Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Sources and Searches
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Assessment of Study Quality and Data Synthesis
3. Results
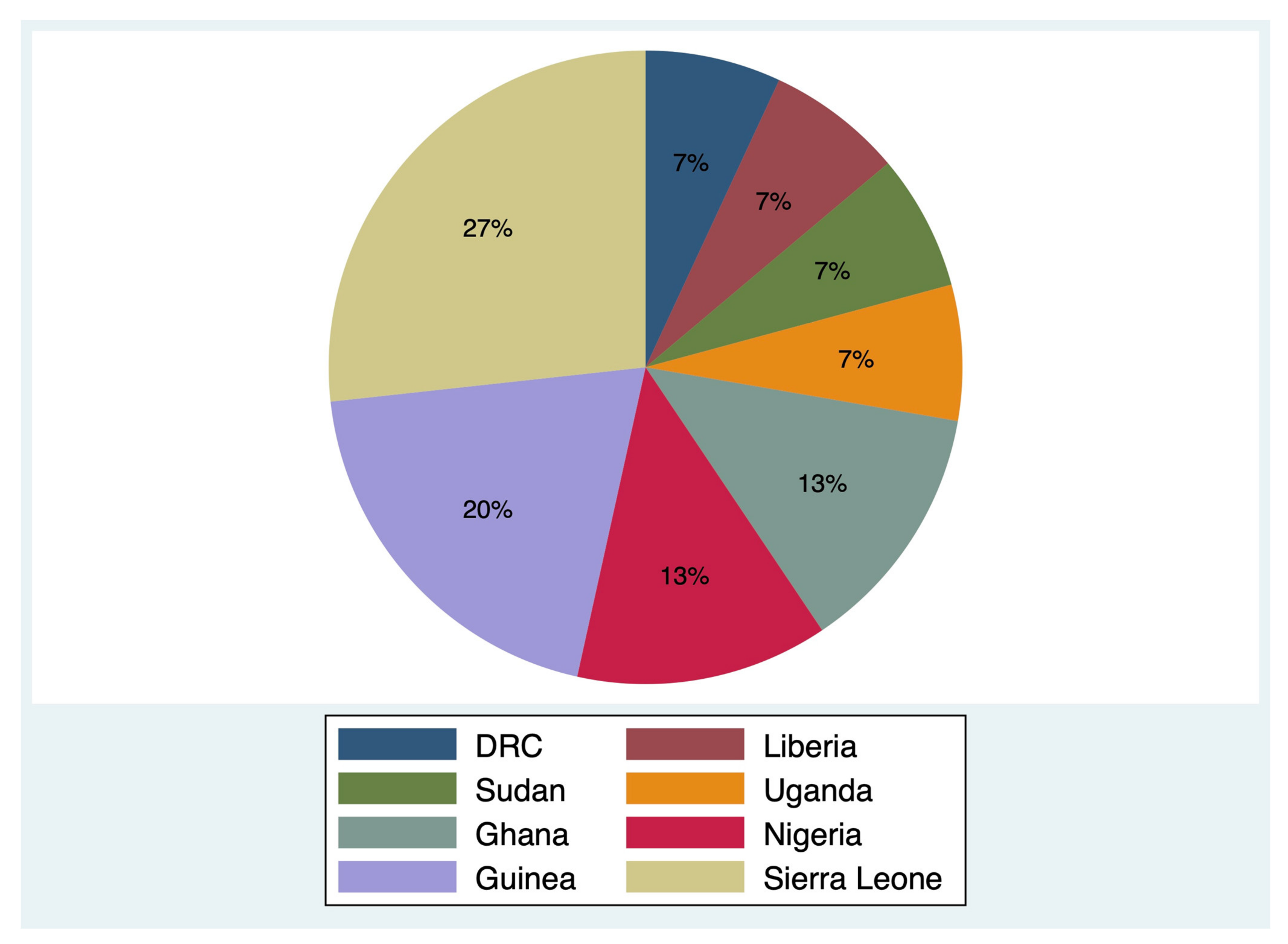
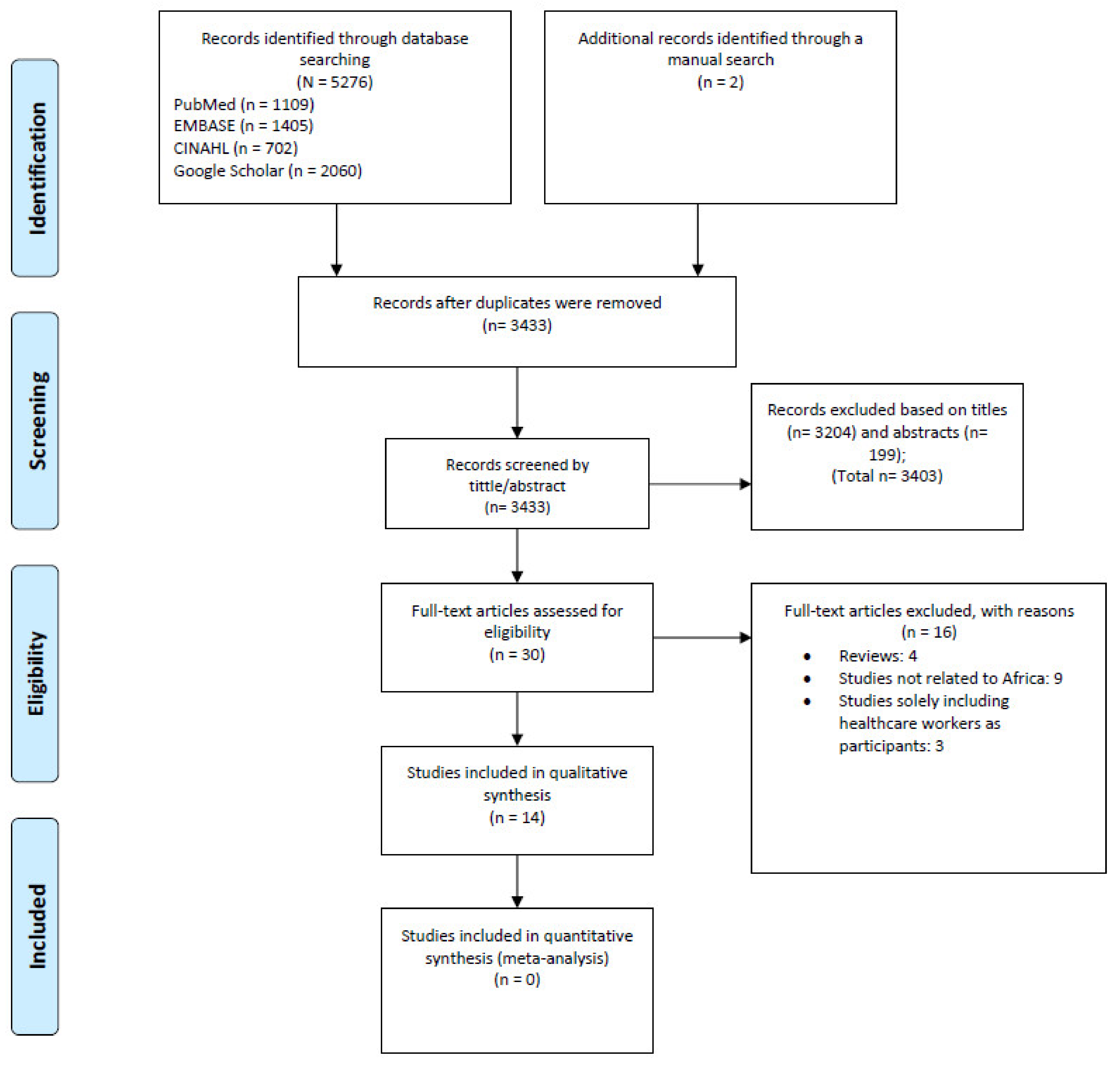
3.1. Study Selection and Description of Studies
| First Author (Year) | Study Design | Setting | Study Population (Focus Groups) | Age (Year) | Sample Size | KAP Element | Data Collection Instrument | Data Collection Period | Study Quality (Score) * |
|---|---|---|---|---|---|---|---|---|---|
| Claude (2018) [16] | CS and qualitative research | DRC | Community, IDPs, pygmy, and HCWs | ≥25 and 15–24 | 582 | K; A; P | Prepared by the authors with reference to previously published KAP | 2018 | High (9) |
| Winters (2018) [17] | CS | Sierra Leone | Community | 21–35 | 10,604 | K; A; P | Prepared by the authors | 2014–2015 | High (9) |
| Tenkorang (2018) [18] | CS | Ghana | Community | 18–69 | 800 | K; P | Prepared by the author | 2016 | High (9) |
| Jalloh (2017) [21] | CS | Sierra Leone | Community | ≥25 and 15–24 | 1413 | K; A; P | Prepared by the authors with reference to previously published KAP | 2014 | High (10) |
| Jalloh (2017) [20] | CS | Guinea | Community | ≥25 and 15–24 | 6273 | K; A; P | NR | 2015 | High (9) |
| Jalloh (2017) [19] | CS | Sierra Leone and Guinea | Community | 35–40 | 1137 | K; A; P | Prepared by the authors with reference to previously published KAP | 2015 | High (8) |
| Mohamed (2017) [22] | CS | Sudan | Community | >18 | 1255 | K; A; P | Prepared by the authors | 2015 | High (8) |
| Nyakarahuka (2017) [29] | CS | Uganda | Community | 33 | 740 | K; A | Prepared by the authors | 2015 | High (10) |
| Jiang (2016) [23] | CS | Sierra Leone | Community | NR | 466 | K; A; P | Prepared by the authors | 2015 | Moderate (7) |
| Adongo ** (2016) [24] | Qualitative research | Ghana | Community and nurses | NR | 235 | K; A | Prepared by the authors | 2015 | - |
| Iliyasu (2015) [25] | CS | Nigeria | Community and HCWs | 32 | 1035 | K; A; P | Prepared by the authors with reference to previously published KAP | 2014 | High (9) |
| Buli (2015) [26] | CS | Guinea | Community | ≥25 and 18–24 | 358 | K; A; P | Prepared by the authors | 2014–2015 | Moderate (7) |
| Gidado (2015) [27] | CS | Nigeria | Community | 34 | 5322 | K; A; P | Prepared by the authors | 2014 | Moderate (7) |
| Kobayashi (2015) [28] | CS | Liberia | Community | 32 | 609 | K; A; P | NR | 2014 | High (8) |
3.2. Methodological Assessment of Included Studies
3.3. Knowledge, Awareness, and Rumors about EBOLA
3.4. Attitudes, Beliefs, and Misconceptions
3.5. Practices on Ebola Prevention and Treatment
4. Discussion
4.1. Limited Biomedical Knowledge about Ebola
4.2. Perceived Causes of Ebola That Are beyond the Bio-Medical Science Paradigm and Intention to Choose Unorthodox Treatment
