Surgical Treatment of Vitiligo
Abstract
:1. Introduction
2. Methods for Seeking References
3. Dermatosurgery
4. Tissue Grafts
4.1. Mini-Punch Graft (MPG)
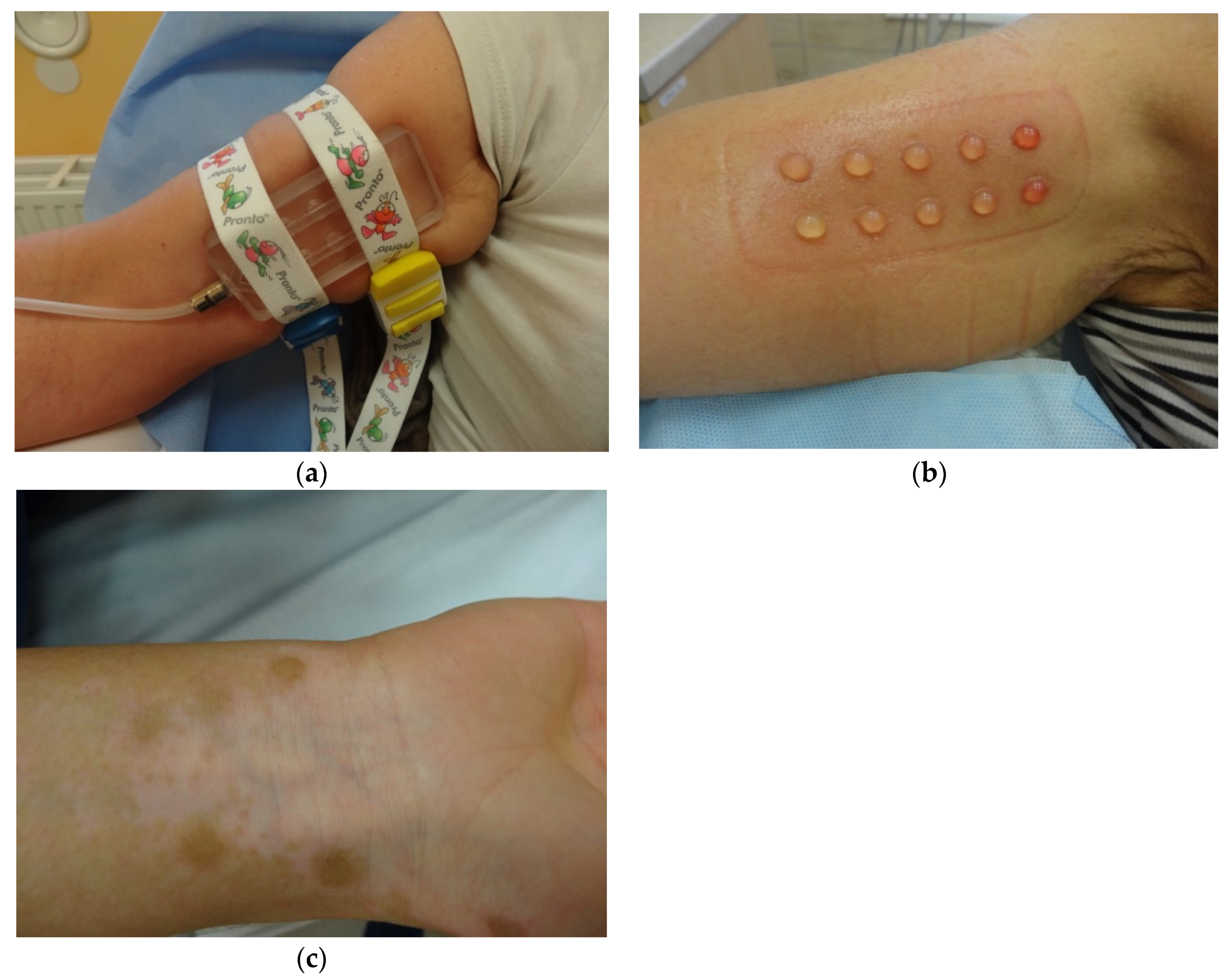
4.2. Suction Blister Epidermal Grafting (SBEG)
4.3. Split-Thickness Skin Grafting (STSG)
4.4. Epidermal Curettage Technique (ECT)
4.5. Smash Grafting
4.6. Flip-Top Grafting
4.7. Hair Follicle Graft
5. Cellular Grafts
5.1. Cultured Melanocyte Graft
5.2. Cultured Epidermal Graft
5.3. Noncultured Melanocyte-Keratinocyte Suspension
5.4. Noncultured Follicular Root Sheath Suspension
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaei, N.; Gavalas, N.; Weetman, A.; Kemp, E. Autoimmunity as an aetiological factor in vitiligo. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Oiso, N.; Fukai, K.; Kabashima, K.; Kawada, A.; Suzuki, T. Generalized vitiligo and associated autoimmune diseases in Japanese patients and their families. Allergol. Int. 2011, 60, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kwon, H.S.; Jung, H.M.; Kim, G.M.; Bae, J.M. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: A nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Krishnaram, A.S.; Saigal, A.; Adityan, B. Alopecia areata—Vitiligo overlap syndrome: An emerging clinical variant. Indian J. Dermatol. Venereol. Lepropol. 2013, 79, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Ongenae, K.; Beelaert, L.; Geel, N.; Naeyaert, J.-M. Psychosocial effects of vitiligo. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qiu, D.; Yang, H.; Liu, W. The prevalence and odds of depression in patients with vitiligo: A meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1343–1351. [Google Scholar] [CrossRef]
- Hamidizadeh, N.; Ranjbar, S.; Ghanizadeh, A.; Parvizi, M.M.; Jafari, P. Evaluating prevalence of depression, anxiety and hopelessness in patients with Vitiligo on an Iranian population. Health Qual. Life Outcomes 2020, 18, 20. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef]
- Helalat, M.; Rawashdeh, B.; Odiebat, H.; Smadi, R.; Zyod, I. Punch Minigrafting for Stable Vitiligo: Our Experience at the Jordanian Royal Medical Services. J. R. Med. Serv. 2012, 19, 81–86. [Google Scholar]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.; Jouary, T.; et al. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2012, 168, 5–19. [Google Scholar] [CrossRef]
- Ashwini, P.K.; Sushmitha, D.J.; Veeranna, S. Vitiligo with special emphasis on vitiligo surgery. Arch. Med. Health Sci. 2020, 8, 140. [Google Scholar] [CrossRef]
- Dillon, A.B.; Sideris, A.; Hadi, A.; Elbuluk, N. Advances in Vitiligo: An Update on Medical and Surgical Treatments. J. Clin. Aesthet. Dermatol. 2017, 10, 15–28. [Google Scholar]
- Khunger, N.; Kathuria, S.D.; Ramesh, V. Tissue grafts in vitiligo surgery—Past, present, and future. Indian J. Dermatol. 2009, 54, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.; El-Barbary, R.; Moftah, N. Updates in surgical treatment of vitiligo. J. Recent Adv. Med. 2021, 2, 118–127. [Google Scholar] [CrossRef]
- Czajkowski, R.; Placek, W.; Flisiak, I.; Krasowska, D.; Maj, J.; Marchlewicz, M.; Reich, A.; Wolska, H.; Rudnicka, L. Vitiligo. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Dermatol. Rev./Przegląd Dermatol. 2019, 106, 1–15. [Google Scholar] [CrossRef]
