Two Cases of Temporomandibular Synovial Chondromatosis Associated with Gli1 Gene Mutation
Abstract
1. Introduction
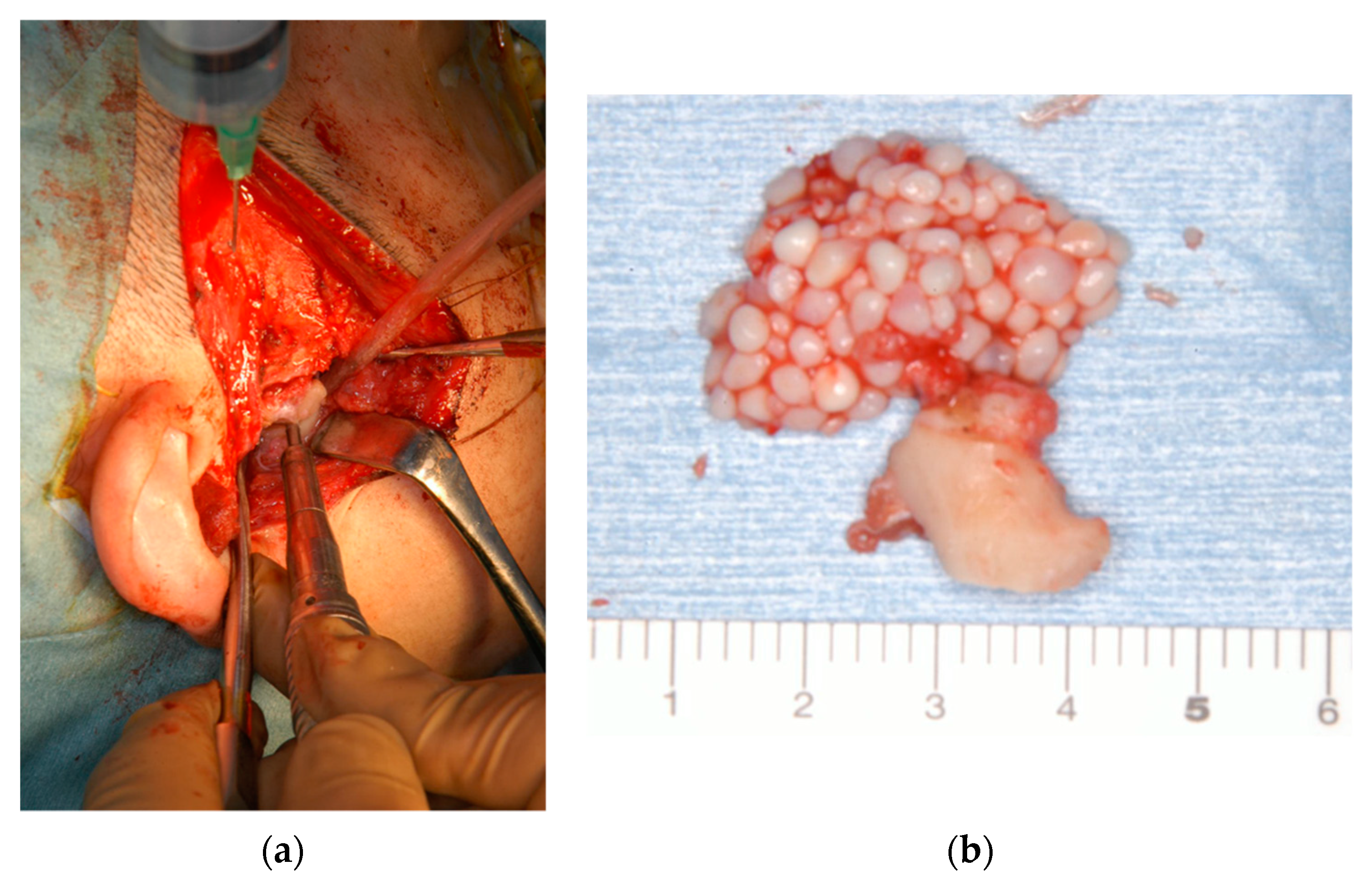
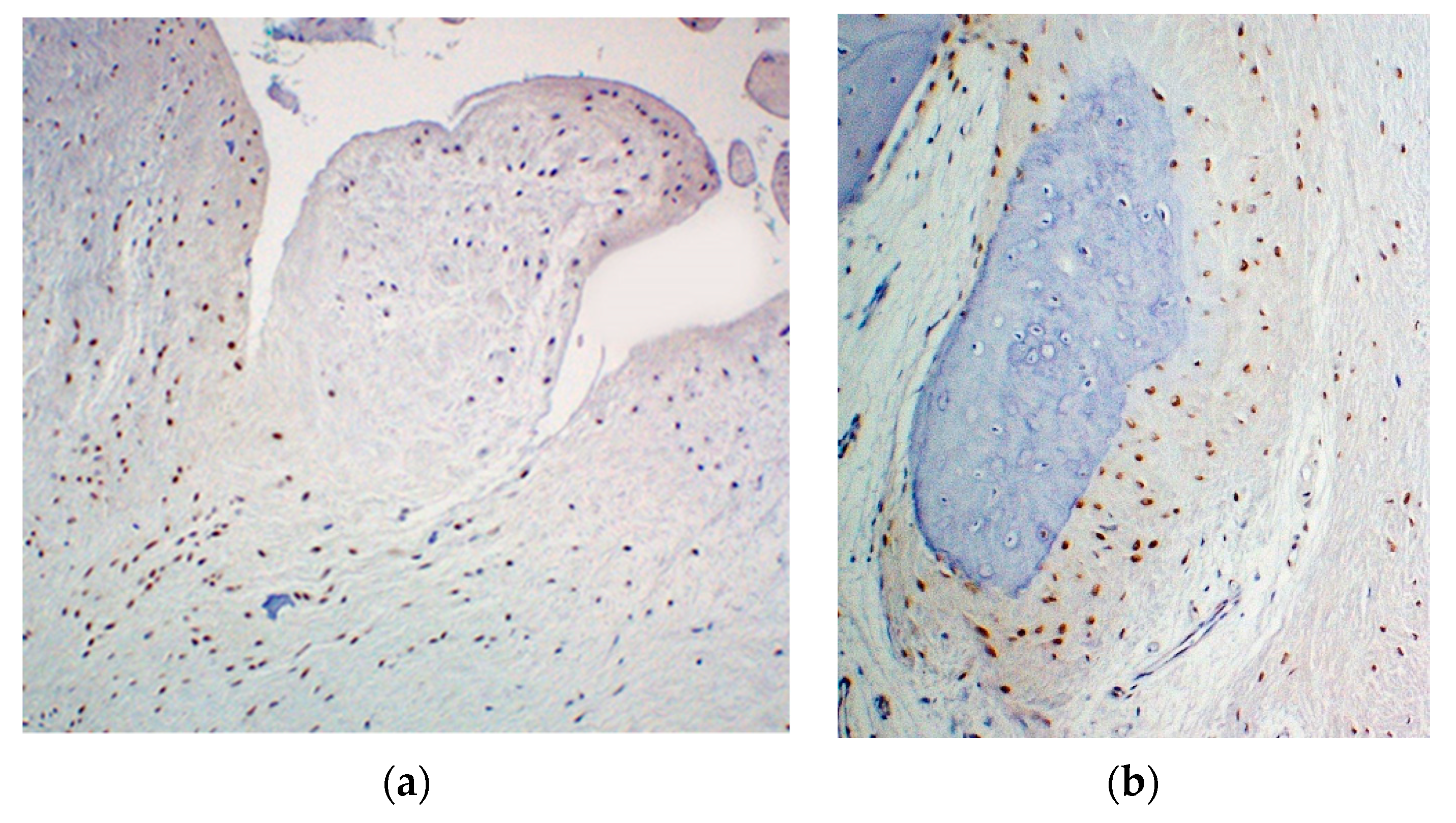
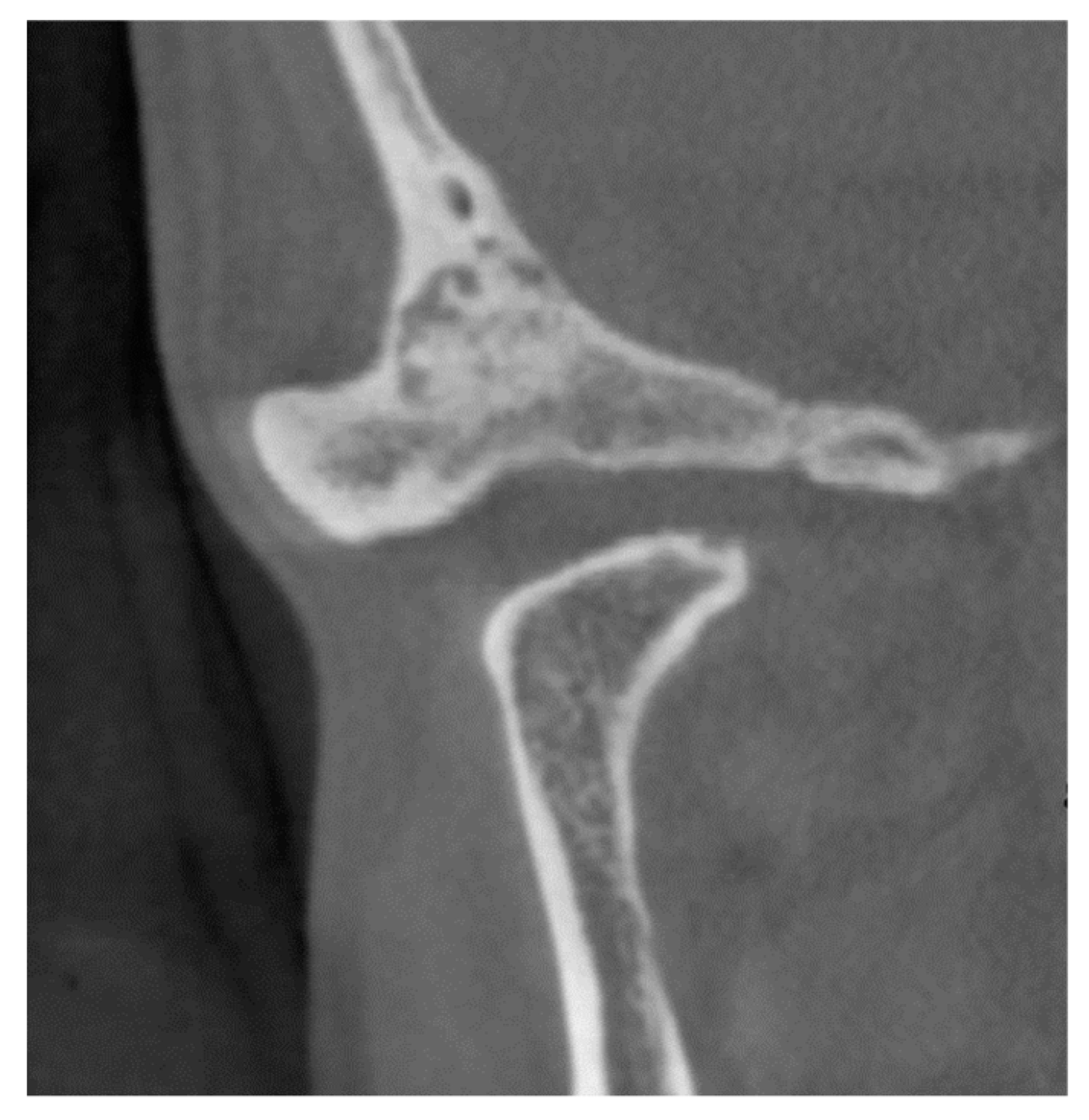
2. Case Reports
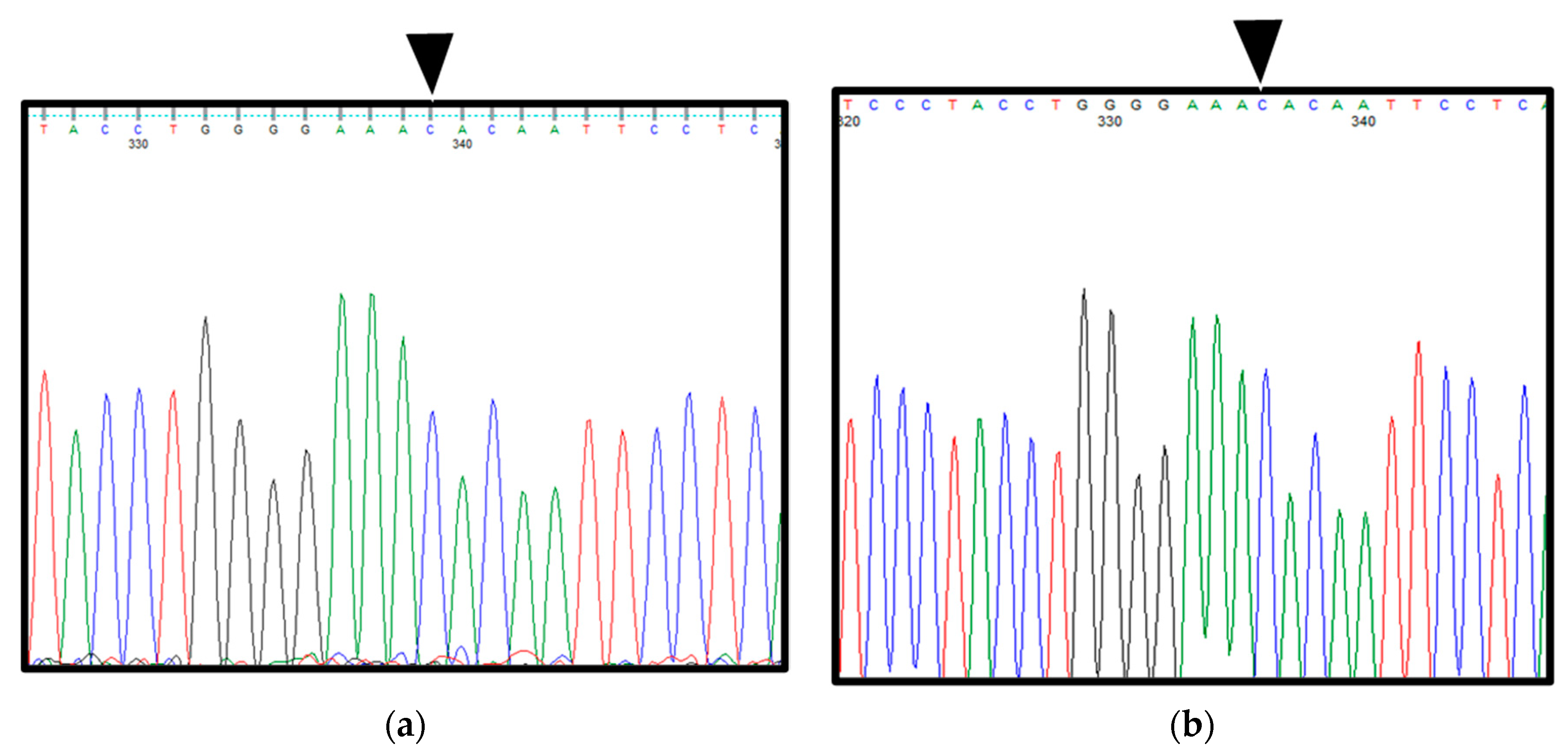
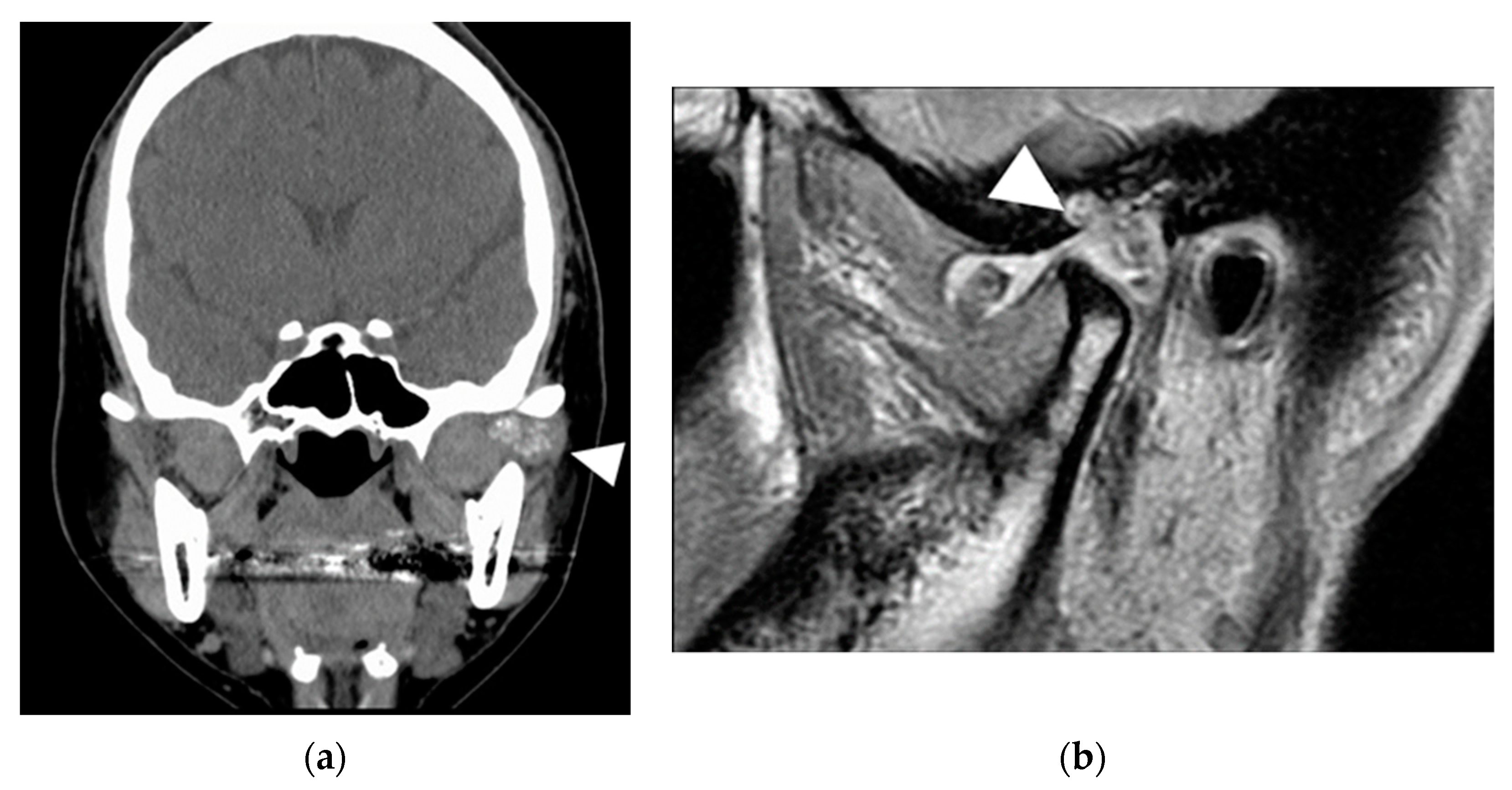
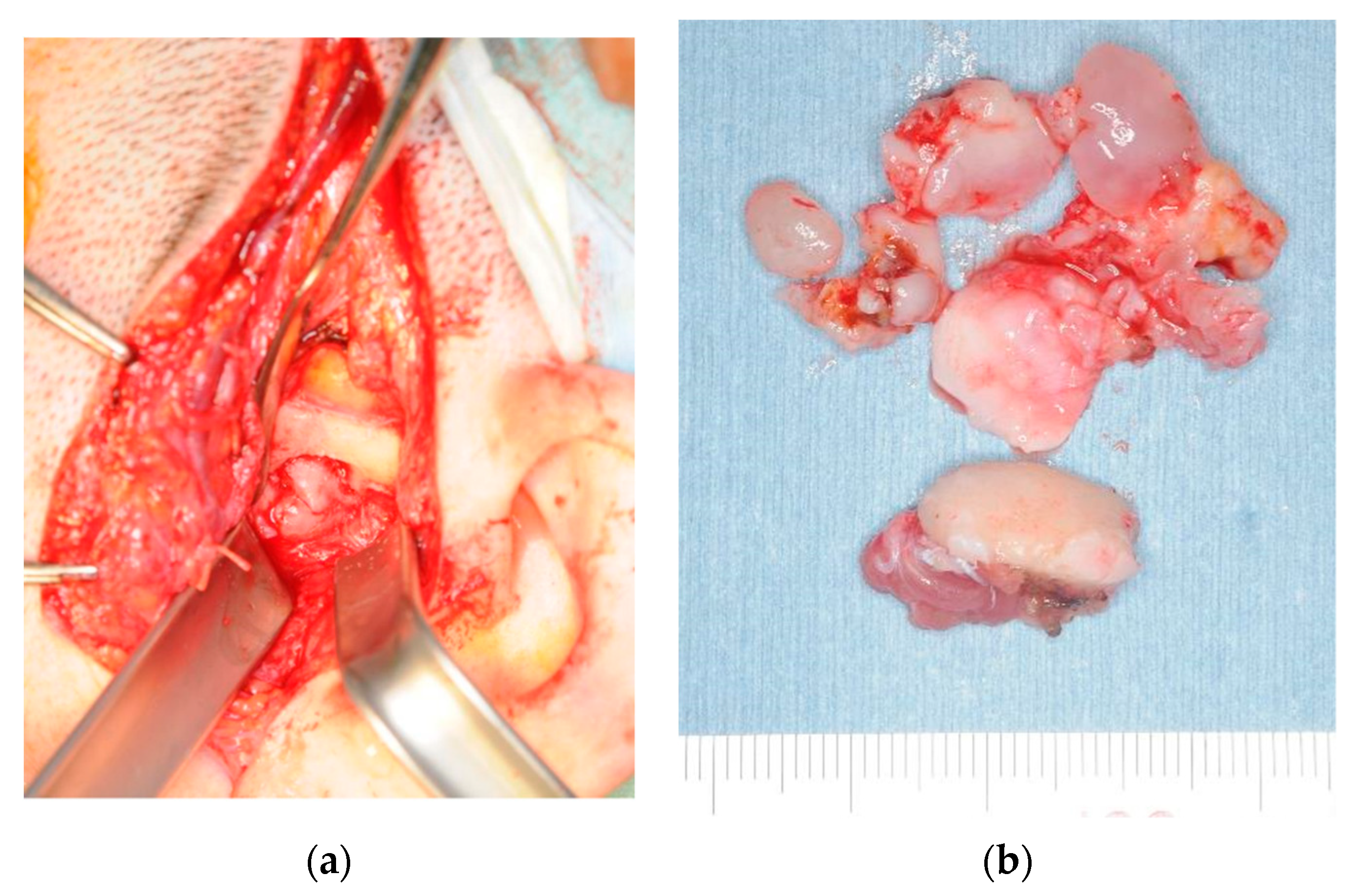
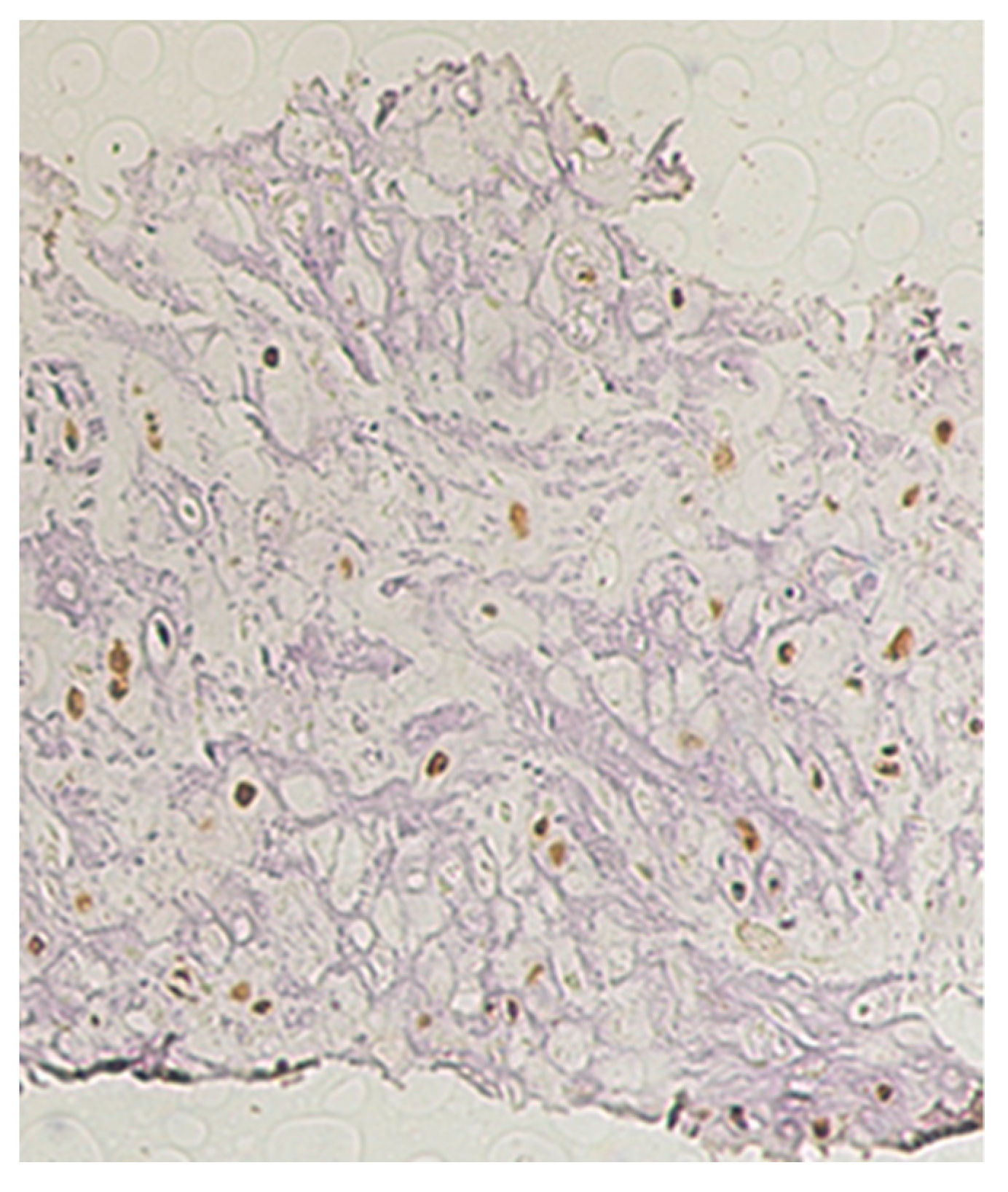
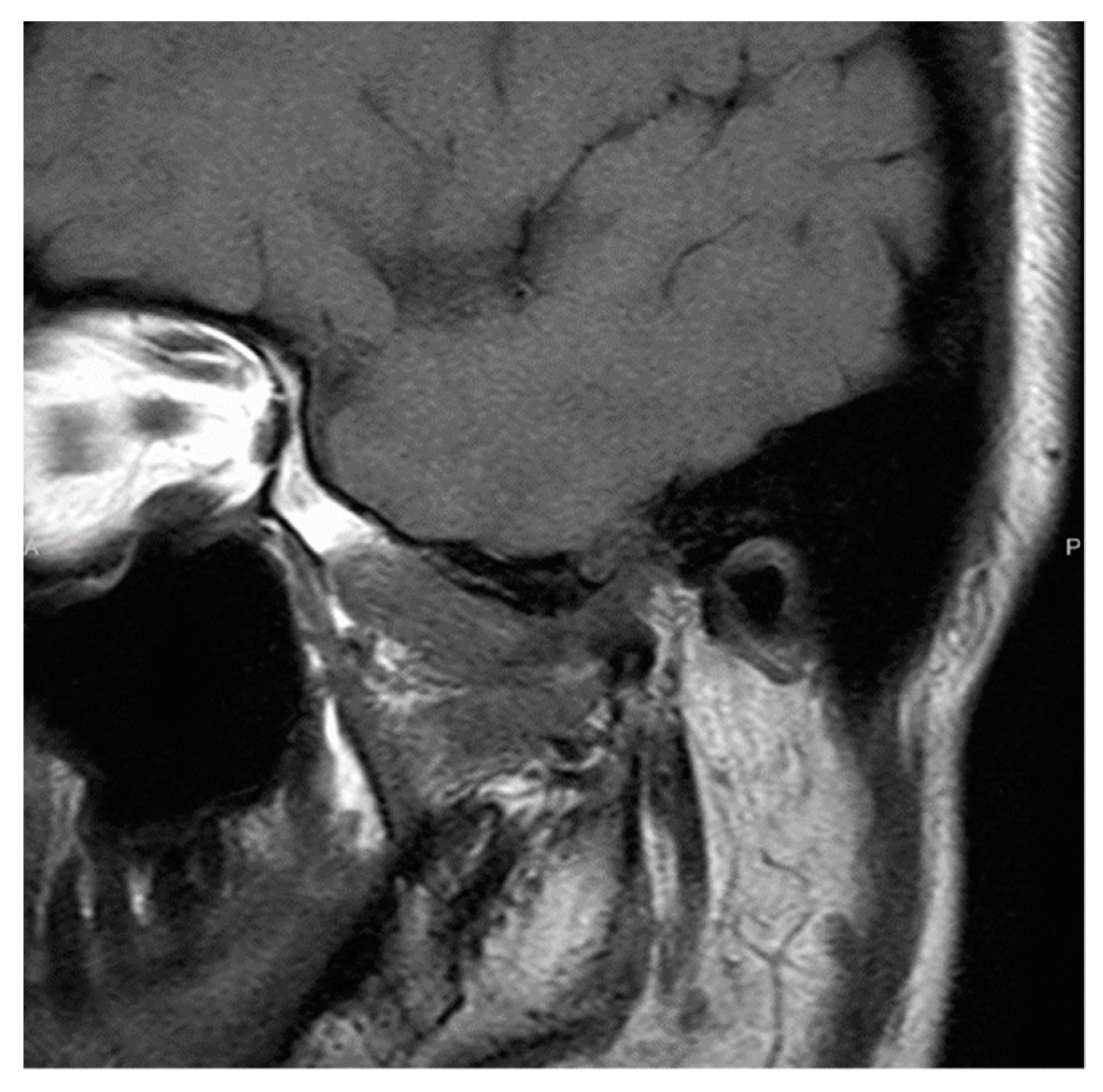
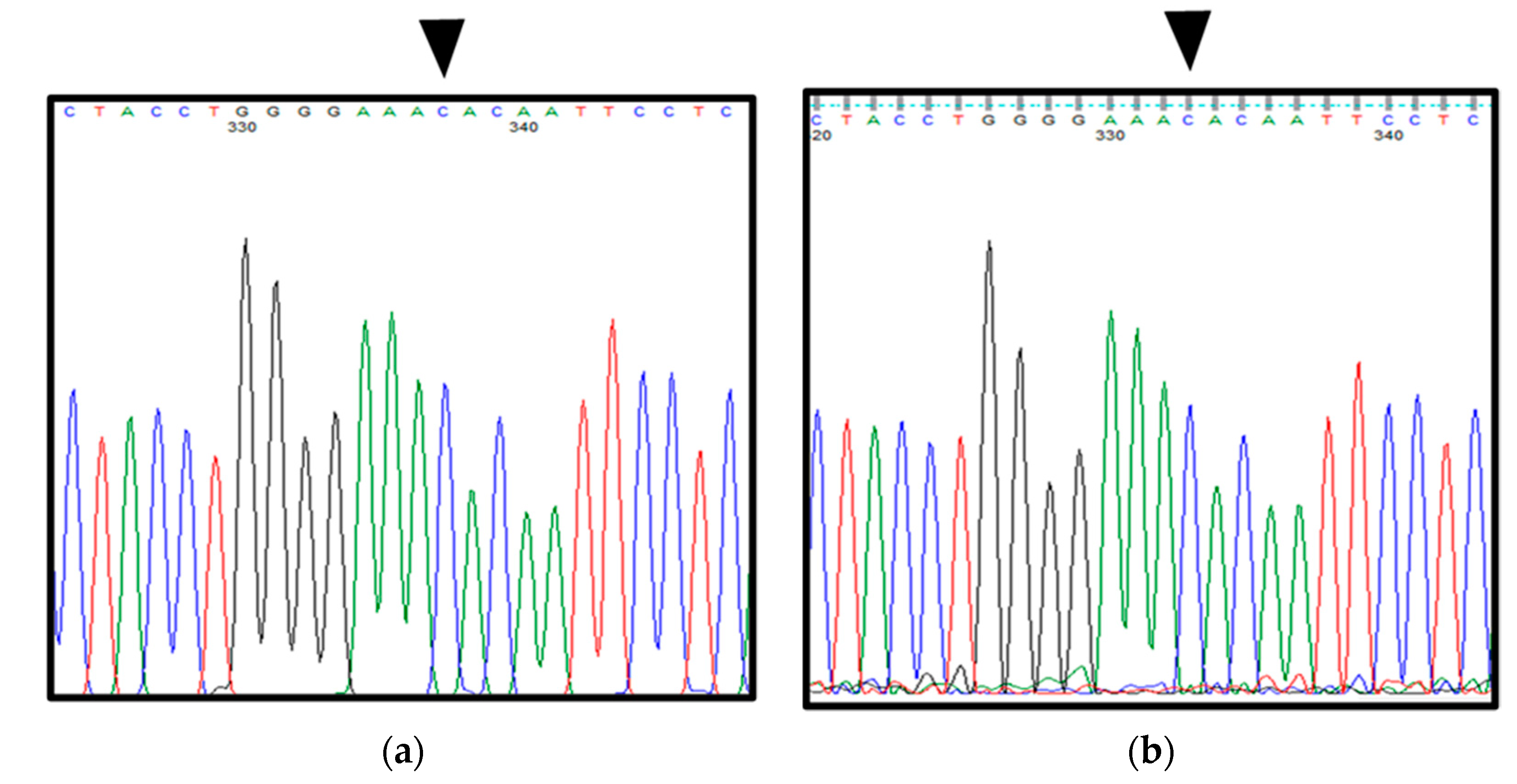
2.1. Patient 1
2.2. Patient 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, F.P.; Dahlin, D.C.; Sullivan, C.R. Articular synovial chondromatosis. J. Bone Jt. Surg. 1962, 44, 77–86. [Google Scholar] [CrossRef]
- Spujet, H.J.; Dorfman, H.D. Lesions of Synovial Origin, Tumors of Bone and Cartilage. In Atlas of Tumor Pathology, Second Series, Fascicle; Armed Forces Institute of Pathology: Washington, DC, USA, 1970; p. 390. [Google Scholar]
