The Role of Extracellular Polymeric Substances in the Toxicity Response of Anaerobic Granule Sludge to Different Metal Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrate
2.2. Metal Oxide NPs and Their Dissolved Metal Ions
2.3. Methanogenic Activity Bioassays and Testing of NPs Toxicity to AGS
2.4. EPS Extraction and Analysis
2.5. Microbial Community Analysis
2.6. Other Analytical Methods and Statistical Analysis
3. Results and Discussion
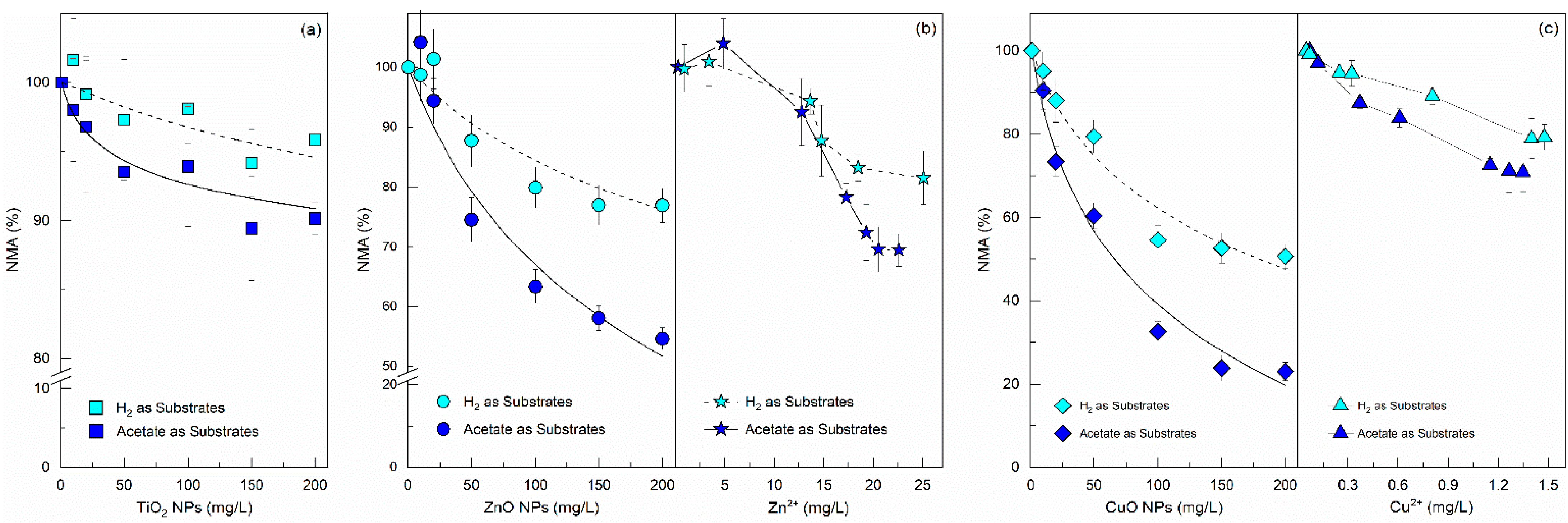
3.1. Effects of NPs Exposure on Methanogenic Activity
3.2. Effect of NPs Exposure on EPS Production
3.3. Possible Effect of NPs on Cytomembrane Integrity
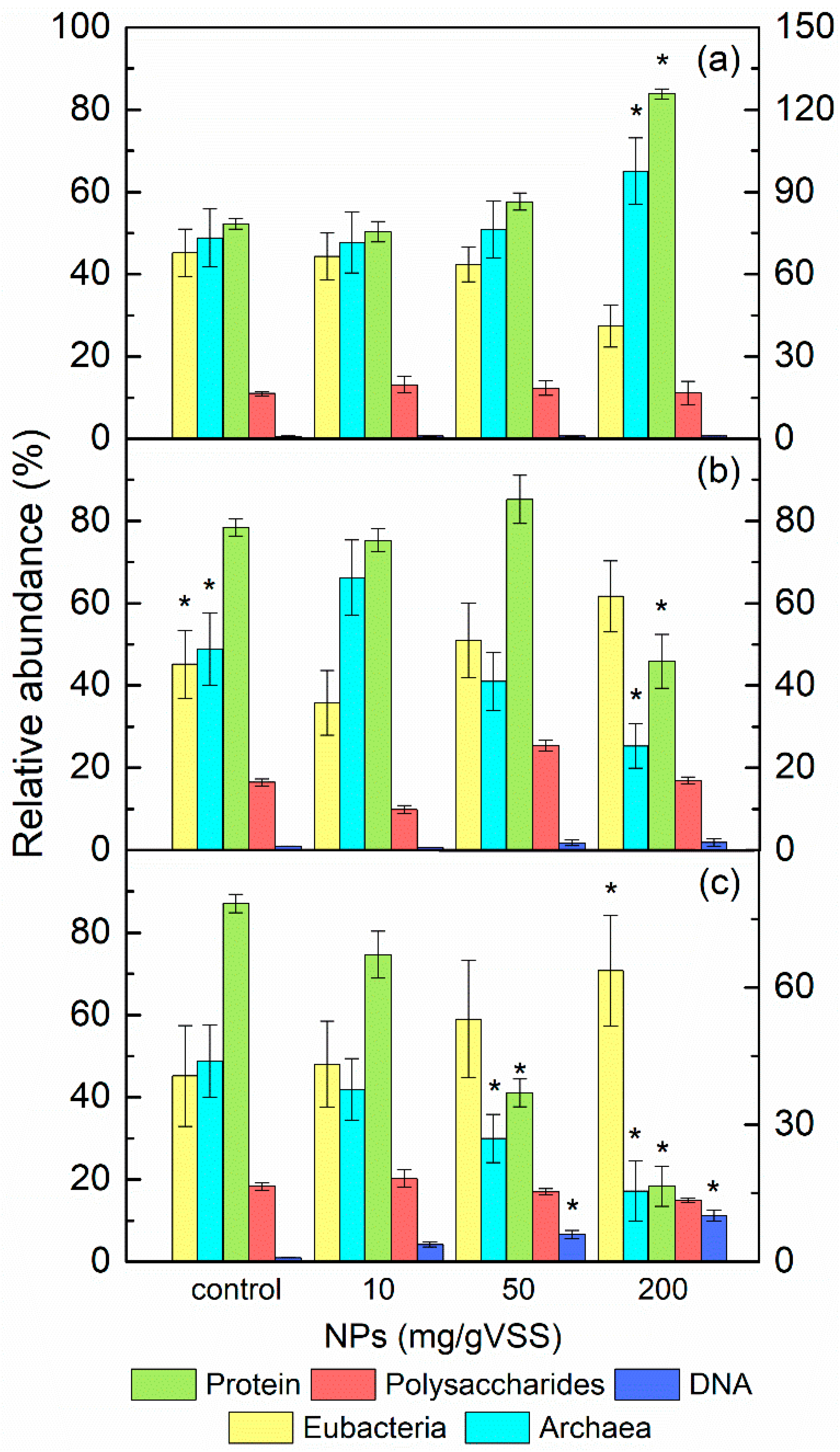
3.4. The Role of EPS on the Diversity and Abundance of Microorganisms in AGS under NPs Exposure
4. Conclusions
- (1)
- The sensitivity of methanogens to each type of metal oxide NP varied with the species. CuO NPs were the most toxic among the studied NPs, since methanogenic activity showed a significant inhibitory effect, consistent over the test period, with the IC50 of 65.7–179 mg/L for CuO NPs and 214 to over 1000 mg/L for ZnO NPs.
- (2)
- The average content of LB-EPS, TB-EPS, and total EPS increased by 69.5%, 38.8%, and 48.4% (calculated based on the test results), respectively, as the concentration of TiO2 NPs increased to 200 mg/L, which provided evidence that AGS prevented the physical restraints caused by TiO2 NPs through secreting more EPS. There is a disparity between the variation trend of LB-EPS and TB-EPS at a ZnO NP exposure concentration of <200 mg/L, which may be explained by the Zn2+ release.