- Eleftheriadou, V.; Atkar, R.; Batchelor, J.; McDonald, B.; Novakovic, L.; Patel, J.V.; Ravenscroft, J.; Rush, E.; Shah, D.; Shah, R.; et al. British Association of Dermatologists guidelines for the management of people with vitiligo 2021. Br. J. Dermatol. 2021, 186, 18–29. [Google Scholar] [CrossRef]
- Falabella, R. Repigmentation of leukoderma by minigrafts of normally pigmented, autologus skin. J. Dermatol. Surg. Oncol. 1978, 4, 916–919. [Google Scholar] [CrossRef]
- Chandrashekar, B.; Madura, C.; Varsha, D. Autologous mini punch grafting: An experience of using motorized power punch in 10 patients. J. Cutan. Aesthetic Surg. 2014, 7, 42. [Google Scholar] [CrossRef]
- Njoo, M.D.; Westerhof, W.; Bos, J.D.; Bossuyt, P.M.M. A Systematic Review of Autologous Transplantation Methods in Vitiligo. Arch. Dermatol. 1998, 134, 1543–1549. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, K. Evolution and evaluation of autologous mini punch grafting in vitiligo. Indian J. Dermatol. 2009, 54, 159–167. [Google Scholar] [CrossRef]
- Mohammad, T.F.; Hamzavi, I.H. Surgical Therapies for Vitiligo. Dermatol. Clin. 2017, 35, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, K.; Malakar, S.; Sarma, N.; Banerjee, U. Repigmentation of vitiligo with punch grafting and narrow-band UV-B (311 nm)—A prospective study. Int. J. Dermatol. 2006, 45, 649–655. [Google Scholar] [CrossRef]
- Salem, S.A.M.; Fezeaa, T.A.; El Khazragy, N.; Soltan, M.Y. Effect of platelet-rich plasma on the outcome of mini-punch grafting procedure in localized stable vitiligo: Clinical evaluation and relation to lesional basic fibroblast growth factor. Dermatol. Ther. 2021, 34, e14738. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T.; Enami, H. Excellent color-matched repigmentation of human vitiligo can be obtained by mini-punch grafting using a machine in combination with ultraviolet therapy. Dermatol. Sin. 2018, 36, 203–206. [Google Scholar] [CrossRef]
- Ragab, M.; El Zagh, O.; Farid, C. Transverse Needling After Autologous Mini-Punch Grafts Improves Repigmentation in Stable Non-Segmental Vitiligo. Clin. Cosmet. Investig. Dermatol. 2021, 14, 827–835. [Google Scholar] [CrossRef]
- Kiistala, U.; Mustakallio, K.K. In-vivo separation of epidermis by production of suction blisters. Lancet 1964, 2, 1444–1445. [Google Scholar] [CrossRef]
- Kar, B.R.; Raj, C. Suction Blister Epidermal Grafting for Vitiligo Involving Angles of Lip: Experience of 112 Patients. J. Cutan. Aesthet. Surg. 2018, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, F.; Kaufmann, R. Suction blister epidermal graft (SBEG)—An easy way to apply this method. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 468–471. [Google Scholar] [CrossRef]
- Anbar, T.S.; El-Fakahany, H.M.; El-Khayyat, M.A.; Abdel-Rahman, A.T.; Saad, E.K. Factors affecting the outcome of the suction blisters using two different harvesting techniques in vitiligo patients. J. Cosmet. Dermatol. 2020, 19, 1723–1729. [Google Scholar] [CrossRef]
- Al-Hadidi, N.; Griffith, J.L.; Al-Jamal, M.S.; Hamzavi, I. Role of Recipient-site Preparation Techniques and Post-operative Wound Dressing in the Surgical Management of Vitiligo. J. Cutan. Aesthet. Surg. 2015, 8, 79–87. [Google Scholar] [CrossRef]
