Healthcare Professionals’ Compliance with the Standard Management Guidelines towards the Use of Biological Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Sampling
- Those who were registered with the appropriate professional body for accreditation;
- Those who provided a written consent form for their voluntary participation in the study.
2.3. Study Instrument
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Demographic Characteristics
3.2. Assessment of Knowledge of Respondents Regarding bDMARDs
3.3. Respondents’ Compliance with the RA Standard Management Guidelines
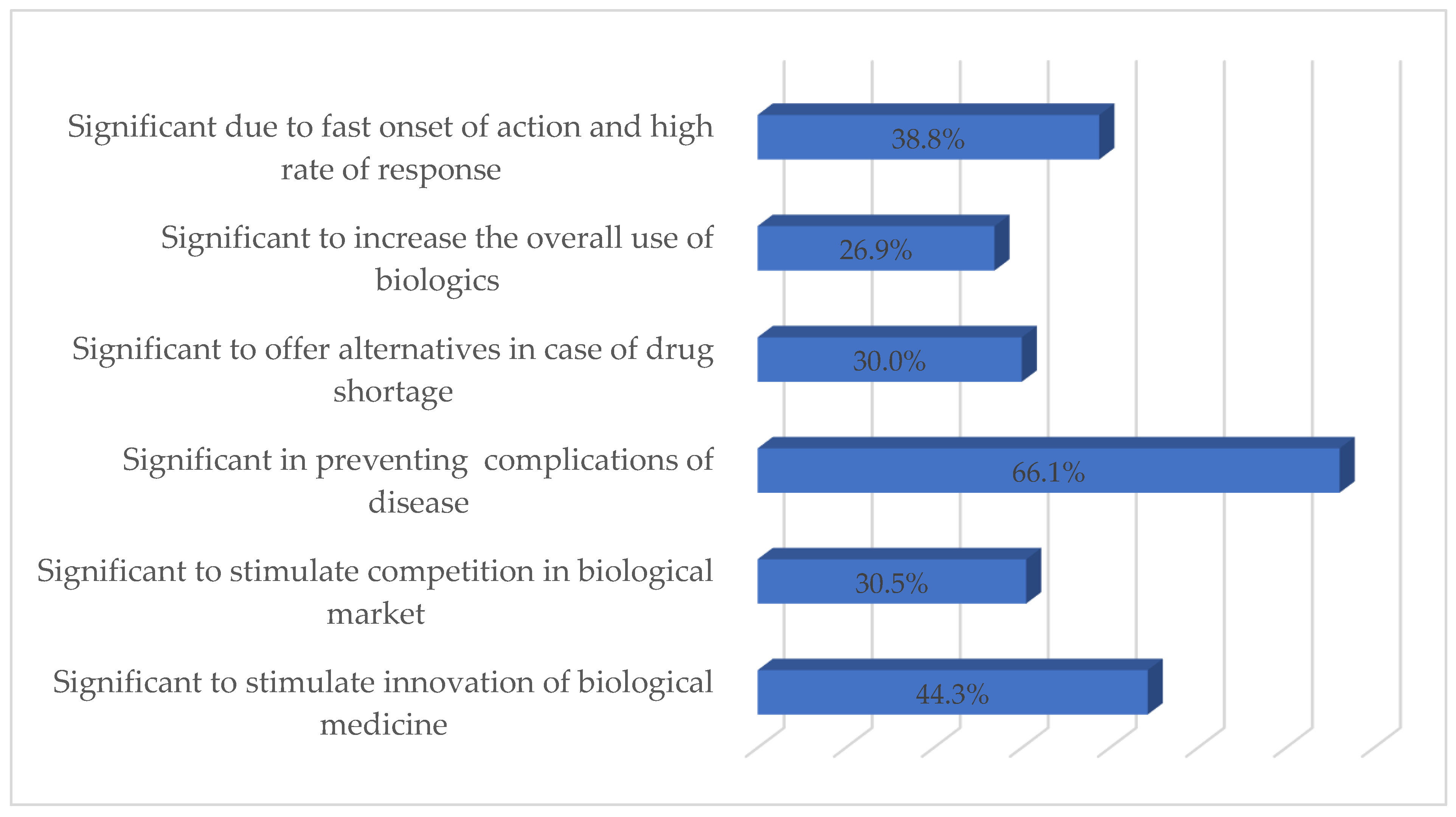
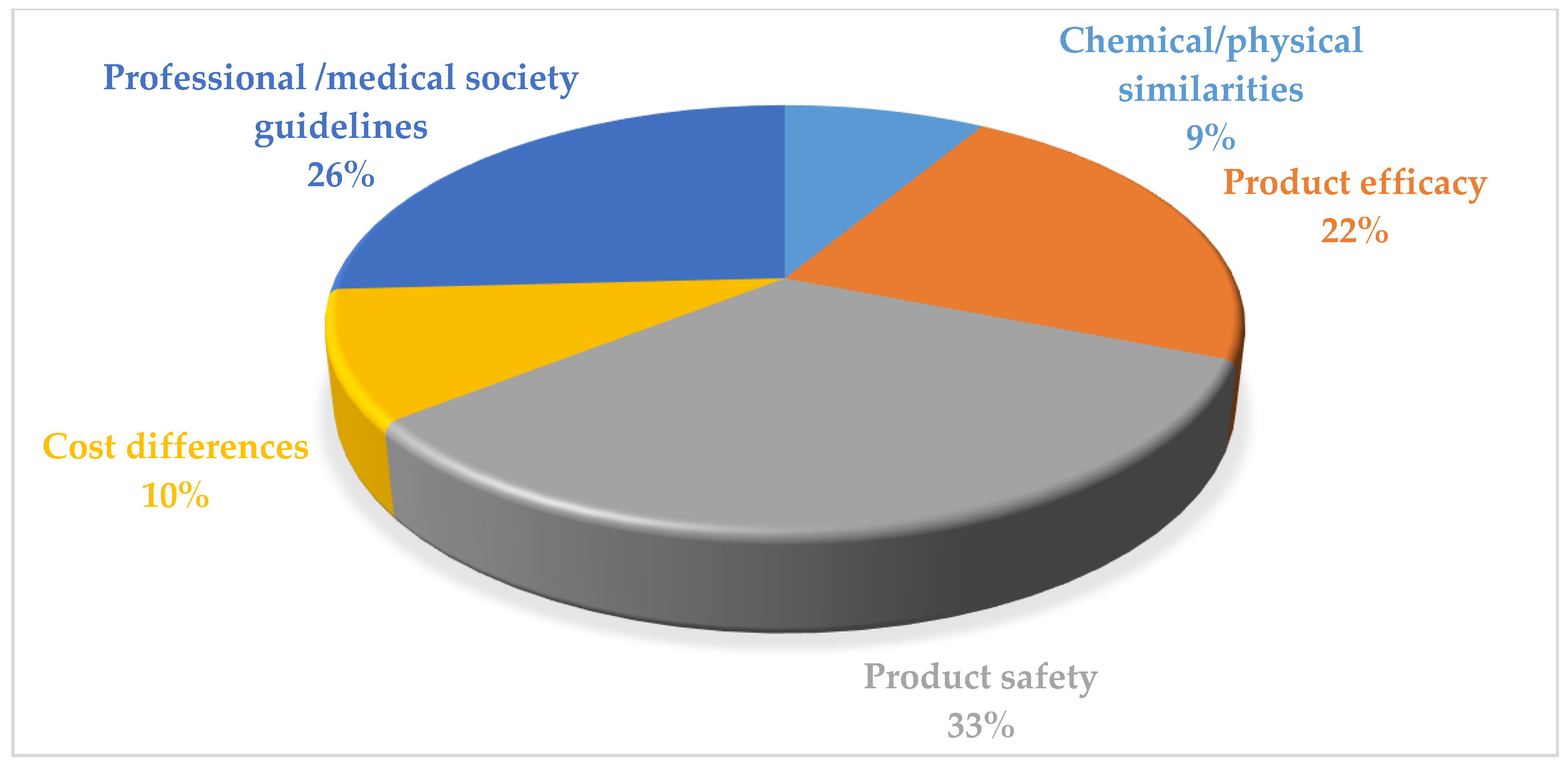
3.4. Respondents’ Perceived Importance and Barriers of Using bDMARDs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conigliaro, P.; Triggianese, P.; De Martino, E.; Fonti, G.L.; Chimenti, M.S.; Sunzini, F.; Viola, A.; Canofari, C.; Perricone, R. Challenges in the treatment of rheumatoid arthritis. Autoimmun. Rev. 2019, 18, 706–713. [Google Scholar] [CrossRef]
- Chaudhari, K.; Rizvi, S.; Syed, B.A. Rheumatoid arthritis: Current and future trends. Nat. Rev. Drug Discov. 2016, 15, 305–306. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.; Rollefstad, S.; Heslinga, M.; McInnes, I.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; McWilliams, D.F. Pain in rheumatoid arthritis. Curr. Pain Headache Rep. 2012, 16, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Fautrel, B.; Den Broeder, A.A. De-intensifying treatment in established rheumatoid arthritis (RA): Why, how, when and in whom can DMARDs be tapered? Best Pract. Res. Clin. Rheumatol. 2015, 29, 550–565. [Google Scholar] [CrossRef]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine. 2019, 86, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Juge, P.-A.; Lee, J.S.; Lau, J.; Kawano-Dourado, L.; Serrano, J.R.; Sebastiani, M.; Koduri, G.; Matteson, E.; Bonfiglioli, K.; Sawamura, M.; et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur. Clin. Respir. J. 2021, 57, e2000337. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Chen, Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J. Drug Target. 2018, 26, 845–857. [Google Scholar] [CrossRef]
- Zhang, A.; Lee, Y.C. Mechanisms for joint pain in rheumatoid arthritis (RA): From cytokines to central sensitization. Curr. Osteoporos. Rep. 2018, 16, 603–610. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Sebastiani, M.; Atzeni, F.; Milazzo, L.; Quartuccio, L.; Scirè, C.; Gaeta, G.B.; Lapadulag, G.; Armignaccoh, O.; Tavioi, M.; Olivieri, I.; et al. Italian consensus Guidelines for the management of hepatitis B virus infections in patients with rheumatoid arthritis. Jt. Bone Spine 2017, 84, 525–530. [Google Scholar] [CrossRef]
