1. Introduction
Nondiabetic hyperglycemia is a dangerous metabolic phenomenon in the hospitalized population [
1,
2,
3] and may occur in up to 32–38% of patients treated in the anesthesiology and intensive care unit [
2,
3]. High glucose levels are the body’s response to stress associated with the underlying disease or surgery. The main processes responsible for stress hyperglycemia are insulin resistance and increased gluconeogenesis [
4,
5]. This is due to the excessive secretion of hormones such as glucagon, growth hormone, glucocorticoids, catecholamines, and proinflammatory cytokines: interleukin 1, interleukin 6, and TNF-alpha [
5,
6]. Hyperglycemia causes a number of adverse reactions for the body, such as an increase in free radical expression and associated oxidative damage to cells, as well as a propensity for thrombosis and associated peripheral perfusion abnormalities, abnormal vascular reactivity, or an impaired immune response [
5,
6]. Hyperglycemia has also been shown to worsen the prognosis of stroke and acute myocardial infarction [
7]. The presence of hyperglycemia in the critically ill population is also favored by extrinsic factors such as administered drugs with hyperglycemic effects and patient nutrition [
4].
Thus, glycemic control in intensive care becomes a therapeutic aim. A 2001 study by Van Den Berghe et al. demonstrated the advantage of strict over liberal glycemic control [
8]. However, in that case, too many hypoglycemic incidents were observed and were associated with an increased risk of death [
9]. The 2009 NICE-SUGAR trial provided evidence that more moderate glycemic control is safer and no less effective [
10,
11]. Hence, the current consensus is that glycemia should be controlled within the range of 140–180 mg/dL [
12] and the signal to initiate insulin therapy is its value >200 mg/dL found twice. Moreover, it was noted that inattentive treatment for glycemic disturbances, causing glycemic fluctuations [
13] and hypoglycemic episodes [
14], is equally dangerous for hospitalized patients. Thus, glycemic variability has become another important parameter with a prognostic value [
15]. The aim of this study was to evaluate the variability of glycemia and its underlying determinants as well as to verify the association with the death rate in patients treated in a multiprofile anesthesiology and intensive care unit (ICU).
2. Materials and Methods
Medical records of 37 patients hospitalized in a multiprofile ICU between 3 January 2020 and 29 February 2020 were prospectively analyzed. Due to the non-interventional, observational nature of the study, the consent of the Bioethics Committee was not required. The following information was assessed: basic demographic data, reason for admission, admission priority according to the guidelines of the Polish Society of Anesthesiology and Intensive Therapy, patient status on admission according to APACHE II, SAPS II, and SOFA scores, the presence of diabetes mellitus and its type, and treatment before admission to ICU. The following were recorded for the entire treatment period: daily glycemic values, insulin doses used, incidence of hypoglycemia (i.e., <50 mg/dL), energy intake provided by nutrition (intra- and parenteral), as well as energy contained in glucose, propofol, and citrate solutions for renal replacement therapy. The total dose and the mean daily dose were calculated for the above parameters. Blood glucose was measured in all patients on admission and 4 times a day, at 6:00, 12:00, 18:00, and 24:00. If blood glucose values were <200 mg/dL in the first two days of stay, then the measurements were limited and performed only 2 times/day at 6:00 and 18:00. If values >200 mg/dL persisted in 4 consecutive measurements despite insulin therapy, measurements were intensified to 8 measurements at 3:00, 6:00, 9:00, 12:00, 15:00, 18:00, 21:00, and 24:00. Insulin therapy was introduced when the glycemic value in two measurements was ≥200 mg/dL, and the insulin doses were adjusted to keep glycemia control between 140–180 mg/dL. The doses of the most commonly used hypoglycemic drugs in the unit (corticosteroids and epinephrine) were analyzed. The variability of glycemia during the treatment period, defined as (1) the standard deviation (SD) of all measurements taken and (2) the coefficient of variation calculated as the quotient of mean glycemia and SD (×100%), was evaluated. Deaths during the ICU treatment period were analyzed.