- Anbar, T.S.; Moftah, N.H.; El-Khayyat, M.A.M.; El-Fakahany, H.M.; Abdel-Rahman, A.T.; Saad, E.K. Syringes versus Chinese cups in harvesting suction-induced blister graft: A randomized split-body study. Int. J. Dermatol. 2018, 57, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Shroff, S.; Gupta, S. Modified technique of suction blistering for epidermal grafting in vitiligo. Int. J. Dermatol. 1999, 38, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.J.; Edmondson, S.-J.; Bystrzonowski, N.; Hachach-Haram, N.; Kanapathy, M.; Richards, T.; Mosahebi, A. The CelluTome epidermal graft-harvesting system: A patient-reported outcome measure and cost evaluation study. Int. Wound J. 2017, 14, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachach-Haram, N.; Bystrzonowski, N.; Kanapathy, M.; Smith, O.; Harding, K.; Mosahebi, A.; Richards, T. A prospective, multicentre study on the use of epidermal grafts to optimise outpatient wound management. Int. Wound J. 2017, 14, 241–249. [Google Scholar] [CrossRef]
- Janowska, A.; Dini, V.; Panduri, S.; Macchia, M.; Oranges, T.; Romanelli, M. Epidermal skin grafting in vitiligo: A pilot study. Int. Wound J. 2016, 13, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Iwanowski, T.; Szlązak, P.; Rustowska, A.; Sokołowska-Wojdyło, M. Efficacy of suction blister epidermal grafting with concomitant phototherapy in vitiligo treatment. Postepy Dermatol. Allergol. 2018, 35, 592–598. [Google Scholar] [CrossRef]
- Hann, S.K.; Im, S.; Bong, H.W.; Park, Y.K. Treatment of stable vitiligo with autologous epidermal grafting and PUVA. J. Am. Acad. Dermatol. 1995, 32, 943–948. [Google Scholar] [CrossRef]
- Kohlhauser, M.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P. Historical Evolution of Skin Grafting—A Journey through Time. Medicina 2021, 57, 348. [Google Scholar] [CrossRef]
- Mulekar, S.V.; Isedeh, P. Surgical interventions for vitiligo: An evidence-based review. Br. J. Dermatol. 2013, 169, 57–66. [Google Scholar] [CrossRef]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. [Updated 31 July 2020]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551561/ (accessed on 10 February 2022).
- Khandapur, S.; Sharma, V.K.; Manchanda, Y. Comparison of mini punch grafting versus split thickness grafting in chronic stable vitiligo. Dermatol. Surg. 2005, 31, 436–441. [Google Scholar] [CrossRef]
- Sameem, F.; Sultan, S.J.; Ahmad, Q.M. Split thickness skin grafting in patients with stable vitiligo. J. Cutan. Aesthet. Surg. 2011, 4, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Lekshmipriya, K. A comparative study of efficacy of split-thickness skin grafting versus autologous melanocyte transfer in the management of stable vitiligo. Med. J. Armed Forces India 2020. [Google Scholar] [CrossRef]
- Machado Filho, C.D.; Timoner, F.R. Epidermal curettage technique (ECT) for tissue harvest from the donor area for melanocyte autologous grafting in cases of vitiligo. Bras. Dermatol. 2014, 89, 681–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado Filho, C.D.; Almeida, F.A.; Proto, R.S.; Landman, G. Vitiligo: Analysis of grafting versus curettage alone, using melanocyte morphology and reverse transcriptase polymerase chain reaction for tyrosinase mRNA. Sao Paulo Med. J. 2005, 123, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Malhotra, S.K.; Kaur, T. Comparative Evaluation of Efficacy of Non-cultured Epidermal Cell Suspension and Epidermal Curettage in Stable Vitiligo. J. Cutan. Aesthet. Surg. 2021, 14, 32–40. [Google Scholar] [CrossRef]