- (3)
- The presence of CuO NPs promoted the ROS generation and LDH leakage of AGS. Thus, the negative impact of NPs was caused not only by the dissolved metal ions, but also by their considerable potential to induce physical restraints or membrane reduction in AGS cells without the presence of sufficient protective effects exerted by EPS.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Smeraldi, J.; Hosseini, T.; Khatib, L.; Olson, B.H.; Rosso, D. Evaluation of Nanocopper Removal and Toxicity in Municipal Wastewaters. Environ. Sci. Technol. 2010, 44, 7808–7813. [Google Scholar] [CrossRef] [PubMed]
- Kiser, M.A.; Ryu, H.; Jang, H.; Hristovski, K.; Westerhoff, P. Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res. 2010, 44, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Bian, Y.; Dong, L.; Zheng, X.; Chen, Y. Long-term effects of copper nanoparticles on volatile fatty acids production from sludge fermentation: Roles of copper species and bacterial community structure. Bioresour. Technol. 2022, 348, 126789. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Nano- and microparticles-induced effect on activated sludge properties. Int. J. Environ. Sci. Technol. 2019, 16, 8663–8670. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. The effect of Fe2NiO4 and Fe4NiO4Zn magnetic nanoparticles on anaerobic digestion activity. Sci. Total. Environ. 2018, 642, 276–284. [Google Scholar] [CrossRef]
- Cervantes-Aviles, P.; Ida, J.; Toda, T.; Cuevas-Rodriguez, G. Effects and fate of TiO2 nanoparticles in the anaerobic treatment of wastewater and waste sludge. J. Environ. Manag. 2018, 222, 227–233. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- McHugh, S.; O’Reilly, C.; Mahony, T.; Colleran, E.; O’Flaherty, V. Anaerobic Granular Sludge Bioreactor Technology. Rev. Environ. Sci. Biotechnol. 2003, 2, 225–245. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, N.; Rene, E.R.; Wu, D.X.; Sun, D.Z.; Qiu, B. Stimulation of anaerobic biofilm development in the presence of low concentrations of toxic aromatic pollutants. Bioresour. Technol. 2019, 281, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Quan, X.C.; Si, X.R.; Wu, Y.C. Responses of anaerobic granule and flocculent sludge to ceria nanoparticles and toxic mechanisms. Bioresour. Technol. 2013, 149, 346–352. [Google Scholar] [CrossRef]
- Mu, H.; Zheng, X.; Chen, Y.G.; Chen, H.; Liu, K. Response of Anaerobic Granular Sludge to a Shock Load of Zinc Oxide Nanoparticles during Biological Wastewater Treatment. Environ. Sci. Technol. 2012, 46, 5997–6003. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. Npj Clean Water 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Cui, F.; Liu, Z.; Li, D. Transport, fate, and long-term impacts of metal oxide nanoparticles on the stability of an anaerobic methanogenic system with anaerobic granular sludge. Bioresour. Technol. 2017, 234, 448–455. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Snaidr, J.; Amann, R.; Huber, I.; Ludwig, W.; Schleifer, K.H. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 1997, 63, 2884–2896. [Google Scholar] [CrossRef] [Green Version]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Et Biophys. Acta-Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Delgado, L.; Torà, J.A.; Casals, E.; González, E.; Puntes, V.; Font, X.; Carrera, J.; Sánchez, A. Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. J. Hazard. Mater. 2012, 199, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-delRisco, M.; Orupõld, K.; Dubourguier, H.-C. Particle-size effect of CuO and ZnO on biogas and methane production during anaerobic digestion. J. Hazard. Mater. 2011, 189, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Estrella, J.; Sierra-Alvarez, R.; Field, J.A. Toxicity assessment of inorganic nanoparticles to acetoclastic and hydrogenotrophic methanogenic activity in anaerobic granular sludge. J. Hazard. Mater. 2013, 260, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Otero-González, L.; Field, J.A.; Sierra-Alvarez, R. Inhibition of anaerobic wastewater treatment after long-term exposure to low levels of CuO nanoparticles. Water Res. 2014, 58, 160–168. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.B.; Gao, Y.Y.; Li, F.; Yang, B.; Wang, M.; Ma, C.Y.; Tian, Q.; Song, X.S.; Sand, W. Granulation process in an expanded granular sludge blanket (EGSB) reactor for domestic sewage treatment: Impact of extracellular polymeric substances compositions and evolution of microbial population. Bioresour. Technol. 2018, 269, 153–161. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef]
- Tong, T.Z.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.-F.; Gray, K.A. Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by high-throughput screening: Effects of environmental factors. Water Res. 2013, 47, 2352–2362. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. The influence of natural organic matter and aging on suspension stability in guideline toxicity testing of silver, zinc oxide, and titanium dioxide nanoparticles with Daphnia magna. Environ. Toxicol. Chem. 2015, 34, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Madela, M.; Grobelak, A.; Neczaj, E. Impact of selected nanoparticles on wastewater treatment efficiency. Desalination Water Treat. 2018, 134, 115–120. [Google Scholar] [CrossRef]