4.3. Reduced Trust in Health Authorities, and Hospitals Seen as a Place to Die
4.4. High-Risk Behaviors Due to Unsafe Funeral/Burial Practices
4.5. Stigma
4.6. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Ebola Virus Disease. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease (accessed on 21 February 2022).
- Vetter, P.; Kaiser, L.; Schibler, M.; Ciglenecki, I.; Bausch, D.G. Sequelae of Ebola virus disease: The emergency within the emergency. Lancet Infect. Dis. 2016, 16, e82–e91. [Google Scholar] [CrossRef]
- Clark, D.V.; Kibuuka, H.; Millard, M.; Wakabi, S.; Lukwago, L.; Taylor, A.; Eller, M.A.; Eller, L.A.; Michael, N.L.; Honko, A.N.; et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 905–912. [Google Scholar] [CrossRef]
- Mate, S.E.; Kugelman, J.R.; Nyenswah, T.G.; Ladner, J.T.; Wiley, M.R.; Cordier-Lassalle, T.; Christie, A.; Schroth, G.P.; Gross, S.M.; Davies-Wayne, G.J.; et al. Molecular evidence of sexual transmission of Ebola Virus. N. Engl. J. Med. 2015, 373, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Awah, P.K.; Boock, A.U.; Kum, K.A. Ebola Virus Diseases in Africa: A commentary on its history, local and global context. Pan Afr. Med. J. 2015, 22 (Suppl. 1), 18. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Stringer, B.; Bark, G.; Heller Perache, A.; Jephcott, F.; Broeder, R.; Kremer, R.; Jimissa, A.S.; Samba, T.T. When Ebola enters a home, a family, a community: A qualitative study of population perspectives on Ebola control measures in rural and urban areas of Sierra Leone. PLoS Negl. Trop. Dis. 2018, 12, e0006461. [Google Scholar] [CrossRef] [PubMed]
- Leach, M. Time to put Ebola in context. Interview with Dr Melissa Leach. Bull. World Health Organ. 2010, 88, 488–489. [Google Scholar] [CrossRef]
- Roca, A.; Afolabi, M.O.; Saidu, Y.; Kampmann, B. Ebola: A holistic approach is required to achieve effective management and control. J. Allergy Clin. Immunol. 2015, 135, 856–867. [Google Scholar] [CrossRef]
- Coltart, C.E.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola outbreak, 2013–2016: Old lessons for new epidemics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160297. [Google Scholar] [CrossRef]
- Hersey, S.; Martel, L.D.; Jambai, A.; Keita, S.; Yoti, Z.; Meyer, E.; Seeman, S.; Bennett, S.; Ratto, J.; Morgan, O.; et al. Ebola Virus Disease—Sierra Leone and Guinea, August 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 981–984. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Ntontolo, N.P.; Ngatu, N.R.; Khatiwada, J.; Ngombe, K.L.; Numbi, O.L.; Nzaji, K.M.; Maotela, K.J.; Ngoyi, M.J.; Suzuki, T.; et al. Local perspectives on Ebola during its tenth outbreak in DR Congo: A nationwide qualitative study. PLoS ONE 2020, 15, e0241120. [Google Scholar] [CrossRef]