- Guillen Botaya, E.; Pino Almero, L.; Molini Menchón, M.O.; Silvestre Muñoz, A.; Moscardó-Navarro, A.; Minguez Rey, M.F. Synovial chondromatosis of the knee. A rare cause of knee pain in pediatric age. Arch. Argent. Pediatr. 2020, 118, e34–e38. [Google Scholar] [PubMed]
- Habusta, S.F.; Tuck, J.A. Synovial Chondromatosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhao, W.; Ruan, Y.; Zhang, W.; Yang, F. Synovial chondromatosis of the temporomandibular joint with 400 loose bodies: A case report and literature review. J. Int. Med. Res. 2021, 49, 3000605211000526. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.A.C.; Ferreira e Costa, R.; de Sousa, S.F.; Chagas, M.R.P.; do Carmo, M.A.V.; de Lacerda, J.C.T. Synovial chondromatosis of the temporo mandibular joint successfully treated by surgery. Head Neck Pathol. 2015, 9, 525–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carls, F.R.; Von Hochstetter, A.; Engelke, W.; Sailer, H.F. Loose bodies in the temporomandibular joint: The advantages of arthroscopy. J. Craniomaxillofac. Surg. 1995, 23, 215–221. [Google Scholar] [CrossRef]
- Miller, M.V.; King, A.; Mertens, F.A. Synovial Chondromatosis. In World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone; Fletcher, C.D.M., Unni, K.K., Eds.; IARC Press: Lyon, France, 2002; p. 246. [Google Scholar]
- Sciot, R.; Bridge, J.A. Synovial Chondromatosis. In World Health Organization Classification of Tumors, WHO Classification of Tumors of Soft Tissue and Bone, 4th ed.; Fletcher, C.D.M., Bridge, J.A., Eds.; IARC Press: Lyon, France, 2013; p. 261. [Google Scholar]
- Priyangana, N.; Suresh, M. Synovial chondromatosis of the temporomandibular Joint. J. Maxillofac. Oral Surg. 2020, 19, 230–234. [Google Scholar] [CrossRef]
