K. A. S., Huy, N. T., & Moji, K. (2022). Non-Invasive Prenatal Testing (NIPT) Implementation in Japan: A Comparison with the United Kingdom, Germany, Italy, Sweden, and Taiwan. International Journal of Environmental Research and Public Health, 19(24), 16404. https://doi.org/10.3390/ijerph192416404