Statistical analysis was performed using procedures available in the licensed MedCalc v18.2 software. Quantitative variables were presented as arithmetic mean and standard deviation (normal distribution) or median and interquartile range (IQR, interquartile range) (distribution deviating from normal). The nature of the distribution of quantitative variables was verified with the Shapiro–Wilk test. Qualitative variables were presented as absolute values and percentage. Differences between quantitative variables were assessed using Student’s t-test/analysis of variance or Mann–Whitney U/Kruskal–Wallis test, depending on the number of groups and the character of distribution. For qualitative variables, the chi-square test was used. Correlations were assessed by Pearson’s linear correlation coefficient or Spearman’s rank. The statistical relationship between dichotomous variables was assessed using odds ratio (OR) analysis. Diagnostic accuracy was assessed using ROC curves and area under curve (AUROC). The criterion for statistical significance was p < 0.05.
3. Results
The study group consisted of 37 patients. The median SAPS II score on admission was 39 (IQR: 28–55). The median treatment duration in the unit was 5 (IQR: 2–8) days. The patients’ characteristics are shown in
Table 1. Sepsis was the cause of admission in two (5%) patients. In priority II, according to the Polish Society of Anesthesiology and Intensive Therapy guidelines, three (8%) patients were admitted.
A history of diabetes mellitus was found in nine (24%) patients (all type 2 diabetes mellitus), of whom two (5%) patients were administered insulin and two (5%) patients were receiving oral hypoglycemic drugs. The mean glycemia on admission was 142.1 ± 56.7 mg/dL and was statistically significantly higher in those with a history of diabetes compared to those without diabetes (190.6 ± 82.0 mg/dL vs. 126 ± 34.2 mg/dL; p < 0.0001). The mean glycemia during the treatment period was 165.7 ± 40.8 mg/dL and was statistically significantly higher in those with a history of diabetes compared to those without diabetes (213.1 ± 32.6 mg/dL vs. 150.5 ± 30.2 mg/dL; p = 0.002). The mean standard deviation (SD) of glycemia during the treatment period was 49.9 ± 21.9 mg/dL and was also statistically significantly higher in subjects with a history of diabetes compared to subjects without diabetes (67.1 ± 15.8 mg/dL vs. 43.1 ± 15.8 mg/dL; p = 0.003). The glycemic coefficient of variation (CV) value was 29.4 ± 9.8% and was similar in subjects with and without a history of diabetes (32.0 ± 9.1% vs. 28.5 ± 10.1%; p = 0.36).
Insulin was used for glycemic control in 28 (75.7%) subjects. The median total mean insulin dose administered was 97 (5–253) units or 12 (1–36) units/day of hospitalization. Hypoglycemia occurred in six (16.2%) subjects, with a total of 13 cases. Hypoglycemia was over 16 times more common in those who received insulin for glycemic control (OR = 16.5; 95% CI: 1–312; p = 0.01).
The median total energy intake administered during the treatment equaled 7820 (IQR: 2126–14274) kcal, with an average of 1078 ± 468 kcal/day of hospitalization. The median total glucose dose administered was 1084 (IQR: 331–1797) g, including an average of 148 ± 73 g glucose/day of hospitalization. Twenty-two (59.5%) patients were treated with glucocorticoids, with a median dose of 850 (550–1344) mg during the entire stay (per hydrocortisone dose). In 15 (40.5%) patients, epinephrine was administered at a median dose of 10.4 (IQR: 1.98–3.7) mg during the entire stay.
Correlations between glycemic variability and selected quantitative variables are shown in
Table 2. Statistically significant correlations are observed between BG variability and age, severity of condition at admission according to SAPS II and SOFA (for SD only), and energy, glucose, insulin, steroid, and epinephrine doses.