- Kachhawa, D.; Kalla, G. Keratinocyte-melanocyte graft technique followed by PUVA therapy for stable vitiligo. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 622–624. [Google Scholar] [CrossRef]
- Krishnan, A.; Kar, S. Smashed skin grafting or smash grafting—A novel method of vitiligo surgery. Int. J. Dermatol. 2012, 51, 1242–1247. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Krishnan, A.; Singh, S. Smash Grafting. Vitiligo 2018, 28, 261–265. [Google Scholar] [CrossRef]
- McGovern, T.W.; Bolognia, J.; Leffell, D.J. Flip-Top Pigment Transplantation: A Novel Transplantation Procedure for the Treatment of Depigmentation. Arch. Dermatol. 1999, 135, 1305–1307. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Garg, V.K.; Sarkar, R.; Relhan, V. Comparative Study of Flip-top Transplantation and Punch Grafting in Stable Vitiligo. Dermatol. Surg. 2013, 39, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, L.Y.; Wang, G.C. Role of hair follicles in the repigmentation of vitiligo. J. Investig. Dermatol. 1991, 97, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staricco, R.G. Amelanotic melanocytes in the outer root sheath of the human hair follicle. A preliminary report. J. Investig. Dermatol. 1959, 33, 295–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staricco, R.G.; Miller-Milinska, A. Activation of the amelanotic melanocytes in the outer root sheath of the hair follicle following ultra violet rays exposure. J. Investig. Dermatol. 1962, 39, 163–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, P.; Sacchidanand, S.; Nataraj, H.V.; Savitha, A.S. A Study of Hair Follicular Transplantation as a Treatment Option for Vitiligo. J. Cutan. Aesthet. Surg. 2015, 8, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.R. Surgical Treatment for Vitiligo Through Hair Follicle Grafting: How to Make it Easy. Dermatol. Surg. 2001, 27, 685–686. [Google Scholar] [CrossRef]
- Zokaei, S.; Farhud, D.D.; Keykhaei, M.; Zarif Yeganeh, M.; Rahimi, H.; Moravvej, H. Cultured Epidermal Melanocyte Transplantation in Vitiligo: A Review Article. Iran J. Public Health 2019, 48, 388–399. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.Z.; Shi, H.X.; Yang, Y.H.; Tian, T.; Wang, L. Melanocyte spheroids are formed by repetitive long-term trypsinization. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 258–265. [Google Scholar] [CrossRef]
- Pandya, V.; Parmar, K.S.; Shah, B.J.; Bilimoria, F.E. A study of autologous melanocyte transfer in treatment of stable vitiligo. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 393–397. [Google Scholar] [CrossRef]
- O’Connor, N.; Mulliken, J.; Banks-Schlegel, S.; Kehinde, O.; Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981, 317, 75–78. [Google Scholar] [CrossRef]
