High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of g-C3N4/La-N-TiO2 (CN/La-N-TiO2) Composite Nanomaterials
2.3. Spectroscopic and Electron Microscopic Characterization
2.4. Photocatalytic Tests
3. Results and Discussion
3.1. Photocatalysts Characterization
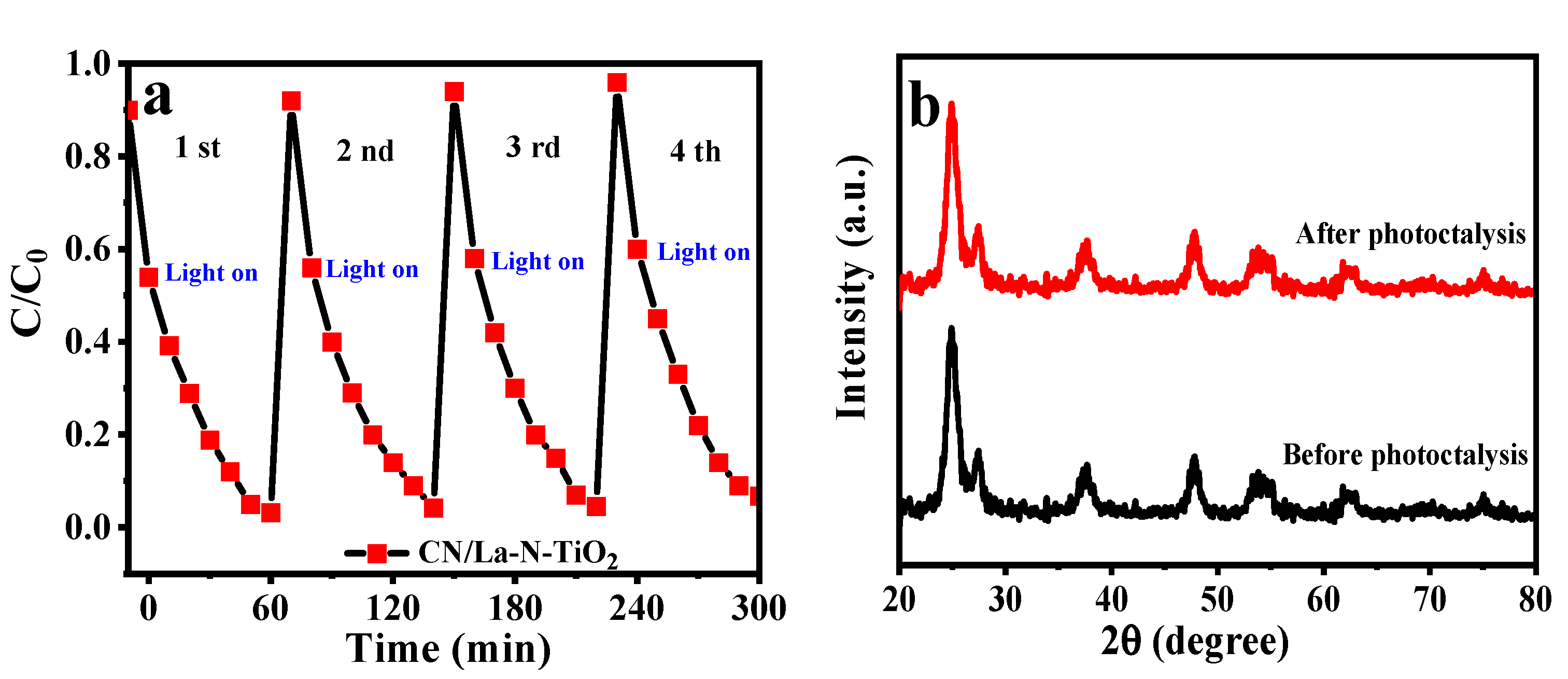
3.1.1. XRD and -FTIR Spectra
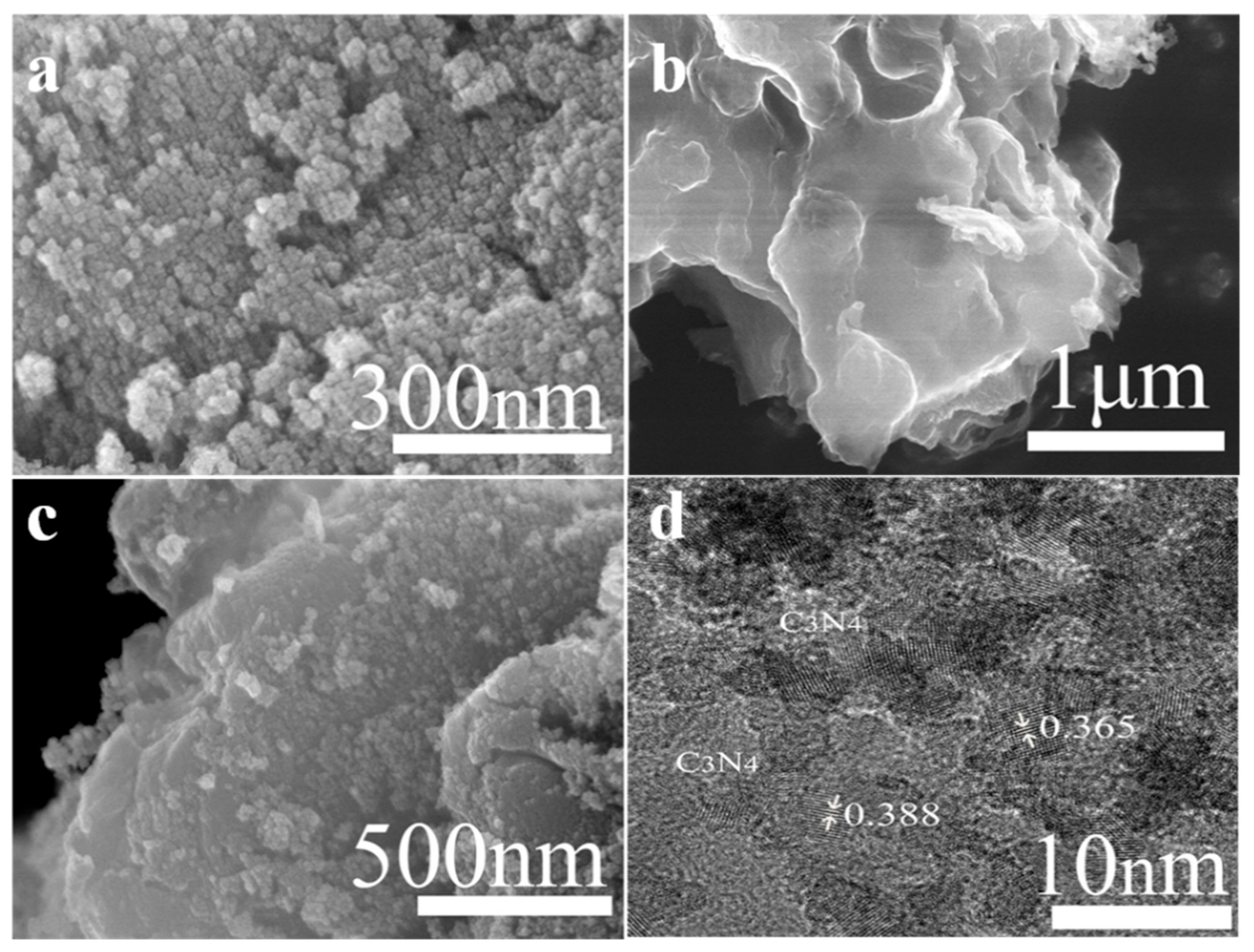
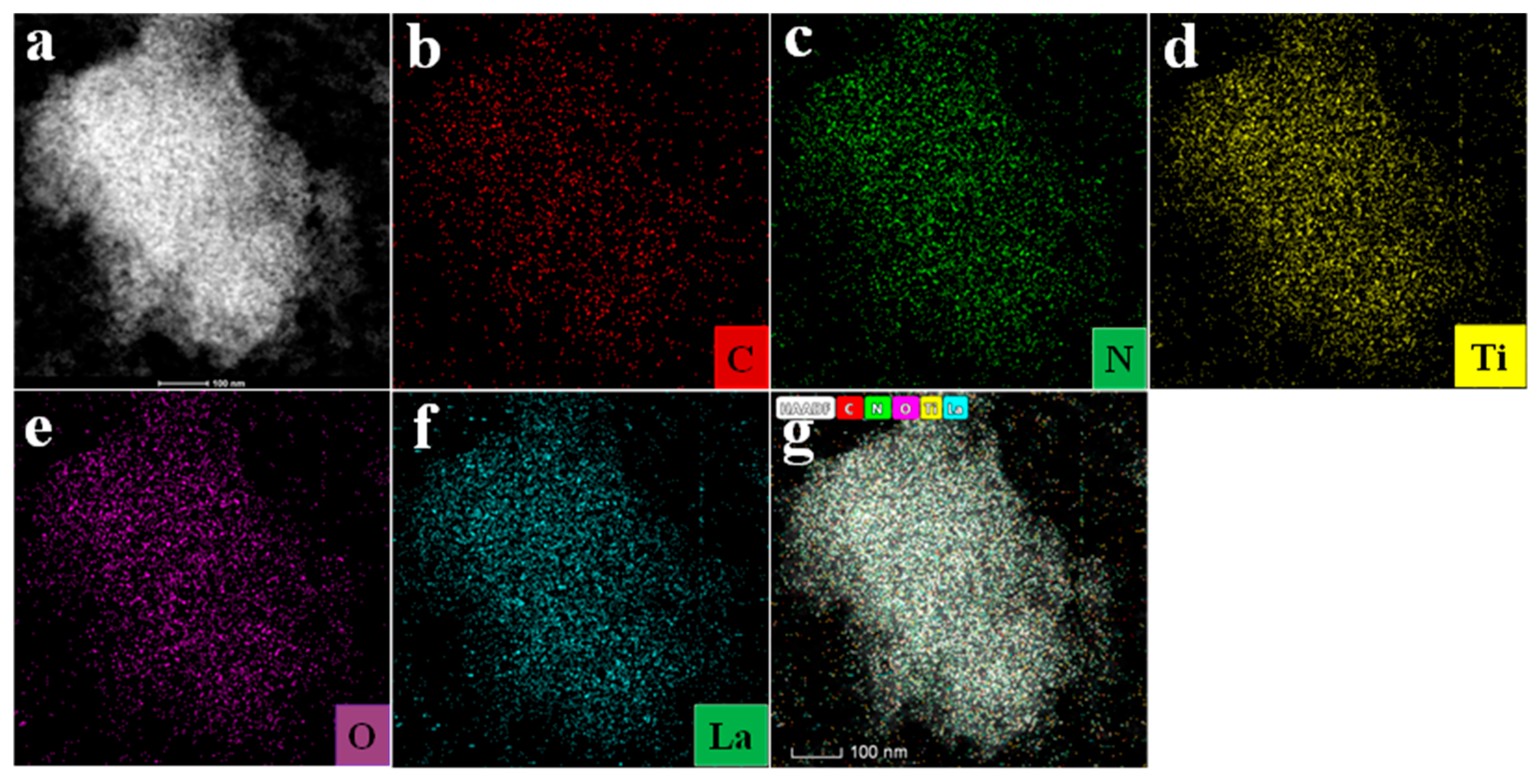
3.1.2. Morphology Analysis
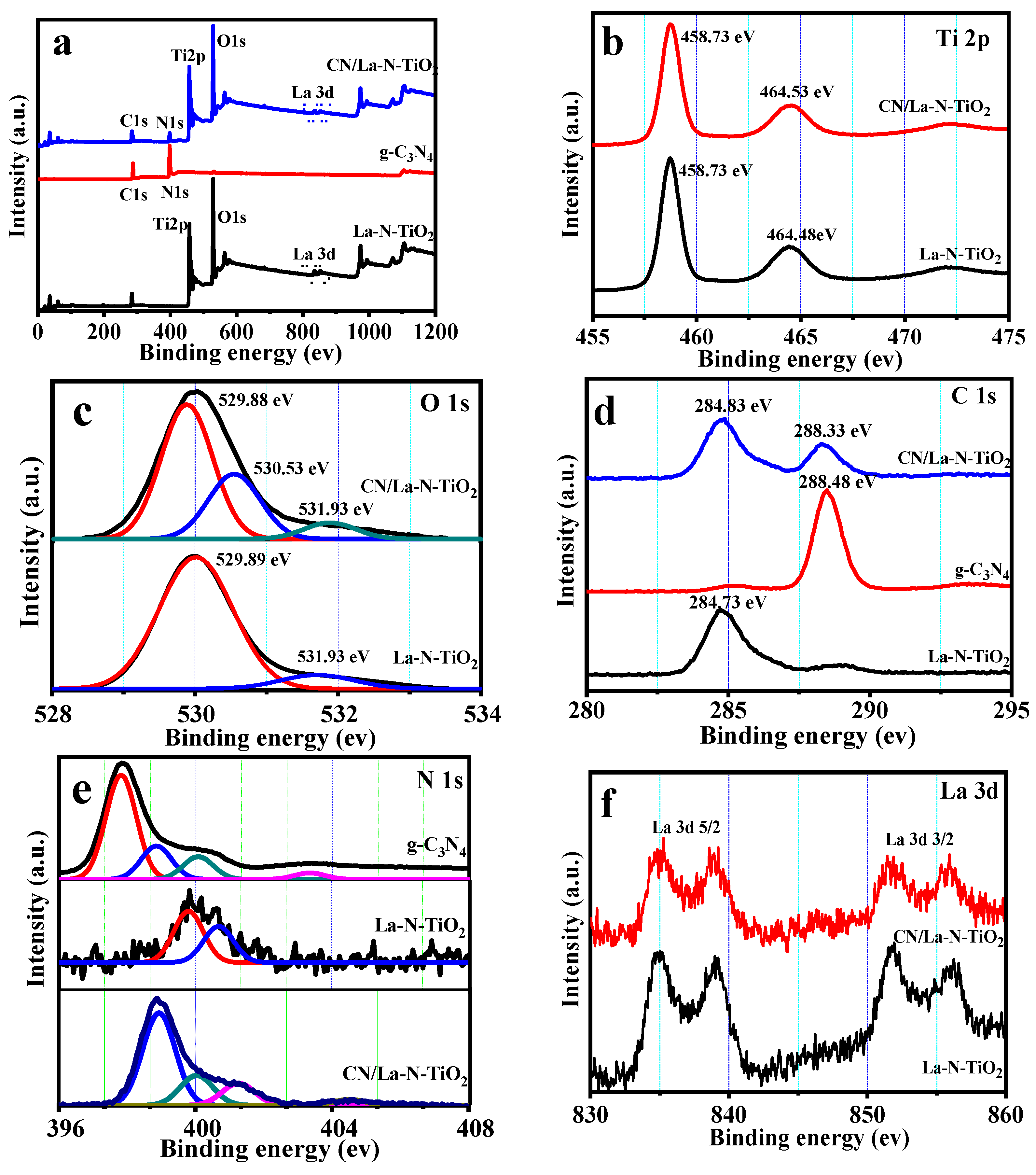
3.1.3. XPS Analysis
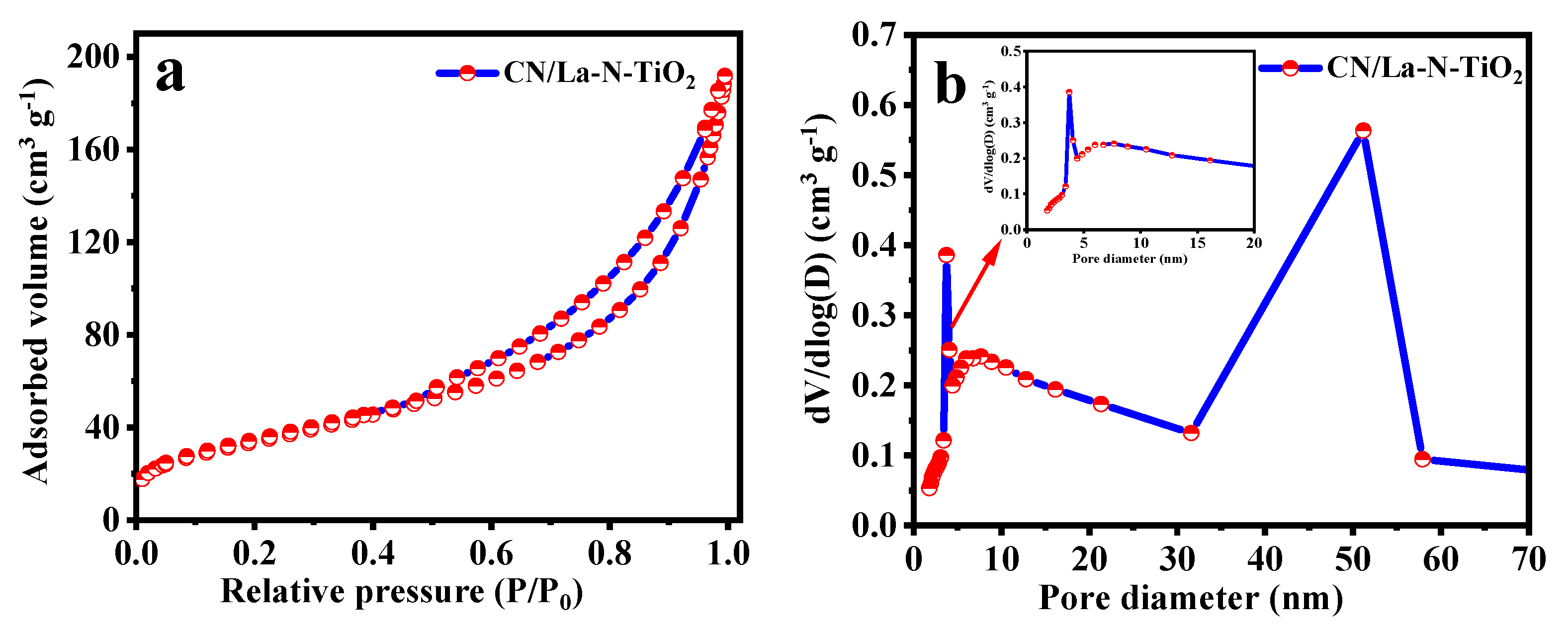
3.1.4. BET Surface Area Analysis
3.1.5. Spectral Properties
3.2. Photocatalytic Performance
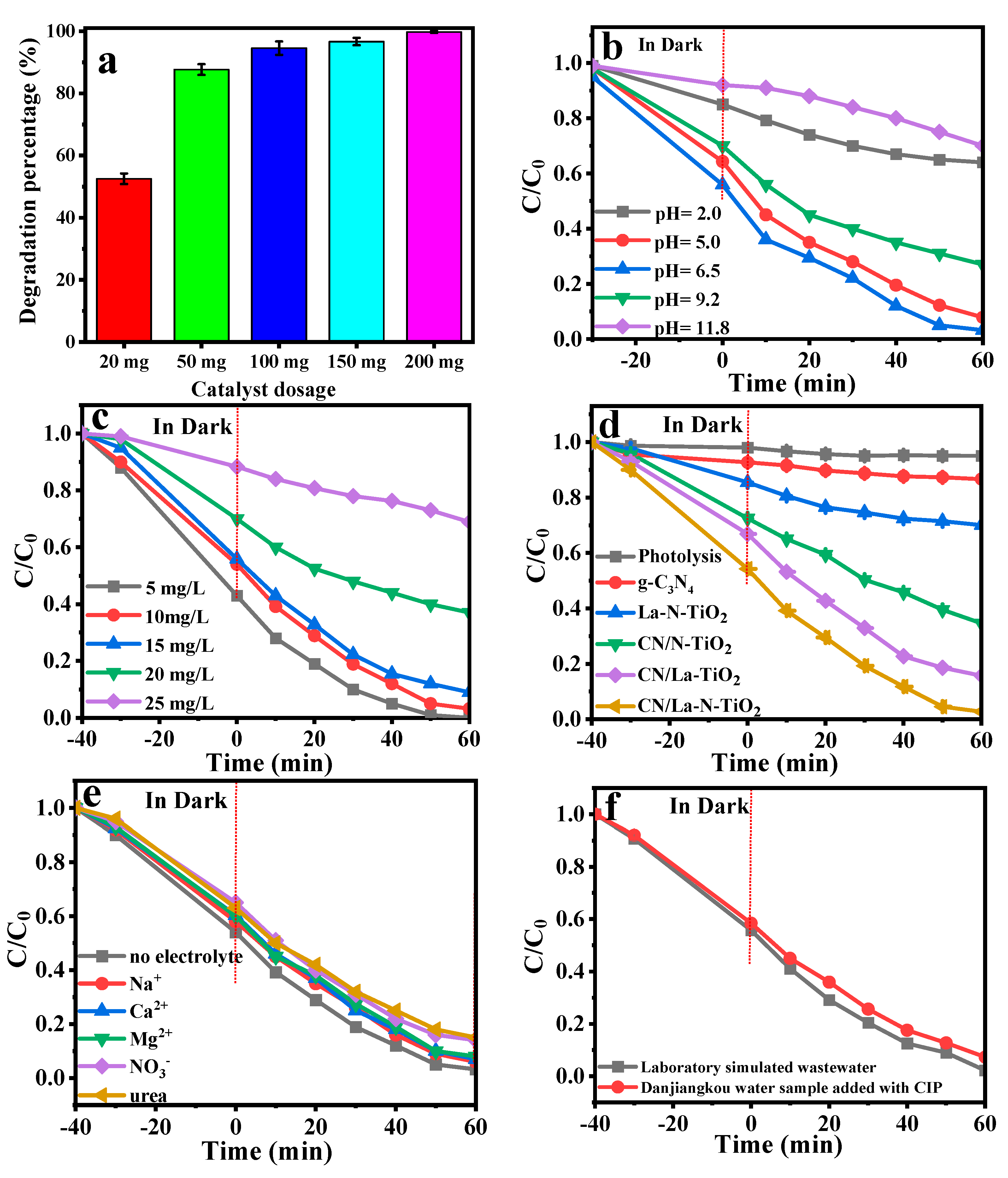
3.2.1. Effect of Catalyst Dosage
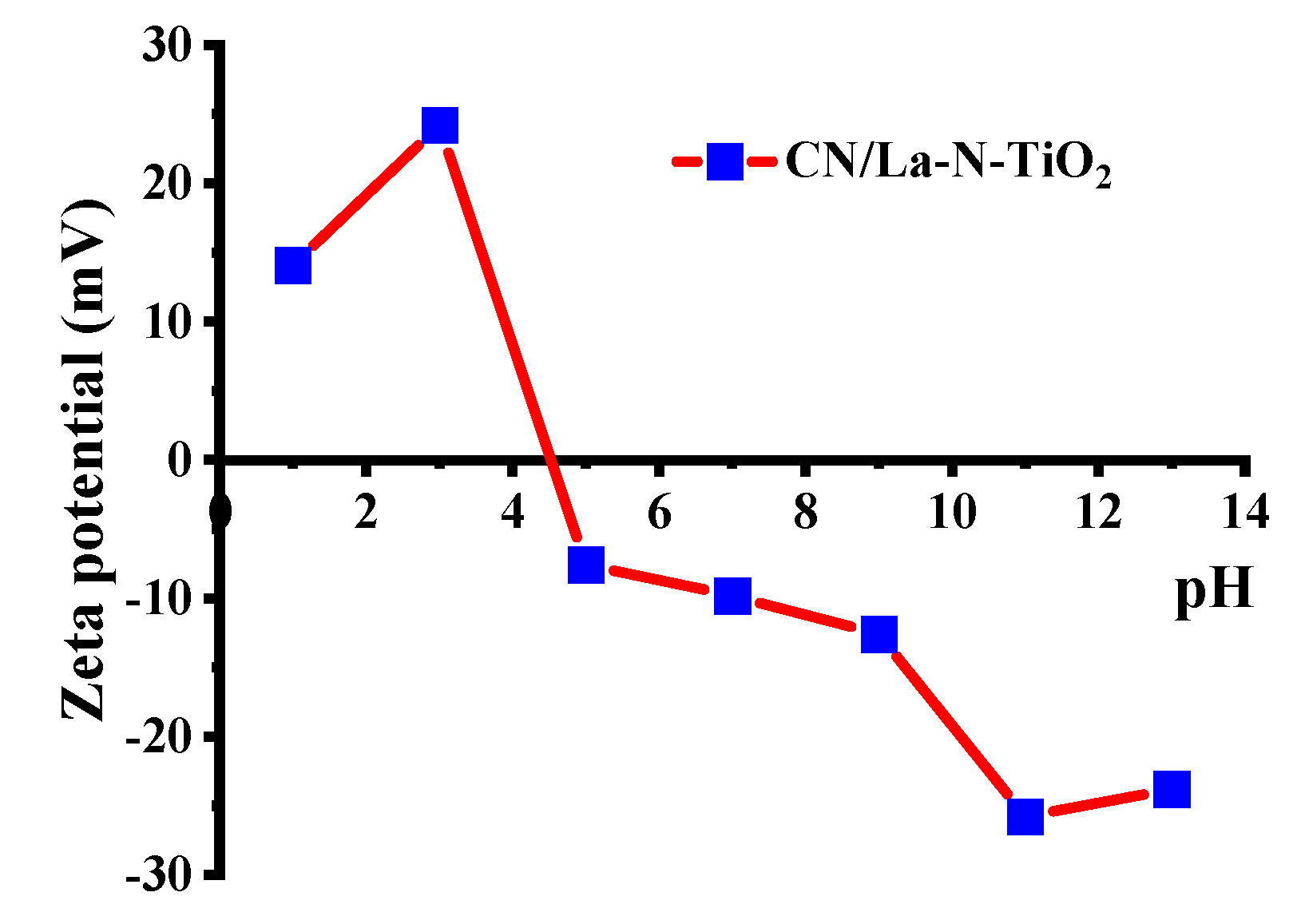
3.2.2. Effect of pH
3.2.3. Effect of Initial CIP Concentrations
3.2.4. Different Photocatalysts
3.2.5. Effect of Coexisting Cations
3.2.6. Degradation in Water Sample of Dangjiangkou
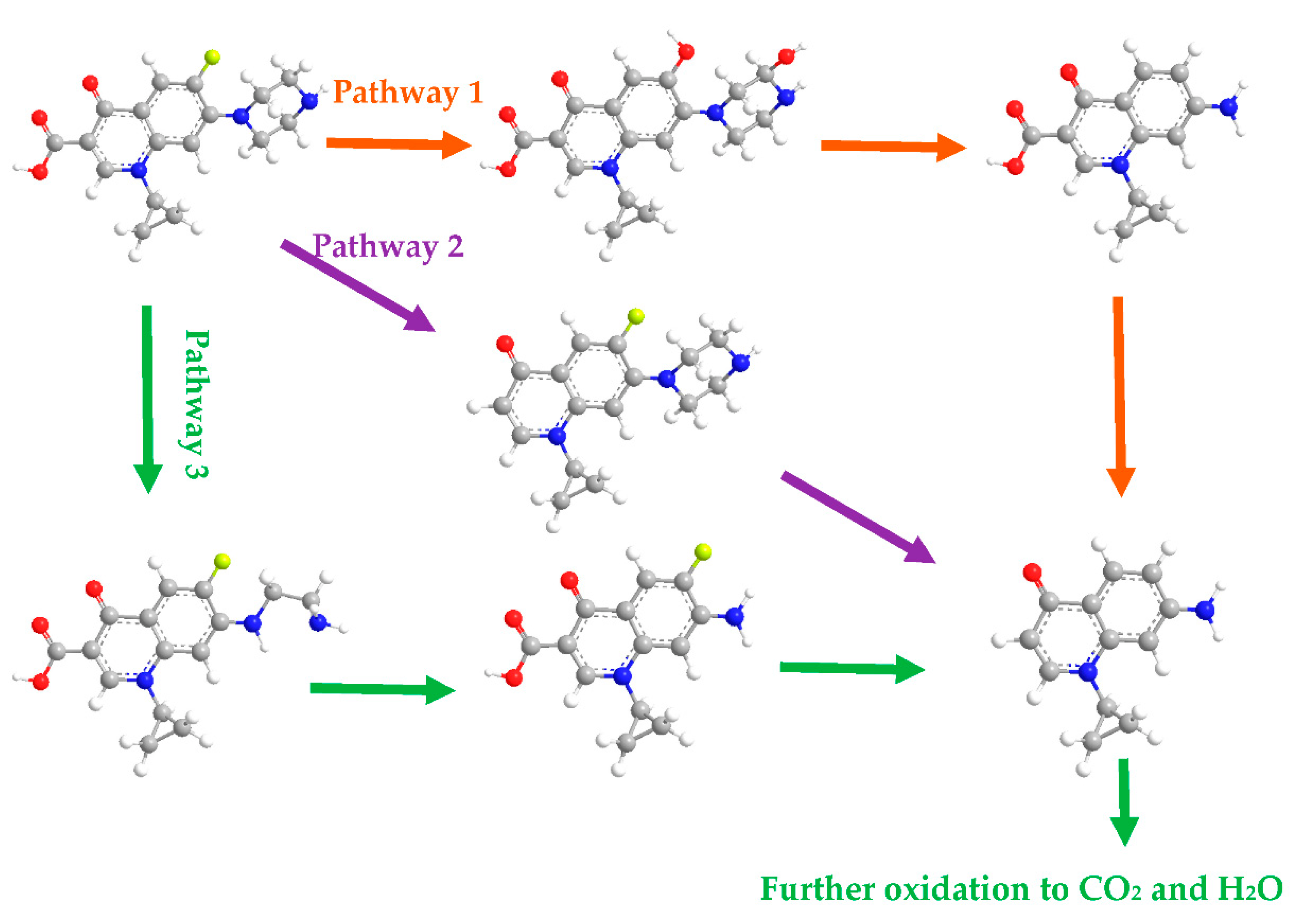
3.2.7. Proposed Pathways of CIP Degradation
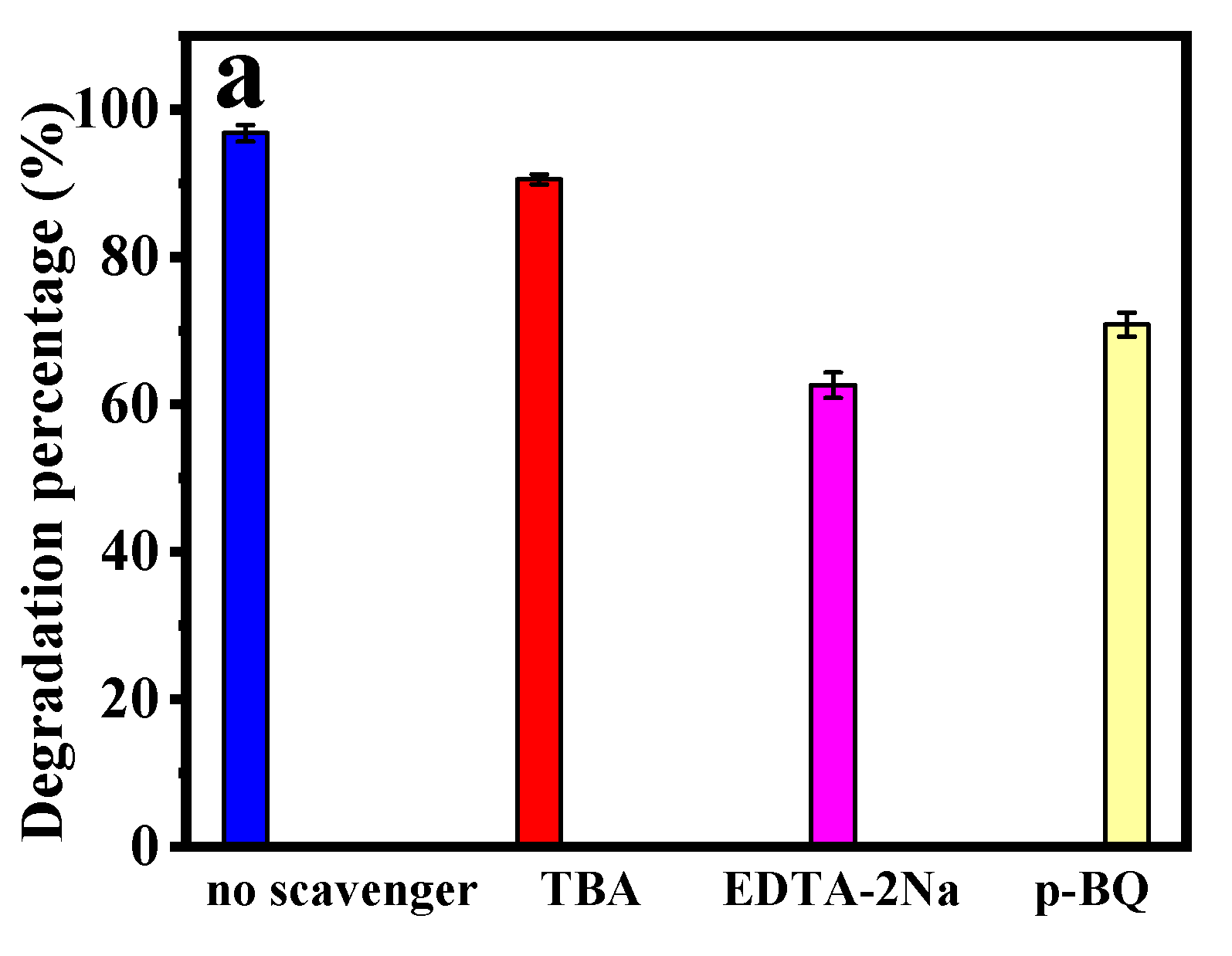
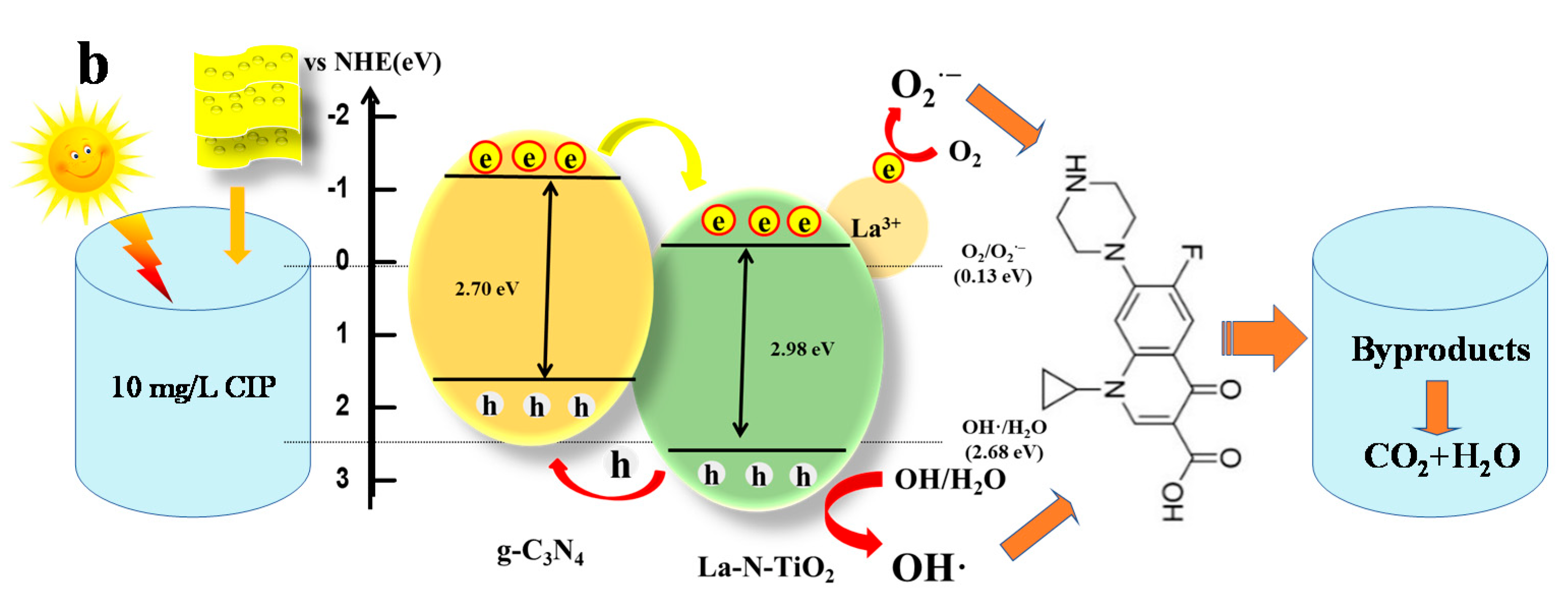
3.2.8. Radical Trapping Experiment and Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, J.; Zeng, L.; Zhu, M. Recent progress on the removal of antibiotic pollutants using photocatalytic oxidation process. Crit. Rev. Environ. Sci. Technol. 2021, 52, 1401–1448. [Google Scholar] [CrossRef]
- Boger, B.; Surek, M.; Vilhena, R.O.; Fachi, M.M.; Junkert, A.M.; Santos, J.M.; Domingos, E.L.; Cobre, A.F.; Momade, D.R.; Pontarolo, R. Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. J. Hazard. Mater. 2020, 402, 123448. [Google Scholar] [CrossRef]
- Feng, M.; Wang, Z.; Dionysiou, D.D.; Sharma, V.K. Metal-mediated oxidation of fluoroquinolone antibiotics in water: A review on kinetics, transformation products, and toxicity assessment. J. Hazard. Mater. 2018, 344, 1136–1154. [Google Scholar] [CrossRef]
