Interventions to Improve Body Composition, Upper and Lower Extremity Muscle Strength, and Balance Ability of Older Female Adults: An Intervention Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Subjects
2.2. Research Materials
2.3. Testing Method
2.3.1. Body Composition
2.3.2. Fitness Test
2.3.3. Sarcopenia Test
2.4. Statistical Analysis
3. Results
3.1. Body Composition Testing
3.2. Performance of Upper and Lower Extremity Muscle Strength
3.3. Performance of Balance Ability
3.4. Analysis of Upper Limb Handgrip Strength and Lower Limb Gait Speed
3.5. Evaluated Analysis of Sarcopenia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fiber loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A contemporary health problem among older adult populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Meier, N.F.; Lee, D. Physical activity and sarcopenia in older adults. Aging Clin. Exp. Res. 2020, 32, 1675–1687. [Google Scholar] [CrossRef] [Green Version]
- Kreissl, A.; Jorda, A.; Truschner, K.; Skacel, G.; Greber-Platzer, S. Clinically relevant body composition methods for obese pediatric patients. BMC Pediatr. 2019, 19, 84. [Google Scholar] [CrossRef]
- Cunningham, J.J. Body composition and resting metabolic rate: The myth of feminine metabolism. Am. J. Clin. Nutr. 1982, 36, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Sun, Y.; Zhang, T.; Zou, L.; Wu, X.; Wang, D.; Chen, Z. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: A systematic review and meta-analysis. Aging Dis. 2020, 11, 863–873. [Google Scholar] [CrossRef]
- Geidl, W.; Wais, J.; Fangmann, C.; Demisse, E.; Pfeifer, K.; Sudeck, G. Physical activity promotion in daily exercise therapy: The perspectives of exercise therapists in German rehabilitation settings. BMC Sports Sci. Med. Rehabil. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, J.W., III; Merkas, J.; Pilutti, L.A. The effect of exercise training on gait, balance, and physical fitness asymmetries in persons with chronic neurological zonditions: A systematic review of randomized controlled trials. Front. Physiol. 2020, 11, 585765. [Google Scholar] [CrossRef] [PubMed]
- López-Teros, T.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Gait speed and handgrip strength as predictors of incident disability in Mexican older adults. J. Frailty Aging 2014, 3, 109–112. [Google Scholar] [CrossRef]
- Franzon, K.; Zethelius, B.; Cederholm, T.; Kilander, L. The impact of muscle function, muscle mass and sarcopenia on independent ageing in very old Swedish men. BMC Geriatr. 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Ikezoe, T.; Tabara, Y.; Matsuda, F.; Tsuboyama, T.; Ichihashi, N. Differences in lower limb muscle strength and balance ability between sarcopenia stages depend on sex in community-dwelling older adults. Aging Clin. Exp. Res. 2022, 34, 527–534. [Google Scholar] [CrossRef]
- Gadelha, A.B.; Neri, S.G.R.; Oliveira, R.J.; Bottaro, M.; David, A.C. Severity of sarcopenia is associated with postural balance and risk of falls in community-dwelling older women. Exp. Aging Res. 2018, 44, 258–269. [Google Scholar] [CrossRef]