- Katayama, Y.; Kurita, K.; Fukuda, K.; Kondo, T.; Sugita, Y.; Maeda, H. A case of calcium pyrophosphate dehydrate crystal deposition with synovial chondromatosis and calcification of the disc in the temporomandibular joint. J. Oral Maxillofac. Surg. 2012, 58, 366–370. [Google Scholar]
- Murakami, K.; Yamamoto, I.; Matsumoto, T. Synovial ehondromatosis assoeiated with osteochondroma of the mandibular condyl. J. Jpn. Soc. Oral Tumors 2000, 12, 387–390. [Google Scholar] [CrossRef]
- Mese, H.; Nakayama, S.; Saeada, S.; Shimo, T.; Nishiyama, A.; Sasaki, A. Synovial chondromatosis of the temporomandibular joint with pre-auricular swelling. J. Okayama Dent. Soc. 2004, 13, 221–225. [Google Scholar]
- Blankestijn, J.; Panders, A.K.; Vermey, A.; Scherpbier, A.J. Synovial chondromatosis of the temporomandibular joint: Report of three cases and review of the literature. Cancer 1985, 55, 479–485. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Krallman, P.; Neff, J.R.; Nelson, M.; Liu, J.; Bridge, J.A. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet. Cytogenet. 2003, 140, 18–22. [Google Scholar] [CrossRef]
- Totoki, Y.; Yoshida, A.; Hosoda, F.; Nakamura, H.; Hama, N.; Ogura, K.; Yoshida, A.; Fujiwara, T.; Arai, Y.; Toguchida, J.; et al. Unique mutation portraits and frequent COL2A1 gene alteration in chondrosarcoma. Genome Res. 2014, 24, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Amary, F.; Perez-Casanova, L.; Ye, H.; Cottone, L.; Strobl, A.C.; Cool, P.; Miranda, E.; Berisha, F.; Aston, W.; Rocha, M.; et al. Synovial chondromatosis and soft tissue chondroma: Extraosseous cartilaginous tumor defined by FN1 gene rearrangement. Mod. Pathol. 2019, 32, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, C.; Nihous, H.; Macagno, N. Soft tissue tumours with FN1 (Fibronectin 1) fusion gene. Ann. Pathol. 2022. [Google Scholar] [CrossRef]