- United States Agency for International Development (USAID). The KAP Survey Model (Knowledge, Attitudes, and Practices). Available online: https://www.spring-nutrition.org/publications/tool-summaries/kap-survey-model-knowledge-attitudes-and-practices (accessed on 22 May 2020).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Qualitative Checklist. 2018. Available online: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Qualitative-Checklist-2018_fillable_form.pdf (accessed on 14 February 2022).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Claude, K.M.; Underschultz, J.; Hawkes, M.T. Ebola virus epidemic in war-torn eastern DR Congo. Lancet 2018, 392, 1399–1401. [Google Scholar] [CrossRef]
- Winters, M.; Jalloh, M.F.; Sengeh, P.; Jalloh, M.B.; Conteh, L.; Bunnell, R.; Li, W.; Zeebari, Z.; Nordenstedt, H. Risk communication and Ebola-specific knowledge and behavior during 2014–2015 outbreak, Sierra Leone. Emerg. Infect. Dis. 2018, 24, 336–344. [Google Scholar] [CrossRef]
- Tenkorang, E.Y. Effect of knowledge and perceptions of risks on Ebola-preventive behaviours in Ghana. Int. Health 2018, 10, 202–210. [Google Scholar] [CrossRef]
- Jalloh, M.F.; Sengeh, P.; Monasch, R.; Jalloh, M.B.; DeLuca, N.; Dyson, M.; Golfa, S.; Sakurai, Y.; Conteh, L.; Sesay, S.; et al. National survey of Ebola-related knowledge, attitudes and practices before the outbreak peak in Sierra Leone: August 2014. BMJ Glob. Health 2017, 2, e000285. [Google Scholar] [CrossRef]
- Jalloh, M.F.; Robinson, S.J.; Corker, J.; Li, W.; Irwin, K.; Barry, A.M.; Ntuba, P.N.; Diallo, A.A.; Jalloh, M.B.; Nyuma, J.; et al. Knowledge, attitudes, and practices related to Ebola virus disease at the end of a national epidemic—Guinea, August 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1109–1115. [Google Scholar] [CrossRef]
- Jalloh, M.F.; Bunnell, R.; Robinson, S.; Jalloh, M.B.; Barry, A.M.; Corker, J.; Sengeh, P.; VanSteelandt, A.; Li, W.; Dafae, F.; et al. Assessments of Ebola knowledge, attitudes and practices in Forecariah, Guinea and Kambia, Sierra Leone, July–August 2015. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160304. [Google Scholar] [CrossRef]
- Mohamed, M.M.G.; Shwaib, H.M.; Fahim, M.M.; Ahmed, E.A.; Omer, M.K.; Monier, I.A.; Balla, S.A. Ebola hemorrhagic fever under scope, view of knowledge, attitude and practice from rural Sudan in 2015. J. Infect. Public Health 2017, 10, 287–294. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, G.Q.; Tu, W.X.; Zheng, C.J.; Lai, X.H.; Li, X.X.; Wei, Q.; Li, M.; Deng, L.Q.; Huo, X.; et al. Rapid assessment of knowledge, attitudes, practices, and risk perception related to the prevention and control of Ebola virus disease in three communities of Sierra Leone. Infect. Dis. Poverty 2016, 5, 53. [Google Scholar] [CrossRef][Green Version]
- Adongo, P.B.; Tabong, P.T.; Asampong, E.; Ansong, J.; Robalo, M.; Adanu, R.M. Beyond knowledge and awareness: Addressing misconceptions in Ghana’s preparation towards an outbreak of Ebola virus disease. PLoS ONE 2016, 11, e0149627. [Google Scholar] [CrossRef]