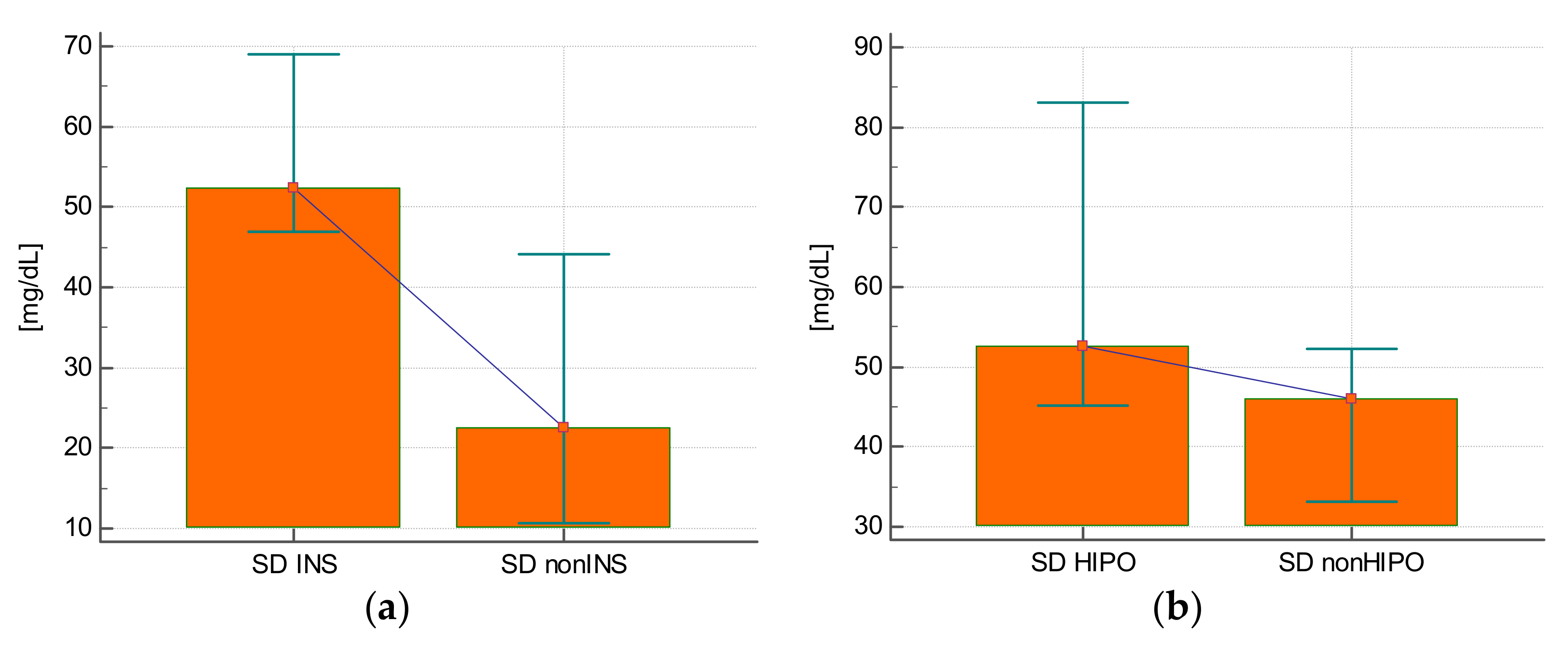
The SD of glycemia during the stay was statistically significantly higher in those who were treated with insulin compared with those who did not require this treatment (25.3 ± 14.3 mg/dL vs. 57.9 ± 17.7 mg/dL;
p < 0.0001) (
Figure 1A), in those who experienced hypoglycemia (63.3 ± 23.1 mg/dL vs. 48.7 ± 20.6 mg/dL;
p = 0.02) (
Figure 1B), and in subjects who received steroids (59.1 ± 20.3 mg/dL vs. 36.6 ± 17.3 mg/dL;
p = 0.001) (
Figure 1C) or epinephrine (61.3 ± 17.2 mg/dL vs. 42.3 ± 21.8 mg/dL;
p = 0.008) (
Figure 1D).