- Cho, K.H.; Bok, S.K.; Kim, Y.-J.; Hwang, S.L. Effect of lower limb strength on falls and balance of the elderly. Ann. Rehabil. Med. 2012, 36, 386–393. [Google Scholar] [CrossRef]
- de Mello, R.; Dalla Corte, R.R.; Gioscia, J.; Moriguchi, E.H. Effects of physical exercise programs on sarcopenia management, dynapenia, and physical performance in the elderly: A systematic review of randomized clinical trials. J. Aging Res. 2019, 2019, 1959486. [Google Scholar] [CrossRef] [Green Version]
- Granic, A.; Hurst, C.; Dismore, L.; Davies, K.; Stevenson, E.; Sayer, A.A.; Aspray, T. Milk and resistance exercise intervention to improve muscle function in community-dwelling older adults at risk of sarcopenia (MIlkMAN): Protocol for a pilot study. BMJ Open 2019, 9, e031048. [Google Scholar] [CrossRef]
- Akehurst, E.; Scott, D.; Rodriguez, J.P.; Gonzalez, C.A.; Murphy, J.; McCarthy, H.; Dorgo, S.; Hayes, A. Associations of sarcopenia components with physical activity and nutrition in Australian older adults performing exercise training. BMC Geriatr. 2021, 21, 276. [Google Scholar] [CrossRef]
- Jefferis, B.J.; Sartini, C.; Lee, I.M.; Choi, M.; Amuzu, A.; Gutierrez, C.; Casas, J.P.; Ash, S.; Lennnon, L.T.; Wannamethee, S.G.; et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health 2014, 14, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardstu, H.B.; Andersen, V.; Fimland, M.S.; Aasdahl, L.; Raastad, T.; Cumming, K.T. Effectiveness of a resistance training program on physical function, muscle strength, and body composition in community-dwelling older adults receiving home care: A cluster-randomized controlled trial. Eur. Rev. Aging Phys. Act. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, D.; Chen, X.; Chen, Y. What intensity of exercise is most suitable for the elderly in China? A propensity score matching analysis. BMC Public Health 2021, 21, 396. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Wai, J.P.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.; Lee, M.C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Owusu, C.; Margevicius, S.; Schluchter, M.; Koroukian, S.M.; Berger, N.A. Short physical performance battery, usual gait speed, grip strength and vulnerable elders survey each predict functional decline among older women with breast cancer. J. Geriatr. Oncol. 2017, 8, 356–362. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Hillside: New York, NY, USA, 1988; pp. 20–26. [Google Scholar]
- Freiberger, E.; Sieber, C.; Pfeifer, K. Physical activity, exercise, and sarcopenia—Future challenges. Wien. Med. Wochenschr. 2011, 61, 416–425. [Google Scholar] [CrossRef]
- Füzéki, E.; Banzer, W. Physical activity recommendations for health and beyond in currently inactive populations. Int. J. Environ. Res. Public Health 2018, 15, 1042. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.; Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Müller, S.; Scharhag, J. The intensity and effects of strength training in the elderly. Dtsch. Arztebl. Int. 2011, 108, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zouita, S.; Zouhal, H.; Ferchichi, H.; Paillard, T.; Dziri, C.; Hackney, A.C.; Laher, I.; Granacher, U.; Ben Moussa Zouita, A. Effects of combined balance and strength training on measures of balance and muscle strength in older women with a history of falls. Front. Physiol. 