- Baniaamam, M.; Paulus, W.J.; Blanken, A.B.; Nurmohamed, M.T. The effect of biological DMARDs on the risk of congestive heart failure in rheumatoid arthritis: A systematic review. Expert Opin. Biol. Ther. 2018, 18, 585–594. [Google Scholar] [CrossRef]
- Romero-Sanchez, C.; Rodriguez, C.; Santos-Moreno, P.; Mesa, A.M.; Lafaurie, G.I.; Giraldo, S.-Q.; De-Avila, J.; Castillo, D.M.; Duran, M.; Chalem, C.P. Is the treatment with biological or non-biological DMARDS a modifier of periodontal condition in patients with rheumatoid arthritis? Curr. Rheumatol. Rev. 2017, 13, 139–151. [Google Scholar] [CrossRef]
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; Van Der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.R.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1101–1136. [Google Scholar] [CrossRef]
- Uthman, I.; Almoallim, H.; Buckley, C.D.; Masri, B.; Dahou-Makhloufi, C.; El Dershaby, Y.; Sunna, N.; Raza, K.; Kumar, K.; Huijer, H.A.-S.; et al. Nurse-led care for the management of rheumatoid arthritis: A review of the global literature and proposed strategies for implementation in Africa and the Middle East. Rheumatol. Int. 2021, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Raosoft, I. Sample Size Calculator; Raosoft Inc.: Seattle, WA, USA, 2020. [Google Scholar]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; Van Der Heijde, D.; Dougados, M.; Van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottois, C.; López-Medina, C.; Dumas, S.; Julien, H.; Sephora, B.; Roux, C.; Moltó, A.; Conort, O.; Dougados, M. POS0273-HPR Pharmacist’s impact on self-management for patients with chronic inflammatory arthritis treated with biological DMARDS. BMJ 2021, 80, 360. [Google Scholar] [CrossRef]
- Shakeel, S.; Hassali, M.A.; Rehman, H.; ur Rehman, A.; Muneswarao, J. Knowledge, attitude, and practice towards biosimilars and interchangeable products: A prescriptive insight by the pharmacists. Int. J. Gen. Med. 2020, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Garneau, K.L.; Iversen, M.D.; Tsao, H.; Solomon, D.H. Primary care physicians’ perspectives towards managing rheumatoid arthritis: Room for improvement. Arthritis Res. Ther. 2011, 13, R189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mok, C.C. Hepatitis B and C infection in patients undergoing biologic and targeted therapies for rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2018, 32, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tseng, C.; Hsu, C.; Tung, C.; Huang, K.; Lu, M.; Lai, N. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib. Int. J. Rheum. Dis. 2021, 24, 1362–1369. [Google Scholar] [CrossRef]