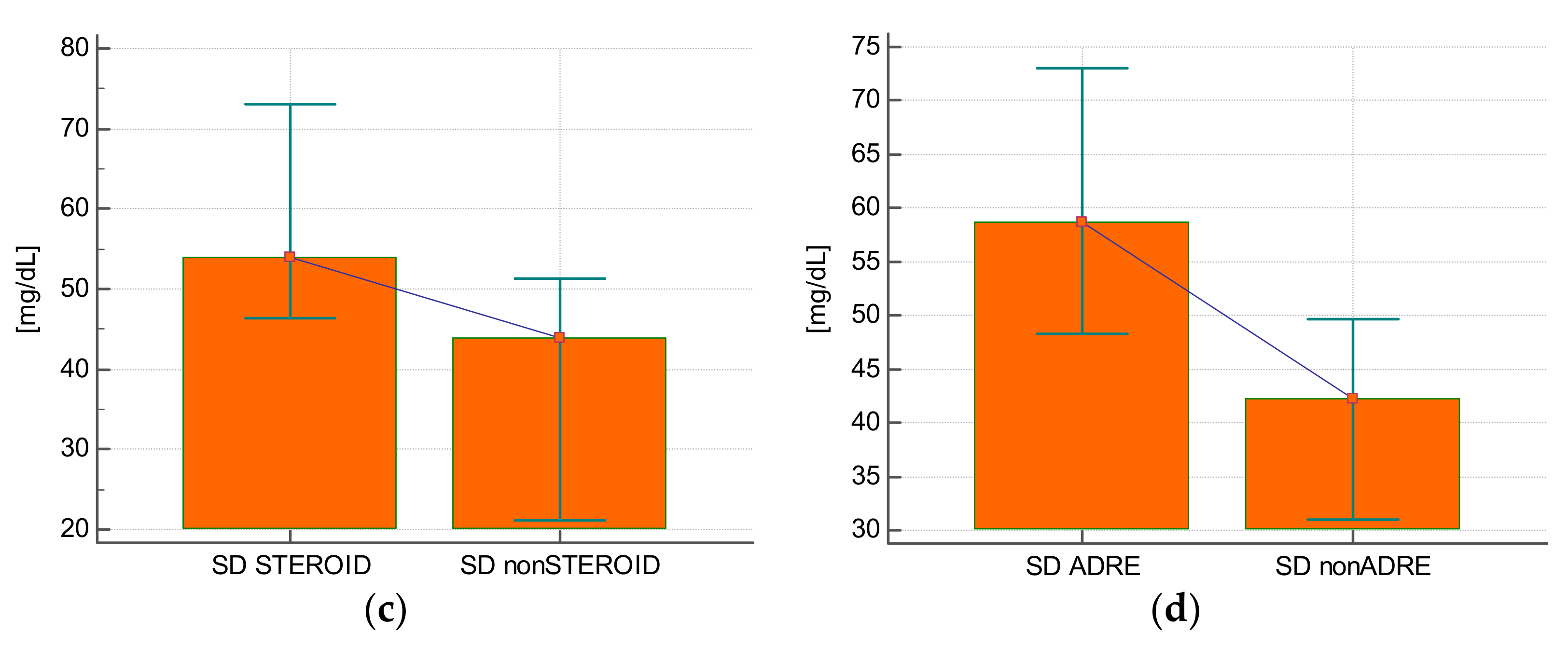
The CV value of glycemia was statistically significantly higher in subjects who received insulin compared with those who did not require this treatment (32.3 ± 7.5% vs. 20.3 ± 11%;
p = 0.0007) (
Figure 2A), in subjects who experienced hypoglycemia (38.8 ± 8.9% vs. 27.6 ± 9%;
p = 0.005) (
Figure 2B), and in those who received steroids (33.6 ± 7.8% vs. 23.2 ± 9.4%;
p = 0.0009) (
Figure 2C) or epinephrine (34.2 ± 7.9% vs. 26.1 ± 9.8%;
p = 0.01) (
Figure 2D).