- Fang, H.H.; Xu, L.C.; Chan, K.Y. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 2002, 36, 4709–4716. [Google Scholar] [CrossRef]
- Gondikas, A.; Gallego-Urrea, J.; Halbach, M.; Derrien, N.; Hassellov, M. Nanomaterial Fate in Seawater: A Rapid Sink or Intermittent Stabilization? Front. Environ. Sci. 2020, 8, 151. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yu, H.Q. Biological hydrogen production in a UASB reactor with granules. I: Physicochemical characteristics of hydrogen-producing granules. Biotechnol. Bioeng. 2006, 94, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Tang, X.; Fan, C.Z.; Hao, W.L.; Zhao, Y.L.; Zeng, Y.J. Cationic polyacrylamide alleviated the inhibitory impact of ZnO nanoparticles on anaerobic digestion of waste activated sludge through reducing reactive oxygen species induced. Water Res. 2021, 205, 117651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, H.; Zheng, X. Chronic Response of Waste Activated Sludge Fermentation to Titanium Dioxide Nanoparticles. Chin. J. Chem. Eng. 2014, 22, 1162–1167. [Google Scholar] [CrossRef]
- Joshi, N.; Ngwenya, B.T.; French, C.E. Enhanced resistance to nanoparticle toxicity is conferred by overproduction of extracellular polymeric substances. J. Hazard. Mater. 2012, 241, 363–370. [Google Scholar] [CrossRef]
- Nemeth, I.; Molnar, S.; Vaszita, E.; Molnar, M. The Biolog EcoPlate (TM) Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities. Nanomaterials 2021, 11, 1777. [Google Scholar] [CrossRef]
- Tijero, J.; Guardiola, E.; Mirada, F.; Cortijo, M. Effect of Cu2+, Ni2+ and Zn2+ on an Anaerobic-Digestion System. J. Environ. Sci. Health A 1991, 26, 799–811. [Google Scholar] [CrossRef]
- Fu, H.M.; Peng, M.W.; Yan, P.; Wei, Z.; Fang, F.; Guo, J.S.; Chen, Y.P. Potential role of nanobubbles in dynamically modulating the structure and stability of anammox granular sludge within biological nitrogen removal process. Sci. Total Environ. 2021, 784, 147110. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Amin Vieira da Costa, N.P.; Libardi, N.; Ribeiro da Costa, R.H. How can the addition of extracellular polymeric substances (EPS)-based bioflocculant affect aerobic granular sludge (AGS)? J. Environ. Manag. 2022, 310, 114807. [Google Scholar] [CrossRef] [PubMed]
- Gomec, C.Y.; Letsiou, I.; Ozturk, I.; Eroglu, V.; Wilderer, P.A. Identification of Archaeal population in the granular sludge of an UASB reactor treating sewage at low temperatures. J. Environ. Sci. Health A 2008, 43, 1504–1510. [Google Scholar] [CrossRef]
- Gu, L.; Li, Q.; Quan, X.; Cen, Y.; Jiang, X. Comparison of nanosilver removal by flocculent and granular sludge and short- and long-term inhibition impacts. Water Res. 2014, 58, 62–70. [Google Scholar] [CrossRef]


| NPs | Supernatant | EPS | Immobilized AGS | ||
|---|---|---|---|---|---|
| Type | Concentration | LB | TB | ||
| TiO2 | 10 | 0.73 ± 0.05 | 7.34 ± 0.25 | 0.62 ± 0.11 | 1.32 ± 0.06 |
| 50 | 3.35 ± 0.15 | 28.53 ± 1.35 | 5.29 ± 0.24 | 12.84 ± 0.58 | |
| 200 | 16.60 ± 0.69 | 85.66 ± 3.66 | 31.06±1.32 | 66.68 ± 3.13 | |
| ZnO | 10 | 1.14 ± 0.04 | 6.22 ± 0.28 | 1.18 ± 0.45 | 1.46 ± 0.06 |
| 50 | 6.35 ± 0.29 | 21.88 ± 0.97 | 11.51 ± 0.52 | 10.27 ± 0.49 | |
| 200 | 16.60 ± 0.72 | 67.69 ± 3.28 | 32.83 ± 1.56 | 82.88 ± 3.72 | |
| CuO | 10 | 0.37 ± 0.02 | 7.12 ± 0.33 | 0.95 ± 0.05 | 1.56 ± 0.06 |
| 50 | 1.36 ± 0.06 | 16.74 ± 0.79 | 9.28 ± 0.46 | 22.64 ± 1.12 | |
| 200 | 10.08 ± 0.44 | 51.83 ± 2.33 | 20.12 ± 0.97 | 117.98 ± 5.57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chang, F.; Li, Z.; Cui, F. The Role of Extracellular Polymeric Substances in the Toxicity Response of Anaerobic Granule Sludge to Different Metal Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2022, 19, 5371. https://doi.org/10.3390/ijerph19095371
Li H, Chang F, Li Z, Cui F. The Role of Extracellular Polymeric Substances in the Toxicity Response of Anaerobic Granule Sludge to Different Metal Oxide Nanoparticles. International Journal of Environmental Research and Public Health. 2022; 19(9):5371. https://doi.org/10.3390/ijerph19095371
Chicago/Turabian StyleLi, Huiting, Fang Chang, Zhendong Li, and Fuyi Cui. 2022. "The Role of Extracellular Polymeric Substances in the Toxicity Response of Anaerobic Granule Sludge to Different Metal Oxide Nanoparticles" International Journal of Environmental Research and Public Health 19, no. 9: 5371. https://doi.org/10.3390/ijerph19095371
APA StyleLi, H., Chang, F., Li, Z., & Cui, F. (2022). The Role of Extracellular Polymeric Substances in the Toxicity Response of Anaerobic Granule Sludge to Different Metal Oxide Nanoparticles. International Journal of Environmental Research and Public Health, 19(9), 5371. https://doi.org/10.3390/ijerph19095371