- Littlejohn, E.A.; Monrad, S.U. Early diagnosis and treatment of rheumatoid arthritis. Prim. Care 2018, 45, 237–255. [Google Scholar] [CrossRef]
- Shirani, F. Primary Care in rheumatoid arthritis. Rheum. Res. 2019, 4, 133–138. [Google Scholar]
- Mathijssen, E.G.; Vriezekolk, J.E.; Popa, C.D.; van den Bemt, B.J. Shared decision making in routine clinical care of patients with rheumatoid arthritis: An assessment of audio-recorded consultations. Ann. Rheum. Dis. 2020, 79, 170–175. [Google Scholar] [CrossRef]
- De Mits, S.; Lenaerts, J.; Vander Cruyssen, B.; Mielants, H.; Westhovens, R.; Durez, P.; Elewaut, D. A nationwide survey on patient’s versus physician s evaluation of biological therapy in rheumatoid arthritis in relation to disease activity and route of administration: The be-raise study. PLoS ONE 2016, 11, e0166607. [Google Scholar] [CrossRef] [Green Version]
- Mahlich, J.; Sruamsiri, R. Preference for shared decision-making in Japanese patients with rheumatoid arthritis. Cogent Med. 2017, 4, 1353262. [Google Scholar] [CrossRef]
- Shakeel, S.; Mohamed, A.H.; Rehman, H.; Iffat, W.; Yasmin, R.; Farrukh, U. Explanatory Findings of Prescribing Biosimilar Medicines in Oncology Care Settings of Pakistan. Lat. Am. J. Pharm. 2020, 39, 2041–2045. [Google Scholar]
- Kalkan, A.; Roback, K.; Hallert, E.; Carlsson, P. Factors influencing rheumatologists’ prescription of biological treatment in rheumatoid arthritis: An interview study. Implement. Sci. 2014, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Aladul, M.I.; Fitzpatrick, R.W.; Chapman, S.R. Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: A qualitative study. BMJ 2018, 8, e023603. [Google Scholar] [CrossRef] [Green Version]
- Leonard, E.; Wascovich, M.; Oskouei, S.; Gurz, P.; Carpenter, D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. J. Manag. Care Spec. Pharm. 2019, 25, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sarnola, K.; Merikoski, M.; Jyrkkä, J.; Hämeen-Anttila, K. Physicians’ perceptions of the uptake of biosimilars: A systematic review. BMJ 2020, 10, e034183. [Google Scholar] [CrossRef] [PubMed]
- Daei, A.; Soleymani, M.R.; Ashrafi-Rizi, H.; Zargham-Boroujeni, A.; Kelishadi, R. Clinical information seeking behavior of physicians: A systematic review. Int. J. Med. Inform. 2020, 139, 104144. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Frequency n (%) |
|---|---|
| Age (Years) | |
| Mean ± SD | 31.8 ± 12.8 |
| Gender | |
| Male | 138 (33.4) |
| Female | 275 (66.5) |
| Organization | |
| Private | 158 (38.3) |