- Hopyan, S.; Nadesan, P.; Yu, C.; Wunder, J.; Alman, B.A. Dysregulation of Hedgehog signaling predisposes to synovial chondro matosis. J. Pathol. 2005, 206, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Huntzicker, E.G.; Estay, I.S.; Zhen, H.; Lokteva, L.A.; Jackson, P.K.; Oro, A.E. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006, 20, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Zacharias, W.J.; Tremelling, M.; Noble, C.L.; Nimmo, E.R.; Tenesa, A.; Cornelius, J.; Torkvist, L.; Kao, J.; Farrington, S.; et al. Analysis of Germline Gli1 variation implicates Hedgehog signaling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008, 5, e239. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Pichler, M.; Absenger, G.; Stotz, M.; Weissmueller, M.; Samonigg, H.; Asslaber, M.; Lax, S.; Leitner, G.; Winder, T.; et al. A functional germline variant in GLI1 implicates Hedgehog signaling in clinical outcome of stage II and III colon carcinoma patients. Clin. Cancer Res. 2014, 20, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Tian, T.; Zhang, R.; Wang, L.; Xu, J.; Fan, L.; Li, J.Y.; Xu, W. Association between polymorphism of Gli1 gene SNP rs2228226 and chronic lymphocytic leukemia in Chinese population. Med. Oncol. 2014, 31, 294. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukutani, T.; Toratani, S.; Kanda, T.; Matsui, K.; Yamasaki, S.; Sumi, K.; Ogawa, I.; Yanamoto, S. Two Cases of Temporomandibular Synovial Chondromatosis Associated with Gli1 Gene Mutation. Int. J. Environ. Res. Public Health 2022, 19, 4702. https://doi.org/10.3390/ijerph19084702
Fukutani T, Toratani S, Kanda T, Matsui K, Yamasaki S, Sumi K, Ogawa I, Yanamoto S. Two Cases of Temporomandibular Synovial Chondromatosis Associated with Gli1 Gene Mutation. International Journal of Environmental Research and Public Health. 2022; 19(8):4702. https://doi.org/10.3390/ijerph19084702
Chicago/Turabian StyleFukutani, Taeko, Shigeaki Toratani, Taku Kanda, Kensaku Matsui, Sachiko Yamasaki, Kensaku Sumi, Ikuko Ogawa, and Souichi Yanamoto. 2022. "Two Cases of Temporomandibular Synovial Chondromatosis Associated with Gli1 Gene Mutation" International Journal of Environmental Research and Public Health 19, no. 8: 4702. https://doi.org/10.3390/ijerph19084702
APA StyleFukutani, T., Toratani, S., Kanda, T., Matsui, K., Yamasaki, S., Sumi, K., Ogawa, I., & Yanamoto, S. (2022). Two Cases of Temporomandibular Synovial Chondromatosis Associated with Gli1 Gene Mutation. International Journal of Environmental Research and Public Health, 19(8), 4702. https://doi.org/10.3390/ijerph19084702