- Pianigiani, E.; Risulo, M.; Andreassi, A.; Taddeucci, P.; Ierardi, F.; Andreassi, L. Autologous epidermal cultures and narrow-band ultraviolet B in the surgical treatment of vitiligo. Dermatol. Surg. 2006, 31, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Plott, R.T.; Brysk, M.M.; Newton, R.C.; Raimer, S.S.; Rajaraman, S. A Surgical Treatment for Vitiligo: Autologous Cultured-Epithelial Grafts. J. Dermatol. Surg. Oncol. 1989, 15, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, Y.; Surleve-Bazeille, J.E. Autologous grafting with noncultured melanocytes: A simplified method for treatment of depigmented lesions. J. Am. Acad. Dermatol. 1992, 26 Pt 1, 191–194. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, X.; Hong, W.; Fu, L.; Qian, G.; Xu, A.E. A retrospective study of long term follow-up of 2283 vitiligo patients treated by autologous, non-cultured melanocyte-keratinocyte transplantation. Aging 2021, 13, 5415–5425. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, D.; Esmat, S. Autologous non-cultured melanocyte–keratinocyte transplantation in the treatment of vitiligo: Patient selection and perspectives. Clin. Cosmet. Investig. Dermatol. 2018, 11, 521–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, V.S.; van den Bol, L.L.C.; van Geel, N.; Bekkenk, M.W.; Luiten, R.M.; Wolkerstorfer, A. Donor to recipient ratios in the surgical treatment of vitiligo and piebaldism: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, B.M.; Esmat, S.; Bassiouny, D.; Zaki, N.S.; Sobhi, R.; Saleh, M.; Abdel-Halim, D.; Hegazy, R.; Gawdat, H.; Samir, N.; et al. Effect of Procedural-Related Variables on Melanocyte–Keratinocyte Suspension Transplantation in Nonsegmental Stable Vitiligo. Dermatol. Surg. 2017, 43, 226–235. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Griffith, J.L.; Huggins, R.H.; Henderson, M.D.; Kerr, H.A.; Jacobsen, G.; Mulekar, S.V.; Lim, H.W.; Hamzavi, I.H. Long-term follow-up of patients undergoing autologous noncultured melanocyte-keratinocyte transplantation for vitiligo and other leukodermas. J. Am. Acad. Dermatol. 2017, 77, 318–327. [Google Scholar] [CrossRef]
- Mohanty, S.; Kumar, A.; Dhawan, J.; Sreenivas, V.; Gupta, S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br. J. Dermatol. 2011, 164, 1241–1246. [Google Scholar] [CrossRef]
- Kumaresan, M. Single-hair follicular unit transplant for stable vitiligo. J. Cutan. Aesthet. Surg. 2011, 4, 41–43. [Google Scholar] [CrossRef]
- Qiu, W.; Chuong, C.-M.; Lei, M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp. Dermatol. 2019, 28, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Liao, Z.K.; Jia, H.Y.; Liu, X.M.; Wan, J.; Lei, T.C. The regional distribution of melanosomes in the epidermis affords a localized intensive photoprotection for basal keratinocyte stem cells. J. Dermatol. Sci. 2021, 103, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, A.; Mohanty, S.; Sahni, K.; Kumar, R. Extracted hair follicle outer root sheath cell suspension for pigment cell restoration in vitiligo. J. Cutan. Aesthet. Surg. 2013, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Vanscheidt, W.; Hunziker, T. Repigmentation by outer-root-sheath-derived melanocytes: Proof of concept in vitiligo and leucoderma. Dermatology 2009, 218, 342–343. [Google Scholar] [CrossRef]