| Public sector | 255 (61.7) |
| Professional Specialty | |
| Physicians | 302 (73.1) |
| Pharmacists | 111 (26.8) |
| Practice Area | |
| Primary patient care | 110 (26.6) |
| Secondary patient care | 28 (6.7) |
| Tertiary patient care | 275 (66.5) |
| Experience | |
| <5 years | 239 (57.8) |
| 5–10 years | 68 (16.4) |
| 10–15 years | 66 (15.9) |
| 15–20 years | 30 (7.2) |
| 20 years and above | 10 (2.4) |
| Knowledge Item | Correct Response | Gender | Organization | Specialty | Practice Area | Experience | |
|---|---|---|---|---|---|---|---|
| Physicians (n = 302) | Pharmacists (n = 111) | ||||||
| Indications and use | 276 (91.3) | 99 (89.1) | 0.001 * | ||||
| Types/examples | 244 (80.7) | 93 (83.7) | 0.001 * | ||||
| Drug target | 171 (56.6) | 96 (86.4) | 0.005 * | 0.029 * | 0.004 * | ||
| Mechanism of action | 160 (52.9) | 80 (72.0) | 0.013 * | ||||
| General principles for use | 226 (74.8) | 79 (71.1) | 0.007 * | 0.001 * | |||
| Monitoring requirements | 258 (85.4) | 82 (73.8) | 0.004 * | 0.003 * | |||
| Contraindications | 195 (64.5) | 71 (63.9) | |||||
| General precautions | 214 (70.8) | 84 (75.6) | 0.002 * | 0.001 * | |||
| Benefits and risks | 189 (62.5) | 88 (79.2) | 0.001 * | ||||
| Adverse effects | 177 (58.6) | 86 (77.4) | |||||
| To What Extent Do You Agree or Disagree with the Following Statements? | Strongly Agree n (%) | Agree n (%) | Neutral n (%) | Disagree n (%) | Strongly Disagree n (%) |
|---|---|---|---|---|---|
| DMARD therapy should initiated immediately upon the RA diagnosis being confirmed. | 101 (24.4) | 172 (41.6) | 97 (23.4) | 21 (5.0) | 22 (5.3) |
| Patients need access to multiple medications with different modes of action to combat the heterogeneity of RA; they may need multiple consecutive therapies during their life course. | 147 (35.5) | 168 (40.6) | 56 (13.5) | 33 (7.9) | 9 (2.1) |
| Monitoring should be regular in active disease and therapy should be adjusted in the case of no improvement. | 103 (24.9) | 167 (40.4) | 125 (30.2) | 13 (3.1) | 5 (1.2) |
| Methotrexate should be included in the initial treatment strategy. | 56 (13.5) | 243 (58.8) | 86 (20.8) | 25 (6.0) | 3 (0.7) |
| If the use of methotrexate is contraindicated in a patient, leflunomide or sulfasalazine should be included in the initial treatment strategy. | 164 (39.7) | 152 (36.8) | 88 (21.3) | 5 (1.2) | 4 (0.9) |
| Short-term glucocorticoids should be considered when initiating or changing csDMARDs. | 103 (24.9) | 168 (40.6) | 116 (28.0) | 22 (5.3) | 4 (0.9) |
| If the treatment target is not accomplished with the first csDMARD, other csDMARDs strategies should be considered. | 106 (25.6) | 227 (54.9) | 70 (16.9) | 7 (1.6) | 3 (0.7) |