- Iliyasu, G.; Ogoina, D.; Otu, A.A.; Dayyab, F.M.; Ebenso, B.; Otokpa, D.; Rotifa, S.; Olomo, W.T.; Habib, A.G. A Multi-site knowledge attitude and practice survey of Ebola virus disease in Nigeria. PLoS ONE 2015, 10, e0135955. [Google Scholar] [CrossRef]
- Buli, B.G.; Mayigane, L.N.; Oketta, J.F.; Soumouk, A.; Sandouno, T.E.; Camara, B.; Toure, M.S.; Conde, A. Misconceptions about Ebola seriously affect the prevention efforts: KAP related to Ebola prevention and treatment in Kouroussa Prefecture, Guinea. Pan Afr. Med. J. 2015, 22 (Suppl. 1), 11. [Google Scholar] [CrossRef]
- Gidado, S.; Oladimeji, A.M.; Roberts, A.A.; Nguku, P.; Nwangwu, I.G.; Waziri, N.E.; Shuaib, F.; Oguntimehin, O.; Musa, E.; Nzuki, C.; et al. Public knowledge, perception and source of information on Ebola virus disease—Lagos, Nigeria; September, 2014. PLoS Curr. 2015, 7, ecurrents.outbreaks.0b805cac244d700a47d6a3713ef2d6db. [Google Scholar] [CrossRef]
- Kobayashi, M.; Beer, K.D.; Bjork, A.; Chatham-Stephens, K.; Cherry, C.C.; Arzoaquoi, S.; Frank, W.; Kumeh, O.; Sieka, J.; Yeiah, A.; et al. Community knowledge, attitudes, and practices regarding Ebola virus disease—Five counties, Liberia, September–October, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 714–718. [Google Scholar]
- Nyakarahuka, L.; Skjerve, E.; Nabadda, D.; Sitali, D.C.; Mumba, C.; Mwiine, F.N.; Lutwama, J.J.; Balinandi, S.; Shoemaker, T.; Kankya, C. Knowledge and attitude towards Ebola and Marburg virus diseases in Uganda using quantitative and participatory epidemiology techniques. PLoS Negl. Trop. Dis. 2017, 11, e0005907. [Google Scholar] [CrossRef]
- Stehling-Ariza, T.; Rosewell, A.; Moiba, S.A.; Yorpie, B.B.; Ndomaina, K.D.; Jimissa, K.S.; Leidman, E.; Rijken, D.J.; Basler, C.; Wood, J.; et al. The impact of active surveillance and health education on an Ebola virus disease cluster—Kono District, Sierra Leone, 2014–2015. BMC Infect. Dis. 2016, 16, 611. [Google Scholar] [CrossRef]
- Buseh, A.G.; Stevens, P.E.; Bromberg, M.; Kelber, S.T. The Ebola epidemic in West Africa: Challenges, opportunities, and policy priority areas. Nurs. Outlook 2015, 63, 30–40. [Google Scholar] [CrossRef]
- Hodel, E.M.; Kabanywanyi, A.M.; Malila, A.; Zanolari, B.; Mercier, T.; Beck, H.P.; Buclin, T.; Olliaro, P.; Decosterd, L.A.; Genton, B. Residual antimalarials in malaria patients from Tanzania—Implications on drug efficacy assessment and spread of parasite resistance. PLoS ONE 2009, 4, e8184. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Moore, K.A.; Kelley, N.S.; Brosseau, L.M.; Wong, G.; Murphy, F.A.; Peters, C.J.; LeDuc, J.W.; Russell, P.K.; Van Herp, M.; et al. Transmission of Ebola viruses: What we know and what we do not know. MBio 2015, 6, e00137. [Google Scholar] [CrossRef]