- Shah, A.N.; Marfatia, R.K.; Saikia, S.S. A Study of Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension for Transplantation in Vitiligo. Int. J. Trichol. 2016, 8, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Orentreich, N. Autograft Repigmentation of Leukoderma. Arch. Dermatol. 1972, 105, 734. [Google Scholar] [CrossRef]
- Sanchez, D.P.; Sonthalia, S. Koebner Phenomenon. [Updated 21 November 2021]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553108/ (accessed on 10 March 2022).
- Barona, M.I.; Arrunátegui, A.; Falabella, R.; Alzate, A. An epidemiologic case-control study in a population with vitiligo. J. Am. Acad. Dermatol. 1995, 33, 621–625. [Google Scholar] [CrossRef]
- Liu, W.; Ma, D.-L. Koebner phenomenon in vitiligo after suction blister epidermal grafting. Can. Med. Assoc. J. 2019, 191, E968. [Google Scholar] [CrossRef] [Green Version]
- Mulekar, S.V. Koebner Phenomenon in Vitiligo: Not Always an Indication of Surgical Failure. Arch. Dermatol. 2007, 143, 799. [Google Scholar] [CrossRef]
- Mineiro dos Santos Garrett, N.F.; Carvalho da Costa, A.C.; Barros Ferreira, E.; Damiani, G.; Diniz dos Reis, P.E.; Inocêncio Vasques, C. Prevalence of dermatological toxicities in patients with melanoma undergoing immunotherapy: Systematic review and meta-analysis. PLoS ONE 2021, 16, e0255716. [Google Scholar] [CrossRef]
- Phan, K.; Charlton, O.; Smith, S.D. New onset vitiligo in a patient with hidradenitis suppurativa treated with adalimumab. Dermatol. Ther. 2020, 33, e13347. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Bae, J.M.; Lee, R.W.; Kim, S.H.; Parsad, D.; Pourang, A.; Hamzavi, I.; Shourick, J.; Ezzedine, K. Surgical Interventions for Patients With Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. 2021, 157, 307–316. [Google Scholar] [CrossRef] [PubMed]
| Tissue Grafts | Cellular Grafts |
|---|---|
| Mini-punch graft | Cultured melanocyte graft |
| Suction blister epidermal graft | Cultured epidermal graft |
| Split-thickness skin graft | Noncultured epidermal melanocyte suspension |
| Epidermal curettage technique | |
| Smash graft | Noncultured follicular root sheath suspension |
| Flip-top pigment grafting | |
| Hair follicle graft |
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Mini-punch graft |
|
| Helalat et al., 2012 [9] |
| Eleftheriadou et al., 2021 [16] | ||
|
| Chandrashekar et al., 2014 [18] | |
| Suction blister epidermal graft |
|
| Kar et al., 2018 [27] |
|
| Angeletti et al., 2019 [28] | |
|
| Iwanowski et al., 2018 [36] | |
| Split-thickness skin grafting |
|
| Khandapur et al., 2005 [41] |
|
| Chopra et al., 2020 [43] | |
| Epidermal curettage technique |
| The author does not predict any particular disadvantages | Machado et al., 2014 [44] |
|
| Tyagi et al., 2021 [46] | |
| Smash graft |
|
| Kar et al., 2018 [50] |
| Flip-top pigment grafting |
|
| Mohammad et al., 2017 [21] |
|
| Sharma et al., 2013 [52] | |
| Hair follicle graft |
|
| Thakur et al., 2015 [56] |
|
| Sardi et al., 2001 [57] | |
| Cultured melanocyte graft |
|
| Zokaei et al., 2019 [58] |
|
| Pandya et al., 2005 [60] | |
| Cultured epidermal graft |
|
| Pianigiani et al., 2006 [62] |
|
| Plott et al.,1989 [63] | |
| Noncultured melanocyte-keratinocyte suspension(noncultured epidermal cell suspension) |
|
| Bassiouny et al., 2018 [66] |
|
| El-Zawahry et al., 2017 [68] | |