| If the treatment target is not achieved with the first csDMARD strategy, then a bDMARD should be added. | 122 (29.5) | 201 (48.6) | 66 (15.9) | 21 (5.0) | 3 (0.7) |
| bDMARDs should be combined with a csDMARD in patients who cannot use csDMARDs as a co-medication. | 150 (36.3) | 132 (31.9) | 120 (29.0) | 5 (1.2) | 6 (1.4) |
| If a bDMARD has failed, treatment with another bDMARD should be considered. | 104 (25.1) | 191 (46.2) | 85 (20.5) | 19 (4.6) | 14 (3.3) |
| Treatment goals of RA patients should be based on a shared decision between the patient and the rheumatologist. | 118 (28.5) | 122 (29.5) | 129 (31.2) | 39 (9.4) | 5 (1.2) |
| Treatment decisions are based on disease activity, safety issues, and other patient factors such as co-morbidities and progression of structural damage. | 101 (24.4) | 184 (44.5) | 81 (19.6) | 18 (4.3) | 29 (7.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeel, S.; Iffat, W.; Qamar, A.; Rehman, H.; Ghuman, F.; Butt, F.; ur Rehman, A.; Madléna, M.; Paulik, E.; Gajdács, M.; et al. Healthcare Professionals’ Compliance with the Standard Management Guidelines towards the Use of Biological Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Patients. Int. J. Environ. Res. Public Health 2022, 19, 4699. https://doi.org/10.3390/ijerph19084699
Shakeel S, Iffat W, Qamar A, Rehman H, Ghuman F, Butt F, ur Rehman A, Madléna M, Paulik E, Gajdács M, et al. Healthcare Professionals’ Compliance with the Standard Management Guidelines towards the Use of Biological Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Patients. International Journal of Environmental Research and Public Health. 2022; 19(8):4699. https://doi.org/10.3390/ijerph19084699
Chicago/Turabian StyleShakeel, Sadia, Wajiha Iffat, Ambreen Qamar, Hina Rehman, Faiza Ghuman, Fareeha Butt, Anees ur Rehman, Melinda Madléna, Edit Paulik, Márió Gajdács, and et al. 2022. "Healthcare Professionals’ Compliance with the Standard Management Guidelines towards the Use of Biological Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Patients" International Journal of Environmental Research and Public Health 19, no. 8: 4699. https://doi.org/10.3390/ijerph19084699
APA StyleShakeel, S., Iffat, W., Qamar, A., Rehman, H., Ghuman, F., Butt, F., ur Rehman, A., Madléna, M., Paulik, E., Gajdács, M., & Jamshed, S. (2022). Healthcare Professionals’ Compliance with the Standard Management Guidelines towards the Use of Biological Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Patients. International Journal of Environmental Research and Public Health, 19(8), 4699. https://doi.org/10.3390/ijerph19084699