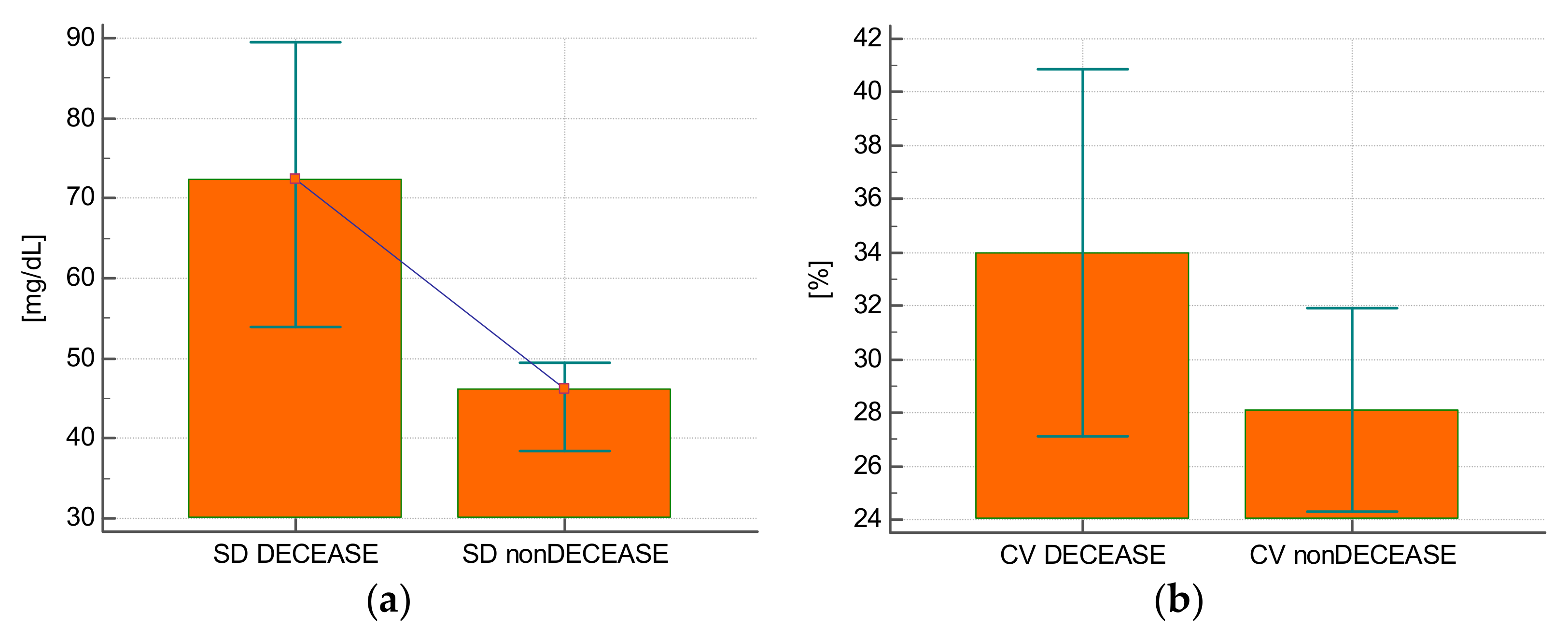
Eight (21.6%) patients died during their stay in the intensive care unit. The glycemia SD value was higher in those who died (68.9 ± 18.6 mg/dL vs. 44.7 ± 20 mg/dL;
p = 0.004) (
Figure 3A). The SD allowed to predict the risk of death with a good diagnostic accuracy (AUROC = 0.806; 95% CI: 0.643–0.917;
p = 0.0014). The glycemia CV was comparable for those who died and those who were discharged from the unit (34 ± 8.2% vs. 28.1 ± 10%;
p = 0.13) (
Figure 3B). The CV was not predictive of the risk of death (AUROC = 0.690; 95% CI: 0.517–0.831;
p = 0.09).