- Qian, Q.Z.; Guang, G.Y.; Chang, G.P.; You, S.L.; Jian, L.Z. Comprehensive evaluation of antibiotics emission and fate in the river basins of china: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar]
- Huang, F.; An, Z.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009–2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Ma, D.; Wang, Y.; Gao, B.; Yue, Q.; Xu, X. The application of UV/O3 process on ciprofloxacin wastewater containing high salinity: Performance and its degradation mechanism. Chemosphere 2021, 276, 130220. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yan, Z.; Ou, Y.; Peng, B.; Zhang, L.; Shao, J.; Lin, Y.; Zhang, J. Effects of different carbon sources on the removal of ciprofloxacin and pollutants by activated sludge: Mechanism and biodegradation. J. Environ. Sci. 2022, 111, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.T.; Chang, P.H.; Wang, Y.S.; Tsai, Y.; Jean, J.S.; Li, Z.; Krukowski, K. Removal of ciprofloxacin from water by birnessite. J. Hazard. Mater. 2013, 250, 362–369. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Jia, M.; Wang, S.; Huang, Y.; Chen, C. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway. Chem. Eng. J. 2015, 274, 290–297. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Xie, B.; Li, Y.; Zhan, S.; Tian, Y. Synergistic degradation of antimicrobial agent ciprofloxacin in water by using 3D CeO2/RGO composite as cathode in electro-Fenton system. J. Electroanal. Chem. 2017, 784, 6–12. [Google Scholar] [CrossRef]
- Egbedina, A.O.; Adebowale, K.O.; Olu-Owolabi, B.I.; Unuabonah, E.I.; Adesina, M.O. Green synthesis of ZnO coated hybrid biochar for the synchronous removal of ciprofloxacin and tetracycline in wastewater. RSC Adv. 2021, 11, 18483–18492. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Yin, S.; Li, H.; Xu, H.; Zhang, Q.; Li, H. Controllable synthesis of Bi4O5Br2 ultrathin nanosheets for photocatalytic removal of ciprofloxacin and mechanism insight. J. Mater. Chem. A 2015, 3, 15108–15118. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. H2O2 assisted degradation of antibiotic norfloxacin over simulated solar light mediated Bi2WO6: Kinetics and reaction pathway. Chem. Eng. J. 2016, 296, 310–318. [Google Scholar] [CrossRef]
- Ding, Q.; Lam, F.L.Y.; Hu, X. Complete degradation of ciprofloxacin over g-C3N4-iron oxide composite via heterogeneous dark Fenton reaction. J. Environ. Manage. 2019, 244, 23–32. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Yan, Y.; Wang, W.; Ren, Y.; Li, X. Synthesis of highly porous g-C3N4 nanotubes for efficient photocatalytic degradation of sulfamethoxazole. Mater. Today Commun. 2021, 27, 102288. [Google Scholar] [CrossRef]
- Gad-Allah, T.A.; Ali, M.E.; Badawy, M.I. Photocatalytic oxidation of ciprofloxacin under simulated sunlight. J. Hazard. Mater. 2011, 186, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Rahmani-Aliabadi, A.; Nezamzadeh-Ejhieh, A. A visible light FeS/Fe2S3/zeolite photocatalyst towards photodegradation of ciprofloxacin. J. Photochem. Photobiol. A Chem. 2018, 357, 1–10. [Google Scholar] [CrossRef]
- Gan, Y.; Wei, Y.; Xiong, J.; Cheng, G. Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J. 2018, 349, 1–16. [Google Scholar] [CrossRef]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2017, 308, 377–385. [Google Scholar] [CrossRef]
- Chen, M.; Yao, J.; Huang, Y.; Gong, H.; Chu, W. Enhanced photocatalytic degradation of ciprofloxacin over Bi2O3/(BiO)2CO3 heterojunctions: Efficiency, kinetics, pathways, mechanisms and toxicity evaluation. Chem. Eng. J. 2018, 334, 453–461. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Zhou, Y.; Liu, Y.; Peng, B.; Feng, H. Construction of plasmonic Ag modified phosphorous-doped ultrathin g-C3N4 nanosheets/BiVO4 photocatalyst with enhanced visible-near-infrared response ability for ciprofloxacin degradation. J. Hazard. Mater. 2018, 344, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@g-C3N4 hollow core@shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Gandamalla, A.; Manchala, S.; Verma, A.; Fu, Y.P.; Shanker, V. Microwave-assisted synthesis of ZnAl-LDH/g-C3N4 composite for degradation of antibiotic ciprofloxacin under visible-light illumination. Chemosphere 2021, 283, 131182. [Google Scholar] [CrossRef]
- Liu, X.; Lv, P.; Yao, G.; Ma, C.; Tang, Y.; Wu, Y.; Huo, P.; Pan, J.; Shi, W.; Yan, Y. Selective degradation of ciprofloxacin with modified NaCl/TiO2 photocatalyst by surface molecular imprinted technology. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 420–426. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Jiang, Z.; Rong, J.; Pan, J.; Zhang, T.; Yang, D. Preparation of ternary combined ZnO-Ag2O/porous g-C3N4 composite photocatalyst and enhanced visible-light photocatalytic activity for degradation of ciprofloxacin. Chem. Eng. Res. Des. 2016, 111, 253–261. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Naushad, M.; Veses, R.C.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Solar-driven photodegradation of 17-β-estradiol and ciprofloxacin from waste water and CO2 conversion using sustainable coal-char/polymeric-g-C3N4/RGO metal-free nano-hybrids. New J. Chem. 2017, 41, 10208–10224. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Wang, C. Significantly enhanced visible light photocatalytic efficiency of phosphorus doped TiO2 with surface oxygen vacancies for ciprofloxacin degradation: Synergistic effect and intermediates analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Kuila, S.K.; Gorai, D.K.; Gupta, B.; Gupta, A.K.; Tiwary, C.S.; Kundu, T.K. Lanthanum ions decorated 2-dimensional g-C3N4 for ciprofloxacin photodegradation. Chemosphere 2021, 268, 128780. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Geng, J.; Li, H.; Bao, R.; Chen, H.; Wang, W.; Xia, J.; Wong, W. Exceedingly high photocatalytic activity of g-C3N4/Gd-N-TiO2 composite with nanoscale heterojunctions. Sol. Energy Mater. Sol. Cells 2017, 168, 91–99. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, J.; Chen, C.; Chen, H.; Geng, J.; Li, H. One-step synthesis of a visible-light driven C@N-TiO2 porous nanocomposite: Enhanced absorption, photocatalytic and photoelectrochemical performance. J. Phys. Chem. Solids 2020, 136, 109169. [Google Scholar] [CrossRef]
- Bao, R.; Yu, Y.; Chen, H.; Wang, W.; Xia, J.; Li, H. Effects of Rare Earth Elements and Nitrogen Co-Doped on the Photocatalytic Performance of TiO2. Cryst. Res. Technol. 2018, 53, 1700138. [Google Scholar] [CrossRef]
- Li, J.; Du, L.; Jia, S.; Sui, G.; Zhang, Y.; Zhuang, Y.; Li, B.; Xing, Z. Synthesis and photocatalytic properties of visible-light-responsive, three-dimensional, flower-like La–TiO2/g-C3N4 heterojunction composites. RSC Adv. 2018, 8, 29645–29653. [Google Scholar] [CrossRef] [Green Version]
- Meksi, M.; Turki, A.; Kochkar, H.; Bousselmi, L.; Guillard, C.; Berhault, G. The role of lanthanum in the enhancement of photocatalytic properties of TiO2 nanomaterials obtained by calcination of hydrogenotitanate nanotubes. Appl. Catal. B Environ. 2016, 181, 651–660. [Google Scholar] [CrossRef]
- Yu, Y.; Piao, L.; Xia, J.; Wang, W.; Geng, J.; Chen, H.; Xing, X.; Li, H. A facile one-pot synthesis of N-La codoped TiO2 porous materials with bio-hierarchical architectures and enhanced photocatalytic activity. Mater. Chem. Phys. 2016, 182, 77–85. [Google Scholar] [CrossRef]