| Noncultured follicular root sheath suspension |
|
| Gupta et al., 2013 [74] |
|
| Shah et al., 2016 [76] |
| Method | Type of the Article | Number of Patients | Definition of Efficacy (%) | Repigmentation | Follow up | References |
|---|---|---|---|---|---|---|
| MPG | Original article | 29 | Excellent repigmentation (>75%) | Postoperatively 58.6% of patients | 12 months | Helalat et al., 2012 [9] |
| Original article | 10 | No definition in the article | 86.7% of sites repigmented with excellent cosmetic colour match | 6 months | Chandrashekar et al., 2014 [18] | |
| Original article (an intrapatient comparative prospective interventional single-center open-label study) | 17 | Excellent repigmentation (>90%) | PRP/MPG/phototherapy: 0% of patients MPG/phototherapy: 0% of patches PRP/MPG/phototherapy: 52.9% of patients MPG/phototherapy: 41.2% of patches PRP—platelet-rich-plasma MPG—mini-punch grafting | 8 weeks 20 weeks | Salem et al., 2021 [23] | |
| Clinical trial report (a comparative prospective study) | 20 | Excellent cosmetic matching: moderate to excellent (50–100%) The authors did not specify the exact percentage of repigmentation considered as “excellent” | Line 1: 47.4% of lesions Line 2: 33.3% of lesions Line 3: 55.6% of lesions Line 4: 18.2 of lesions line-1—mini-punch grafting, line-2—needling, line-3—combined grafting and needling line-4—control group receiving non-procedural treatment | 3 months 6 months | Ragab et al., 2021 [25] | |
| SBEG | Original article (prospective study conducted in patients who presented with angle of lip vitiligo) | 112 | Complete repigmentation: The authors did not specify the exact percentage of repigmentation considered as “complete” | 88.2% of patients 88.5% of patients 93.1% of patients 87.5% of patients 89.5% of patients | 3 months 6 months 12 months 18 months 24 months | Kar et al.,2018 [27] |
| Original article | 10 | Complete repigmentation (>90%) | 0% of patients 7% of patients | 3 months 6 months | Iwanowski et al., 2018 [36] | |
| STSG | Original article | 64 | Number of lesions showing excellent repigmentation (>75%) | Group 1 (MPG): Face: 50%, Trunk: 88.8% Extremities: 17.6% Group 2 (STSG): Face: 91% Trunk: 100% Extremities: 76.4% | 3 months | Khandapur et al., 2005 [41] |
| Original article (prospective single-center study) | 22 | Excellent repigmentation (≥75%) | Group A (STSG): 40% of patches Group B (autologous noncultured melanocyte transfer): 42.5% of patches | 6 months | Chopra et al., 2020 [43] | |
| Epideraml curettage tachnique | Original article | 20 | Excellent repigmentation of lesions (>75%) | Group A (noncultured epidermal cell suspension): 0%,0%,8%,12% of lesions Group B (epidermal curettage): 0%, 0%, 25%, 60% of lesions | 2 weeks 4 weeks 8 weeks 12 weeks | Tyagi et al., 2021 [46] |
| Smash graft | No available data | |||||
| Flip-top pigment grafting | Original article | 20 | Excellent repigmentation (>90%) | PG: 0% of patients FTT: 0% of patients PG: 0% of patients FTT: 0% of patients PG: 50% of patients FTT: 65% of patients PG—punch grafting FTT—flip-top transplantation | 1 months 3 months 6 months | Sharma et al., 2013 [52] |
| Hair follicle graft | Original article (a prospective study) | 50 | Excellent improvement of the lesions (75–100%) | 33.3% of lesions | 6 months | Thakur et al., 2015 [56] |