- Kasereka, M.C.; Hawkes, M.T. The cat that kills people: Community beliefs about Ebola origins and implications for disease control in Eastern Democratic Republic of the Congo. Pathog. Glob. Health 2019, 113, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Oduyemi, R.O.; Ayegboyin, M.; Salami, K.K. Perceptions of Ebola virus disease in Nigeria: Understanding the influence of imagination on health orientation. Int. J. Nurs. Pract. 2016, 22, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Kelly, J.D. Community Trust and the Ebola Endgame. N. Engl. J. Med. 2015, 373, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, B.; Lidow, N.; Ayscue, P.; Saylors, K.; Mbala, P.; Kumakamba, C.; Kleinman, M. Knowledge and beliefs about Ebola virus in a conflict-affected area: Early evidence from the North Kivu outbreak. J. Glob. Health 2019, 9, 020311. [Google Scholar] [CrossRef]
- Vinck, P.; Pham, P.N.; Bindu, K.K.; Bedford, J.; Nilles, E.J. Institutional trust and misinformation in the response to the 2018–19 Ebola outbreak in North Kivu, DR Congo: A population-based survey. Lancet Infect. Dis. 2019, 19, 529–536. [Google Scholar] [CrossRef]
- Wells, C.R.; Pandey, A.; Ndeffo Mbah, M.L.; Gauzere, B.A.; Malvy, D.; Singer, B.H.; Galvani, A.P. The exacerbation of Ebola outbreaks by conflict in the Democratic Republic of the Congo. Proc. Natl. Acad. Sci. USA 2019, 116, 24366–24372. [Google Scholar] [CrossRef]
- Parker, M.; Hanson, T.M.; Vandi, A.; Babawo, L.S.; Allen, T. Ebola, community engagement, and saving loved ones. Lancet 2019, 393, 2585. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Factors That Contributed to Undetected Spread of the Ebola Virus and Impeded Rapid Containment. 2015. Available online: https://www.who.int/csr/disease/ebola/one-year-report/factors/en (accessed on 25 June 2020).
- Victory, K.R.; Coronado, F.; Ifono, S.O.; Soropogui, T.; Dahl, B.A.; Centers for Disease Control and Prevention (CDC). Ebola transmission linked to a single traditional funeral ceremony—Kissidougou, Guinea, December, 2014–January 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 386–388. [Google Scholar]
- Manguvo, A.; Mafuvadze, B. The impact of traditional and religious practices on the spread of Ebola in West Africa: Time for a strategic shift. Pan Afr. Med. J. 2015, 22 (Suppl. 1), 9. [Google Scholar] [CrossRef]
- Lyons, P.; Winters, M.; Zeebari, Z.; Schmidt-Hellerau, K.; Sengeh, P.; Jalloh, M.B.; Jalloh, M.F.; Nordenstedt, H. Engaging religious leaders to promote safe burial practices during the 2014–2016 Ebola virus disease outbreak, Sierra Leone. Bull. World Health Organ. 2021, 99, 271–279. [Google Scholar] [CrossRef]
- Lee-Kwan, S.H.; DeLuca, N.; Bunnell, R.; Clayton, H.B.; Turay, A.S.; Mansaray, Y. Facilitators and barriers to community acceptance of safe, dignified medical burials in the context of an Ebola epidemic, Sierra Leone, 2014. J. Health Commun. 2017, 22, 24–30. [Google Scholar] [CrossRef]