- Raaja Rajeshwari, M.; Kokilavani, S.; Sudheer Khan, S. Recent developments in architecturing the g-C3N4 based nanostructured photocatalysts: Synthesis, modifications and applications in water treatment. Chemosphere 2021, 291, 132735. [Google Scholar] [CrossRef]
- Deng, H.; Wang, X.C.; Wang, L.; Lia, Z.J.; Liang, P.L.; Ou, J.Z.; Liu, K.; Yuan, L.Y.; Jiang, Z.Y.; Zheng, L.R.; et al. Enhanced photocatalytic reduction of aqueous Re (VII) in ambient air by amorphous TiO2/g-C3N4 photocatalysts: Implications for Tc (VII) elimination. Chem. Eng. J. 2020, 401, 125977. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.; Cheng, H. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Sun, C.; Dai, J.; Zhang, H.; Li, S.; Wang, A. A facile method for preparing porous g-C3N4 nanosheets with efficient photocatalytic activity under visible light. J. Mater. Sci. 2021, 56, 7557–7572. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.J. A reusable, separation-free and biodegradable calcium alginate/g-C3N4 microsphere for sustainable photocatalytic wastewater treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Hu, H.; Hu, J.; Wang, X.; Gan, J.; Su, M.; Ye, W.; Zhang, W.; Ma, X.; Wang, H. Enhanced reduction and oxidation capability over the CeO2/g-C3N4 hybrid through surface carboxylation: Performance and mechanism. Catal. Sci. Technol. 2020, 10, 4712–4725. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Benadict Joseph, X.; Wang, S.F.; Sriram, B.; Antilen Jacob, G.; Ravi, G. Engineering Architecture of 3D-Urchin-like Structure and 2D-Nanosheets of Bi2S3@g-C3N4 as the Electrode Material for a Solid-State Symmetric Supercapacitor. Energy Fuels 2021, 35, 12569–12580. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Zhang, Y.; He, D.; An, Q.; Cao, G. Seed-induced growing various TiO2 nanostructures on g-C3N4 nanosheets with much enhanced photocatalytic activity under visible light. J. Hazard. Mater. 