| Cultured melanocyte graft | Original article | 27 | Excellent repigmentation (>90%) | Cultured melanocyte technique: 50% of patients AMRCS (autologous melanocyte rich cell suspension): 52.2% of patients | 6 months | Pandya et al., 2005 [60] |
| Cultured epidermal graft | Original article | 93 | Complete repigmentation The authors did not specify the exact percentage of repigmentation considered as “complete repigmentation” | 60% of patients | 3 months 6 months 12 months 18 months | Pianigiani et al., 2006 [62] |
| Noncultured melanocyte-keratinocyte suspension | Original article | 20 | Excellent repigmentation of lesions (>75%) | Group A (noncultured epidermal cell suspension): 0%, 0%, 8%, 12% of lesions Group B (epidermal curettage): 0%, 0%, 25%, 60% of lesions | 2 weeks 4 weeks 8 weeks 12 weeks | Tyagi et al., 2021 [46] |
| Original article | 12 | Repigmentation > 90% | 33.3% of patients | 8 days 3 weeks 1 months | Gauthier et al., 1992 [64] | |
| Original article (retrospective review) | 2283 | Long-term excellent repigmentation (>90%) of the skin lesions | 66% of patients—segmental vitiligo 53.5% of patients—undefined vitiligo (focal) 46.5% of patients—non-segmental vitiligo | 12–108 months | Zhang et al., 2021 [65] | |
| Original article (prospective multicenter comparative study) | 37 | Cases with pigmentation ≥ 75% were considered responders | NCECS (noncultured epidermal cell suspension): 20% of patients * ORSHFS (outer root sheath hair follicle suspension): 33.3% of patients * * assesment of the impact of donor tissue variability on the clinical outcome | 18 months | El-Zawahry et al., 2017 [68] | |
| Original article (retrospective review) | 100 | Long-term excellent repigmenattion (>90%) | Segmental/focal vitiligo: 58% of patients Nonsegmental vitiligo: 36% of patients Physical leukoderma: 12% of patients | 12–72 months | Silpa-Archa et al., 2017 [69] | |
| Original article (prospetive study) | 14 | Repigmentation ≥ 90% | 57.1% of patients | 5–15 months | Mohanty et al., 2011 [70] | |
| Noncultured follicular root sheath suspension | Original article | 5 | Repigmentation > 90% | 60% of patients | 6 months | Vanscheidt et al., 2009 [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frączek, A.; Kasprowicz-Furmańczyk, M.; Placek, W.; Owczarczyk-Saczonek, A. Surgical Treatment of Vitiligo. Int. J. Environ. Res. Public Health 2022, 19, 4812. https://doi.org/10.3390/ijerph19084812
Frączek A, Kasprowicz-Furmańczyk M, Placek W, Owczarczyk-Saczonek A. Surgical Treatment of Vitiligo. International Journal of Environmental Research and Public Health. 2022; 19(8):4812. https://doi.org/10.3390/ijerph19084812
Chicago/Turabian StyleFrączek, Alicja, Marta Kasprowicz-Furmańczyk, Waldemar Placek, and Agnieszka Owczarczyk-Saczonek. 2022. "Surgical Treatment of Vitiligo" International Journal of Environmental Research and Public Health 19, no. 8: 4812. https://doi.org/10.3390/ijerph19084812
APA StyleFrączek, A., Kasprowicz-Furmańczyk, M., Placek, W., & Owczarczyk-Saczonek, A. (2022). Surgical Treatment of Vitiligo. International Journal of Environmental Research and Public Health, 19(8), 4812. https://doi.org/10.3390/ijerph19084812