- Mahajan, A.P.; Sayles, J.N.; Patel, V.A.; Remien, R.H.; Sawires, S.R.; Ortiz, D.J.; Szekeres, G.; Coates, T.J. Stigma in the HIV/AIDS epidemic: A review of the literature and recommendations for the way forward. AIDS 2008, 22 (Suppl. 2), S67–S79. [Google Scholar] [CrossRef]
- Drain, P.K. Ebola: Lessons learned from HIV and tuberculosis epidemics. Lancet Infect. Dis. 2015, 15, 146–147. [Google Scholar] [CrossRef][Green Version]
- Lo, T.Q.; Marston, B.J.; Dahl, B.A.; De Cock, K.M. Ebola: Anatomy of an epidemic. Annu. Rev. Med. 2017, 68, 359–370. [Google Scholar] [CrossRef]
- Nuriddin, A.; Jalloh, M.F.; Meyer, E.; Bunnell, R.; Bio, F.A.; Jalloh, M.B.; Sengeh, P.; Hageman, K.M.; Carroll, D.D.; Conteh, L.; et al. Trust, fear, stigma and disruptions: Community perceptions and experiences during periods of low but ongoing transmission of Ebola virus disease in Sierra Leone, 2015. BMJ Glob. Health 2018, 3, e000410. [Google Scholar] [CrossRef]
- James, P.B.; Wardle, J.; Steel, A.; Adams, J. Post-Ebola psychosocial experiences and coping mechanisms among Ebola survivors: A systematic review. Trop. Med. Int. Health 2019, 24, 671–691. [Google Scholar] [CrossRef]
- Glanz, K.; Bishop, D.B. The role of behavioral science theory in development and implementation of public health interventions. Annu. Rev. Public Health 2010, 31, 399–418. [Google Scholar] [CrossRef]
- Masumbuko Claude, K.; Hawkes, M.T. Ebola crisis in Eastern Democratic Republic of Congo: Student-led community engagement. Pathog. Glob. Health 2020, 114, 218–223. [Google Scholar] [CrossRef]
- Sabuni, L.P. Dilemma with the local perception of causes of illnesses in central Africa: Muted concept but prevalent in everyday life. Qual. Health Res. 2007, 17, 1280–1291. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Kidd, S.; Sillah, A.R.; Davis, E.; Mermin, J.; Kilmarx, P.H.; Centers for Disease Control and Prevention (CDC). Improving burial practices and cemetery management during an Ebola virus disease epidemic—Sierra Leone, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 20–27. [Google Scholar]
| First Author, Year | KAP Element | Questionnaire | Authors’ Conclusion |
|---|---|---|---|
| Claude (2018) [16] | K; A; P | Open- and close-ended, and focus group discussion | High knowledge on transmission. However, respondents would practice traditional burials involving physical contact with a family member corpse; hide family members from health authorities. Knowledge among IDPs was low. Armed conflict impeded control efforts in eastern DRC. |
| Winters (2018) [17] | K; A; P | Open- and close-ended | Exposure to information sources was associated with higher knowledge and protective behaviors. Misconceptions and risk behavior were also prevalent. |