2015, 292, 79–89. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Wang, J.; Song, Y. Visible-light-driven elimination of oxytetracycline and Escherichia coli using magnetic La-doped TiO2/copper ferrite/diatomite composite. Environ. Sci. Pollut. Res. 2019, 26, 26593–26604. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Fazio, S.; Guzmán, J.; Colomer, M.T.; Salomoni, A.; Moreno, R. Colloidal stability of nanosized titania aqueous suspensions. J. Eur. Ceram. Soc. 2008, 28, 2171–2176. [Google Scholar] [CrossRef]
- Salma, A.; Thoroe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M. Exploring the potential of nano-zerovalent copper modified biochar for the removal of ciprofloxacin from water. Environmental Nanotechnology. Monit. Manag. 2021, 16, 100604. [Google Scholar]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae-based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]


| Photocatalysts | C0 (mg/L) | pH | Degradation Rate (%) | Light Irradiation Wavelength (λ) | Incident Light Power | Ref |
|---|---|---|---|---|---|---|
| Commercial anatase TiO2 | 1500 ppm | 5.8 | Kapp = 0.02 min−1 | Simulated visible light | Commercial visible metal halide lamp | [24] |
| CeO2–Ag/AgBr | 10 | - | 93.05%/120 min | λ > 420 nm | A 300 W Xe lamp | [25] |
| FeS/Fe2S3/zeolite | 2 | - | 6%/90 min | λ > 380 nm | Tungsten lamp | [26] |
| Mesoporous TiO2 nanocrystallite aggregates | 160 | - | 96.05%/6 h | 200 nm ~1000 nm | A 500 W Xe lamp | [27] |
| ZnO | 4 | 7/10 | 48%/60 min | 365 nm | A xenon lamp | [28] |
| 3D γ-Fe2O3@ZnO core-shell photocatalyst | 10 | 5.8 | 92.5%/60 min | Simulated sunlight | A 300 W Xenon lamp | [29] |
| Bi2O3/(BiO)2CO3 | 10 | 4.0~8.3 | 93.4%/30 min | Simulated solar light | A 300 W Xenon lamp | [30] |