4. Discussion
The aim of this study was to evaluate the variability of glycemia, its underlying determinants, and to verify its association with the incidence of death in patients treated in the anesthesiology and intensive care unit.
The analyses showed that both mean glycemia on admission and mean glycemia during the stay were significantly higher in patients with a history of diabetes. The mean SD of glycemia during the stay was significantly higher in patients with a history of diabetes compared with patients without this illness, while the CV of glycemia was similar. This stays in agreement with previous studies showing that HBA1c levels are strongly associated with mean glycemic value on admission to the ICU, increase in glycemic variability, and incidence of hypoglycemia [
16]. The authors of the cited studies highlight individuals with prediabetic conditions, who have not yet developed a tolerance for acute hyperglycemic states, and therefore identify this group as requiring greater attention in achieving optimal glycemic control.
Hypoglycemia was sixteen times more frequent in patients receiving insulin therapy. This is a dangerous prognostic phenomenon for patients [
17] because even mild hypoglycemia (without signs of neuroglycopenia) increases mortality. [
18] Glucose control monitoring (GCM), which significantly reduces hypoglycemic incidents, seems to be a solution to this problem [
19]. Improvements in glycemic variability when GCM is used have also been documented in patients diagnosed with diabetes [
20]. However, GCM has been shown to not always reduce glycemic variability. It does not affect variability as long as there is an implemented and well-adhered-to insulin-dosing algorithm in the unit, combined with an experienced unit team and frequent blood glucose measurements. However, it is useful in less experienced or frequently rotating teams [
21]. We also showed a positive correlation between SD of glycemia and the severity of condition on admission (according to SAPS II and SOFA) and patient age. Previous authors have reached similar conclusions, in groups of patients with and without a prior diagnosis of diabetes [
22]. Higher mean glucose levels in patients with SOFA ≥9 points are correlated with stress-induced hyperglycemia, and thus increased gluconeogenesis and insulin resistance, as well as with older patient age, which also translates into increased insulin resistance compared to younger groups [
22,
23,
24].
The SD and CV values of glycemia were higher if the patient developed a hypoglycemic episode, and was treated with insulin, steroids, or epinephrine. This is in agreement with the observation that a bolus of hydrocortisone is associated with greater variability in glycemia, as well as greater variability in insulin levels and greater need for boluses of this drug [
25]. CV has also been identified in previous studies as an independent predictor better associated with hypoglycemia than SD is [
26].
The relationship between glycemic variability and total energy intake throughout the day was also found to be statistically significant. This is supported by previous studies that showed a negative correlation between total caloric supply and total serum insulin concentration, and a positive correlation between caloric supply and exogenously administered insulin [
27]. Parenteral nutrition, more often than enteral nutrition, induces hyperglycemia. This may be due to the lack of incretin effect, as well as a more pronounced proinflammatory response [
28].
Finally, it was the SD value of glycemia that proved to be a better predictor of death in our study population. The SD value may also be a better indicator of variability than CV is when we aim to assess the potential effects of glycemic variability without the influence of hypoglycemia [
29].
Limitations of Inference
Our study has several limitations. First of all, it is a single-center study on a heterogeneous, relatively small number of participants. However, similar works in this context exist in the literature, and our observations are consistent with the results of other researchers. Secondly, insulin resistance was not assessed, yet it may be of unmitigated importance for the pathophysiological interpretation of the results. The degree of diabetes control by HBA1c was also not assessed; however, the presence of diabetes had no significant effect on glycemic variability. This is due to the different background of hyperglycemia in chronically present diabetes from that in the acute, critical condition. The lack of an automated glycemic delivery system (SGC, Space Glucose Control® system) can be considered as another limitation of work. However, SGC cannot fully eliminate the risk of glycemic fluctuations, as it requires frequent glycemic measurements and their entry into the system. Lastly, blood glucose measurements were performed both in arterial blood and in capillary blood drawn from a finger, which carries the risk of measurement error, with a margin of measurement error of up to 20%.