2020, 11, 619016. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, A.; Paoli, A.; Andretta, M.; Vidorin, F.; Marcolin, G. Are static and dynamic postural balance assessments two sides of the same coin? A cross-sectional study in the older adults. Front. Physiol. 2021, 12, 681370. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sousa, M.A.; Venegas-Sanabria, L.C.; Chavarro-Carvajal, D.A.; Cano-Gutierrez, C.A.; Izquierdo, M.; Correa-Bautista, J.E.; Ramírez-Vélez, R. Gait speed as a mediator of the effect of sarcopenia on dependency in activities of daily living. J. Cachexia Sarcopenia Muscle 2019, 10, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, P.; Han, Y.; Pang, J.; Wu, S.; Gong, H.; Zhu, J.; Li, J.; Zhang, T. Sarcopenia-related features and factors associated with lower muscle strength and physical performance in older Chinese: A cross sectional study. BMC Geriatr. 2016, 16, 45. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, G.F.; Landolfo, C.; Niebauer, J.; Ozemek, C.; Arena, R.; Lavie, C.J. Promoting physical activity and exercise: JACC health promotion series. J. Am. Coll. Cardiol. 2018, 72, 1622–1639. [Google Scholar] [CrossRef]
- Li, K.W.; Yu, R.F. Assessment of grip force and subjective hand force exertion under handedness and postural conditions. Appl. Ergon. 2011, 42, 929–933. [Google Scholar] [CrossRef]
- Bai, C.H.; Alizargar, J.; Peng, C.Y.; Wu, J.P. Combination of exercise training and resveratrol attenuates obese sarcopenia in skeletal muscle atrophy. Chin. J. Physiol. 2020, 63, 101–112. [Google Scholar] [CrossRef]
- Sousa, N.; Mendes, R.; Abrantes, C.; Sampaio, J. Differences in maximum upper and lower limb strength in older adults after a 12 week intense resistance training program. J. Hum. Kinet. 2011, 30, 183–188. [Google Scholar] [CrossRef] [PubMed]
| Variable | CG (n = 15) M ± SD | EG (n = 15) M ± SD | t | p-Value |
|---|---|---|---|---|
| Age (years) | 72.1 ± 5.7 | 71.8 ± 6.1 | −0.74 | 0.591 |
| Height (cm) | 157.1 ± 8.2 | 157.8 ± 7.0 | 0.61 | 0.623 |
| Weight (kg) | 68.0 ± 13.5 | 68.5 ± 10.4 | 0.83 | 0.477 |
| Course | Posture and Motions | Operation Method |
|---|---|---|
| Cardiopulmonary function | Step-ups 20 (times) × 3 (set) | 30 cm high steps: go up the steps with one foot, stand with both feet together, go down the steps with one foot, stand with both feet together and repeat. |
| Lower body strength | Chair squats 15 (times) × 3(set) | Place a chair behind you and start in the standing position; squat down and raise your hands horizontally, and then squat down and touch the chair with your buttocks and immediately rise into a standing position. Do this once. |
| Pistol squat 10 (times) × 3 (set) | Stand on one foot and leave the other foot off the ground (you can hold a support) and perform 10 deep squats on the left and right feet. | |
| Standing lunges 20 (times) × 3 (set) | Start in a standing position; put your feet together. Step on one foot (with a stride of more than 60 cm), retract the leg that has been stepped out, and step out with the other foot. Repeat the motion with your left and right feet 20 times. | |