| Tenkorang (2018) [18] | K; P | Close-ended | High level of Ebola knowledge and awareness. However, misconceptions remained present. |
| Jalloh (2017) [21] | K; A; P | Open- and close-ended | Awareness of Ebola was high. However, misconceptions and stigma towards Ebola survivors were common. |
| Jalloh (2017) [20] | K; A; P | Open- and close-ended | Awareness of the cause of Ebola, its transmission, and prevention was high. However, nearly half of participants believed that Ebola could be transmitted by air or through mosquito bites. Stigma towards Ebola survivors was also prevalent. |
| Jalloh (2017) [19] | K; A; P | Open- and close-ended | High knowledge on prevention. However, some respondents endorsed stigma towards Ebola survivors. |
| Mohamed (2017) [22] | K; A; P | Open- and close-ended | Poor knowledge, a fair attitude, and suboptimal practices on Ebola. |
| Nyakarahuka (2017) [29] | K; A | Close-ended | Moderate knowledge about EVD and 60% of respondents had a positive towards practices to prevent and control Ebola. |
| Jiang (2016) [23] | K; A; P | Close-ended | After training, knowledge was high, and attitudes related to prevention was satisfactory. However, symptoms and transmission modes needed public education. |
| Adongo (2016) [24] | K; A | Focus group discussion and semi structured in-depth interviews | High level of Ebola knowledge and awareness. However, misconceptions on transmission were present. Potential stigma towards people who might be infected with Ebola or work with Ebola patients. |
| Iliyasu (2015) [25] | K; A; P | Self-administered | Ebola-related KAP was at suboptimal levels. However, myths and misconceptions remained present |
| Buli (2015) [26] | K; A; P | Close-ended | High level of Ebola awareness. However, comprehensive knowledge about Ebola was low. Misconceptions remained present. |
| Gidado (2015) [27] | K; A; P | Close-ended | Existence of gap in Ebola knowledge and perception. Misconceptions and stigma towards Ebola survivors were also prevalent. |
| Kobayashi (2015) [28] | K; A; P | Close-ended | Awareness of Ebola was high. However, knowledge of symptoms of Ebola was poor and stigma towards Ebola survivors and Ebola treatment units were common |
| Knowledge Gaps, Misconceptions and Rumors | Number of Studies, n (%) | Countries (% of Participants) * |
|---|---|---|
| Unaware of the transmission mode | 10 (71) | DRC (11%) [16], Ghana (-) [24], Sierra Leone (60–75%) [19,21], Guinea (10%) [26], Liberia (7–35%) [28], Nigeria (27%) [25], Sudan (30%) [22], and Uganda (49%) [29] |
| A person could contract Ebola from the air | 8 (57) | DRC (-) [16], Ghana (-) [24], Guinea (27%) [20], Guinea (26%) [26], Nigeria (12–42%) [25], Sierra Leone (19%) [19], Sudan (39%) [22], and Uganda (17%) [29] |
| Mosquito bites or houseflies can transmit Ebola | 6 (43) | DRC (-) [16], Ghana (-) [24], Guinea (61%) [19], Guinea (49%) [20], Guinea (27%) [26], and Uganda (11%) [29] |