5. Conclusions
High glycemic variability results from an imbalance between energy delivery and insulin therapy and may be associated with poor prognosis of patients treated in the anesthesiology and intensive care unit.
Author Contributions
Conceptualization, Ł.K.; methodology, C.K.; validation, Ł.K.; formal analysis, A.K., C.K. and Ł.K.; investigation, A.K., C.K. and M.K.; resources, A.K., C.K. and M.K.; data curation, A.K.; writing—original draft preparation, A.K., C.K. and M.K.; writing—review and editing, Ł.K.; visualization, A.K.; supervision, Ł.K.; project administration, Ł.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Due to the non-interventional, observational nature of the study, the consent of the Bioethics Committee was not required.
Informed Consent Statement
Due to the non-interventional, observational nature of the study, the informed consent was not required.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.B.; Kongable, G.L.; Potter, D.J.; Abad, V.J.; Leija, D.E.; Anderson, M. Inpatient glucose control: A glycemic survey of 126 U.S. hospitals. J. Hosp. Med. 2009, 4, E7–E14. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Potter, D.J.; Kongable, G.L.; Cook, C.B. Update on inpatient glycemic control in hospitals in the United States. Endocr. Pract. 2011, 17, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.; Preiser, J. Toward understanding tight glycemic control in the ICU: A systematic review and metaanalysis. Chest 2010, 137, 544–551. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.; Malhotra, A.; Bistrian, B. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Marik, P.; Raghavan, M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004, 30, 748–756. [Google Scholar] [CrossRef]
- Norhammar, A.; Rydén, L.; Malmberg, K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 1999, 22, 1827–1831. [Google Scholar] [CrossRef]
- Garber, A.; Moghissi, E.; Bransome, E., Jr.; Clark, N.G.; Clement, S.; Cobin, R.H.; Furnary, A.P.; Hirsch, I.B.; Levy, P.; Roberts, R.; et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr. Pract. 2004, 10, 77–82. [Google Scholar] [CrossRef][Green Version]
- Finfer, S.; Liu, B.; Chittock, D.R.; Norton, R.; Myburgh, J.A.; McArthur, C.; Mitchell, I.; Foster, D.; Dhingra, V.; Henderson, W.R.; et al. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118. [Google Scholar] [CrossRef]
- Bouillon, R. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef]
- Finfer, S.; Chittock, D.; Yu Shuo Su, S.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, D.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, E.S.; Korytkowski, M.T.; DiNardo, M.; Einhorn, D.; Hellman, R.; Hirsch, I.B.; Inzucchi, S.E.; Ismail-Beigi, F.; Kirkman, M.S.; Umpierrez, G.E. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009, 32, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Glycemic variability and mortality in critically ill patients: The impact of diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef]
- Smith-Palmer, J.; Brändle, M.; Trevisan, R.; Federici, M.O.; Liabat, S.; Valentine, W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Darier, R.; Montaudon, M.; Beauvieux, M.-C.; Coffin-Boutreux, C.; Coste, P.; Douard, H.; Ouattara, A.; Catargi, B. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care 2019, 42, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S.; Rule, P.; Pappy, L.; Ahmed, A.; Huley-Rodrigues, C.; Prevedello, D.; Preiser, J.-C. The Interaction of Acute and Chronic Glycemia on the Relationship of Hyperglycemia, Hypoglycemia, and Glucose Variability to Mortality in the Critically Ill. Crit. Care Med. 2020, 48, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Bellomo, R.; Jacka, M.J.; Egi, M.; Hart, G.K.; George, C. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit. Care 2009, 13, R91. [Google Scholar] [CrossRef]