| Ag@PCNS/BiVO4 | 10 | - | 92.6%/120 min 17.5%/120 min | λ > 420 nm λ > 760 nm | - | [31] |
| ZnAl-LDH/g-C3N4 | 20 | - | 84.1%/150 min | - | A 35 W Xenon lamp | [35] |
| Ag2CrO4/Ag/BiFeO3@RGO | 10 | 6 | 96%/60 min | Visible light | 450 mW cm−2 | [33] |
| TiO2 nanorod/g-C3N4 nanosheet | 4.97 | 6.3 | 93.4%/60 min | Simulated sunlight | A 500WXenon lamp | [34] |
| TiO2@g-C3N4 hollow | - | - | 74%/120 min | Simulated sunlight | - | [32] |
| CN/La-N-TiO2 | 10 | 6.0–7.0 | 96.8%/60 min | λ > 420 nm | A 300 W Xe lamp | our work |
| Catalysts | C1/at.% | C2/at.% | Ti/at.% | O/at.% | N/at.% | La/at.% | Surface Area (m2/g) | Av Pore Size(nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|---|---|
| La-N-TiO2 | 15.71 | – | 19.51 | 51.62 | 1.55 | 0.6 | 83 [47] | 5.10 | 0.17 |
| g-C3N4 | – | 31.298 | – | – | 66.789 | – | 50 [42] | – | – |
| CN/La-N-TiO2 | 12.64 | 6.94 | 19.15 | 48.69 | 12.52 | 0.06 | 122 | 8.76 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liu, K.; Zhang, Y.; Xing, X.; Li, H. High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin. Int. J. Environ. Res. Public Health 2022, 19, 4793. https://doi.org/10.3390/ijerph19084793
Yu Y, Liu K, Zhang Y, Xing X, Li H. High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin. International Journal of Environmental Research and Public Health. 2022; 19(8):4793. https://doi.org/10.3390/ijerph19084793
Chicago/Turabian StyleYu, Yanmin, Ke Liu, Yangyang Zhang, Xuan Xing, and Hua Li. 2022. "High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin" International Journal of Environmental Research and Public Health 19, no. 8: 4793. https://doi.org/10.3390/ijerph19084793
APA StyleYu, Y., Liu, K., Zhang, Y., Xing, X., & Li, H. (2022). High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin. International Journal of Environmental Research and Public Health, 19(8), 4793. https://doi.org/10.3390/ijerph19084793