| Intending to touch a suspected corpse or attending a traditional burial | 6 (43) | DRC (10%) [16], Ghana (17%) [18], Guinea (38%) [19], Nigeria (37%) [25], Sierra Leone (19%) [19], and Sudan (21%) [22] |
| Stigma towards Ebola survivors | 6 (43) | Ghana (-) [24], Guinea (11%) [19], Liberia (59%) [28], Nigeria (64%) [27], Sierra Leone (9%) [19], and Uganda (53%) [29] |
| Bathing with salt or hot water can prevent Ebola | 6 (43) | DRC (-) [16], Guinea (13%) [19], Sierra Leone (17%) [19], Guinea (22%) [20], Guinea (46%) [26], Nigeria (6%) [27], and Sierra Leone (41%) [21] |
| Prayers or spiritual healers can cure Ebola | 6 (43) | Ghana (-) [24], Guinea (3%) [19], Sierra Leone (1%) [19], Guinea (5%) [20], Guinea (9%) [26], Nigeria (57%) [25], and Sierra Leone (19%) [21] |
| Traditional healers can cure Ebola | 5 (36) | Guinea (15%) [26], Liberia (95%) [28], Guinea (3%) [19], Sierra Leone (1%) [19], Sierra Leone (5%) [21] and Sudan (75%) [22] |
| Ebola seen as a punishment from God | 4 (29) | Ghana (-) [24], Guinea (36%) [26], Guinea (31%) [19], Sierra Leone (8%) [19], and Sierra Leone (8%) [21] |
| Hide Ebola cases in homes | 3 (21) | DRC (17%) [16], Liberia (9%) [28], Guinea (86%) [19], and Sierra Leone (95%) [19] |
| Ebola might be spread by witchcraft | 3 (21) | Guinea (9%) [26] and Uganda (1%) [29] |
| Foreign aid workers spread Ebola | 2 (14) | Liberia (12%) [28] and Uganda (-) [29] |
| Ebola could be cured by drinking salty water or eating bitter kola nut | 2 (14) | Nigeria (93%) [25] and Nigeria (-) [27] |
| Believing that Ebola cannot infect them because of divine protection | 1 (7) | Nigeria (61%) [27] |
| Not aware of Ebola in the community | 1 (7) | Guinea (24%) [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzembo, B.A.; Ntontolo, N.P.; Ngatu, N.R.; Khatiwada, J.; Suzuki, T.; Wada, K.; Kitahara, K.; Ikeda, S.; Miyoshi, S.-I. Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4714. https://doi.org/10.3390/ijerph19084714
Muzembo BA, Ntontolo NP, Ngatu NR, Khatiwada J, Suzuki T, Wada K, Kitahara K, Ikeda S, Miyoshi S-I. Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(8):4714. https://doi.org/10.3390/ijerph19084714
Chicago/Turabian StyleMuzembo, Basilua Andre, Ngangu Patrick Ntontolo, Nlandu Roger Ngatu, Januka Khatiwada, Tomoko Suzuki, Koji Wada, Kei Kitahara, Shunya Ikeda, and Shin-Ichi Miyoshi. 2022. "Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 8: 4714. https://doi.org/10.3390/ijerph19084714
APA StyleMuzembo, B. A., Ntontolo, N. P., Ngatu, N. R., Khatiwada, J., Suzuki, T., Wada, K., Kitahara, K., Ikeda, S., & Miyoshi, S.-I. (2022). Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review. International Journal of Environmental Research and Public Health, 19(8), 4714. https://doi.org/10.3390/ijerph19084714