- Egi, M.; Bellomo, R.; Stachowski, E.; French, C.J.; Hart, G.K.; Taori, G.; Hegarty, C.; Bailey, M. Hypoglycemia and outcome in critically ill patients. Mayo Clin. Proc. 2010, 85, 217–224. [Google Scholar] [CrossRef]
- Blaha, J.; Barteczko-Grajek, B.; Berezowicz, P.; Charvat, J.; Chvojka, J.; Grau, T.; Holmgren, J.; Jaschinski, U.; Kopecky, P.; Manak, J.; et al. Space GlucoseControl system for blood glucose control in intensive care patients—A European multicentre observational study. BMC Anesthesiol. 2016, 16, 8. [Google Scholar] [CrossRef]
- Danne, T.; de Valk, H.W.; Kracht, T.; Walte, K.; Geldmacher, R.; Sölter, L.; Berge, W.V.D.; Welsh, Z.K.; Bugler, J.R.; Lange, K.; et al. Reducing glycaemic variability in type 1 diabetes self-management with a continuous glucose monitoring system based on wired enzyme technology. Diabetologia 2009, 52, 1496–1503. [Google Scholar] [CrossRef][Green Version]
- Brunner, R.; Adelsmayr, G.; Herkner, H.; Madl, C.; Holzinger, U. Glycemic variability and glucose complexity in critically ill patients: A retrospective analysis of continuous glucose monitoring data. Crit. Care 2012, 16, R175. [Google Scholar] [CrossRef] [PubMed]
- Preechasuk, L.; Suwansaksri, N.; Ipichart, N.; Vannasaeng, S.; Permpikul, C.; Sriwijitkamol, A. Hyperglycemia and glycemic vari-ability are associated with the severity of sepsis in nondiabetic subjects. J. Crit. Care 2017, 38, 319–323. [Google Scholar] [CrossRef]
- Leonidou, L.; Michalaki, M.; Leonardou, A.; Polyzogopoulou, E.; Psirogiannis, A.; Kyriazopoulou, V.; Gogos, C.A.; Fouka, K.; Gerolymos, M.; Leonardos, P. Stress-induced hyperglycemia in patients with severe sepsis: A compromising factor for survival. Am. J. Med. Sci. 2008, 336, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lechleitner, M. Obesity and the metabolic syndrome in the elderly—A mini-review. Gerontology 2008, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- van Hooijdonk, R.T.M.; Binnekade, J.M.; Bos, L.D.J.; Horn, J.; Juffermans, N.P.; Abu-Hanna, A.; Schultz, M.J. Associations between bolus infusion of hydrocortisone, glycemic variability and insulin infusion rate variability in critically Ill patients under moderate glycemic control. Ann. Intensive Care 2015, 5, 34. [Google Scholar] [CrossRef]
- Rodbard, D. Hypo- and hyperglycemia in relation to the mean, standard deviation, coefficient of variation, and nature of the glucose distribution. Diabetes Technol. Ther. 2012, 14, 868–876. [Google Scholar] [CrossRef]
- Treskes, N.; Koekkoek, W.A.C.; van Zanten, A.R.H. The Effect of Nutrition on Early Stress-Induced Hyperglycemia, Serum Insulin Levels, and Exogenous Insulin Administration in Critically Ill Patients with Septic Shock: A Prospective Observational Study. Shock 2019, 52, e31–e38. [Google Scholar] [CrossRef]
- Petrov, M.S.; Whelan, K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: A systematic review and meta-analysis. Br. J. Nutr. 2010, 103, 1287–1295. [Google Scholar] [CrossRef]
- Mendez, C.E.; Mok, K.-T.; Ata, A.; Tanenberg, R.J.; Calles-Escandon, J.; Umpierrez, G.E. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care 2013, 36, 4091–4097. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).