| Walk in place with high legs 20 (times) × 3 (set) | Start a standing position, raise the leg in place up to thigh-level, and repeat the operation with the left and right feet 20 times. | |
| Upper body strength | 5-pound dumbbell arm curls 12 (times) × 3(set) | Stand vertically with dumbbells in hand and perform flexion movements with both hands at the same time. |
| 5-pound dumbbell flyers 12 (times) × 3(set) | Stand vertically with dumbbells in your hands and abduct your hands to a horizontal level at the same time. | |
| 5-pound dumbbell shoulder raises 12 (times) × 3(set) | Hold the dumbbells at shoulder height as the starting point, raise both hands vertically at the same time, and then return to the starting point. |
| Assessment Category | Test Item | Test Description |
|---|---|---|
| Lower body strength | 30 s chair stand | Number of full stands in 30 s with arms folded across chest |
| Upper body strength | 30 s arm curl | Number of bicep curls in 30 s holding a hand weight (women’s 5 lb) |
| Aerobic endurance | 2 min step | Number of full steps completed by raising each knee to point midway between the patella and iliac crest (number of times knee reaches target) in 2 min |
| Static balance | Single leg (SL) | Participants must lift one leg off the ground and maintain their balance while standing |
| Dynamic balance | 8-foot up-and-go | Number of seconds required to get up from a seated position, walk 8 feet (2.44 m), turn around, and return to a seated position on the chair |
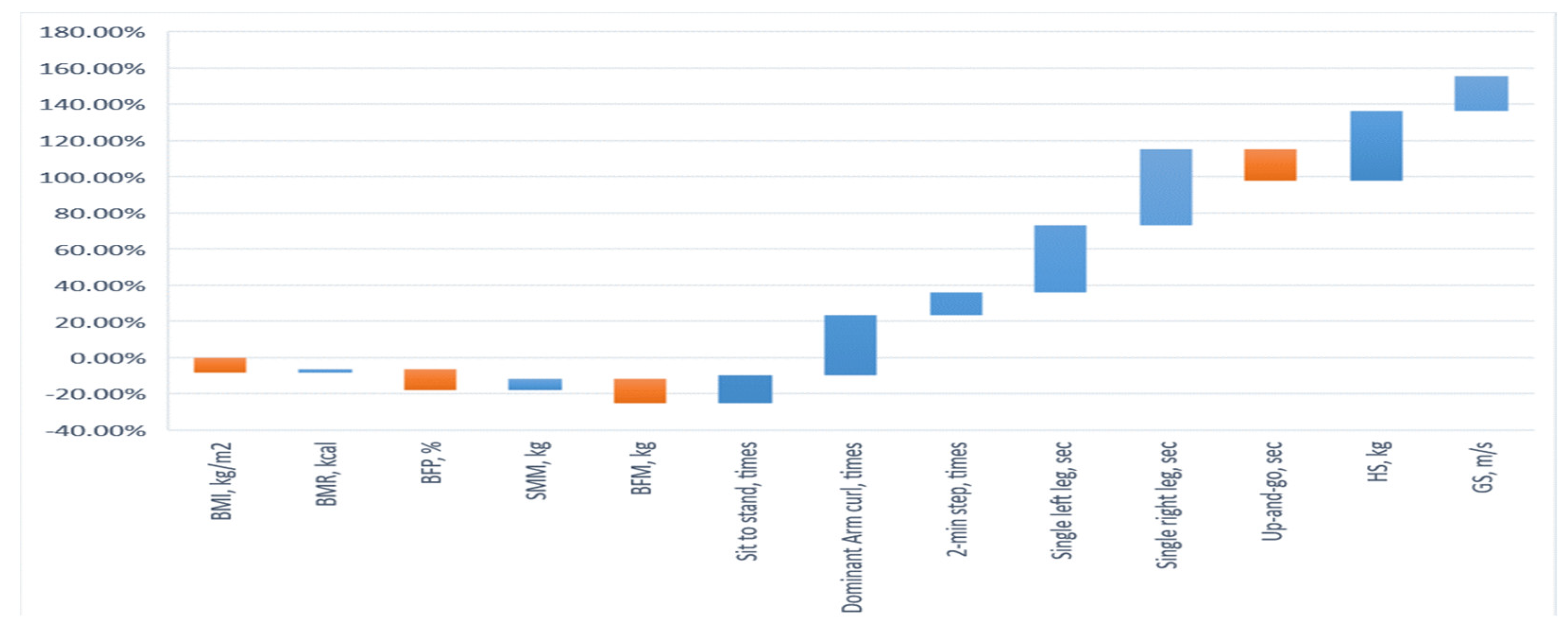
| Variables | CG (n = 15) | EG (n = 15) | Improvement (%) | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| BMI, kg/m2 | 27.29 ± 2.41 | 27.38 ± 2.48 | 27.30 ± 2.14 | 25.22 ± 2.00 | −8.24 |
| BMR, kcal | 1207 ± 66.42 | 1213 ± 72.67 | 1220 ± 94.0 | 1245 ± 101.8 | 2.05 |
| BFP, % | 32.19 ± 5.20 | 32.43 ± 5.56 | 32.30 ± 5.25 | 28.95 ± 4.01 | −11.57 |
| SMM, kg | 26.23 ± 2.52 | 26.01 ± 2.50 | 26.69 ± 2.00 | 28.35 ± 2.25 | 6.23 |
| BFM, kg | 21.79 ± 1.99 | 21.64 ± 1.97 | 21.80 ± 2.08 | 19.18 ± 2.07 | −13.66 |
| Sit to stand, times | 18.93 ± 2.89 | 19.00 ± 3.05 | 18.93 ± 3.13 | 21.87 ± 2.90 | 15.53 |
| Dominant arm curl, times | 20.4 ± 3.00 | 20.27 ± 2.69 | 20.33 ± 3.17 | 27.13 ± 3.64 | 33.45 |
| 2 min step, times | 113.2 ± 7.55 | 112.87 ± 6.25 | 113.13 ± 6.92 | 127.27 ±8.55 | 12.50 |
| Single left leg, sec | 15.76 ± 7.36 | 15.51 ± 6.58 | 15.67 ± 9.39 | 21.47 ± 8.99 | 37.01 |
| Single right leg, sec | 12.91 ± 6.91 | 12.95 ± 6.49 | 12.85 ± 7.76 | 18.24 ± 7.47 | 41.79 |
| Up-and-go, sec | 6.19 ± 0.99 | 6.24 ± 0.94 | 6.29 ± 1.63 | 5.37 ± 0.90 | −17.13 |
| HS, kg | 9.66 ± 0.71 | 9.65 ± 0.69 | 9.64 ± 0.61 | 13.35 ± 1.16 | 38.49 |
| GS, m/s | 0.74 ± 0.05 | 0.73 ± 0.05 | 0.73 ± 0.05 | 0.87 ± 0.09 | 19.18 |
| Variables | CG-Pre | EG-Pre | EG-Pre | EG-Post |
|---|---|---|---|---|
| CG-Post | CG-Pre | EG-Post | CG-Post | |
| BMI, kg/m2 | −1.40 | 0.64 | 6.29 * | −6.50 * |
| BMR, kcal | −1.57 | 1.01 | −5.06 * | 2.44 * |
| BFP, % | −1.51 | 0.383 | 8.48 * | −7.63 * |
| SMM, kg | 1.78 | 1.03 | −8.03 * | 7.93 * |
| BFM, kg | 1.01 | 0.42 | 5.12 * | −5.27 * |
| Sit to stand, times | 0.24 | 0.01 | −3.87 * | 4.01 * |
| Dominant arm curl, times | −0.20 | −0.20 | −6.58 * | 6.15 * |
| 2 min step, times | 0.53 | −0.04 | −3.34 * | 3.04 * |
| Single left leg, sec | −0.11 | −0.08 | −6.65 * | 6.21 * |
| Single right leg, sec | 0.54 | −0.05 | −7.51 * | 5.50 * |
| Up-and-go, sec | −1.48 | 1.03 | 7.09 * | −7.70 * |
| HS, kg | 0.135 | −0.11 | −20.03 * | 8.64 * |
| GS, m/s | 2.18 | −0.32 | −16.55 * | 9.43 * |
| Model | R | R2 | Adjusted R2 | Estimated Standard Error | R2 Change | F Change | Sig |
|---|---|---|---|---|---|---|---|
| 1 | 0.879 (a) | 0.773 | 0.716 | 0.945 | 0.773 | 53.905 * | 0.003 |
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (Constant) | 5.513 | 0.613 | - | 7.342 * | 0.000 |
| BMI | 0.224 | 0.030 | 0.413 | 6.718 * | 0.000 |
| BMR | 0.212 | 0.006 | 0.399 | 6.289 * | 0.000 |
| BFP | 0.318 | 0.001 | 0.532 | 7.163 * | 0.000 |
| SMM | 0.244 | 0.060 | 0.364 | 5.898 * | 0.008 |
| BFM | 0.242 | 0.055 | 0.353 | 4.914 * | 0.017 |
| Dominant arm curl | 0.329 | 0.082 | 0.541 | 7.329 * | 0.000 |
| Model | R | R2 | Adjusted R2 | Estimated Standard Error | R2 Change | F Change | Sig |
|---|---|---|---|---|---|---|---|
| 2 | 0.984 (a) | 0.969 | 0.905 | 0.029 | 0.969 | 36.699 * | 0.001 |
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (Constant) | 6.637 | 0.715 | 6.058 * | 0.000 | |
| BMI | 0.218 | 0.034 | 0.352 | 4.039 * | 0.016 |
| BMR | 0.203 | 0.046 | 0.316 | 3.221 * | 0.037 |
| BFP | 0.212 | 0.038 | 0.340 | 3.791 * | 0.029 |
| SMM | 0.248 | 0.022 | 0.540 | 7.048 * | 0.000 |
| BFM | 0.232 | 0.027 | 0.398 | 5.093 * | 0.003 |
| Sit to stand | 0.225 | 0.031 | 0.377 | 4.757 * | 0.007 |
| 2 min step | 0.233 | 0.025 | 0.415 | 6.831 * | 0.000 |
| Single left leg | 0.209 | 0.039 | 0.338 | 3.824 * | 0.025 |
| Single right leg | 0.213 | 0.036 | 0.342 | 3.833 * | 0.019 |
| Up-and-go | 0.216 | 0.029 | 0.357 | 4.229 * | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-Y.; Wu, C.-E. Interventions to Improve Body Composition, Upper and Lower Extremity Muscle Strength, and Balance Ability of Older Female Adults: An Intervention Study. Int. J. Environ. Res. Public Health 2022, 19, 4765. https://doi.org/10.3390/ijerph19084765
Huang W-Y, Wu C-E. Interventions to Improve Body Composition, Upper and Lower Extremity Muscle Strength, and Balance Ability of Older Female Adults: An Intervention Study. International Journal of Environmental Research and Public Health. 2022; 19(8):4765. https://doi.org/10.3390/ijerph19084765
Chicago/Turabian StyleHuang, Wei-Yang, and Cheng-En Wu. 2022. "Interventions to Improve Body Composition, Upper and Lower Extremity Muscle Strength, and Balance Ability of Older Female Adults: An Intervention Study" International Journal of Environmental Research and Public Health 19, no. 8: 4765. https://doi.org/10.3390/ijerph19084765
APA StyleHuang, W.-Y., & Wu, C.-E. (2022). Interventions to Improve Body Composition, Upper and Lower Extremity Muscle Strength, and Balance Ability of Older Female Adults: An Intervention Study. International Journal of Environmental Research and Public Health, 19(8), 4765. https://doi.org/10.3390/ijerph19084765






