Influence of COMT (rs4680) and DRD2 (rs1076560, rs1800497) Gene Polymorphisms on Safety and Efficacy of Methylphenidate Treatment in Children with Fetal Alcohol Spectrum Disorders
Abstract
:1. Introduction
2. Materials and Methods
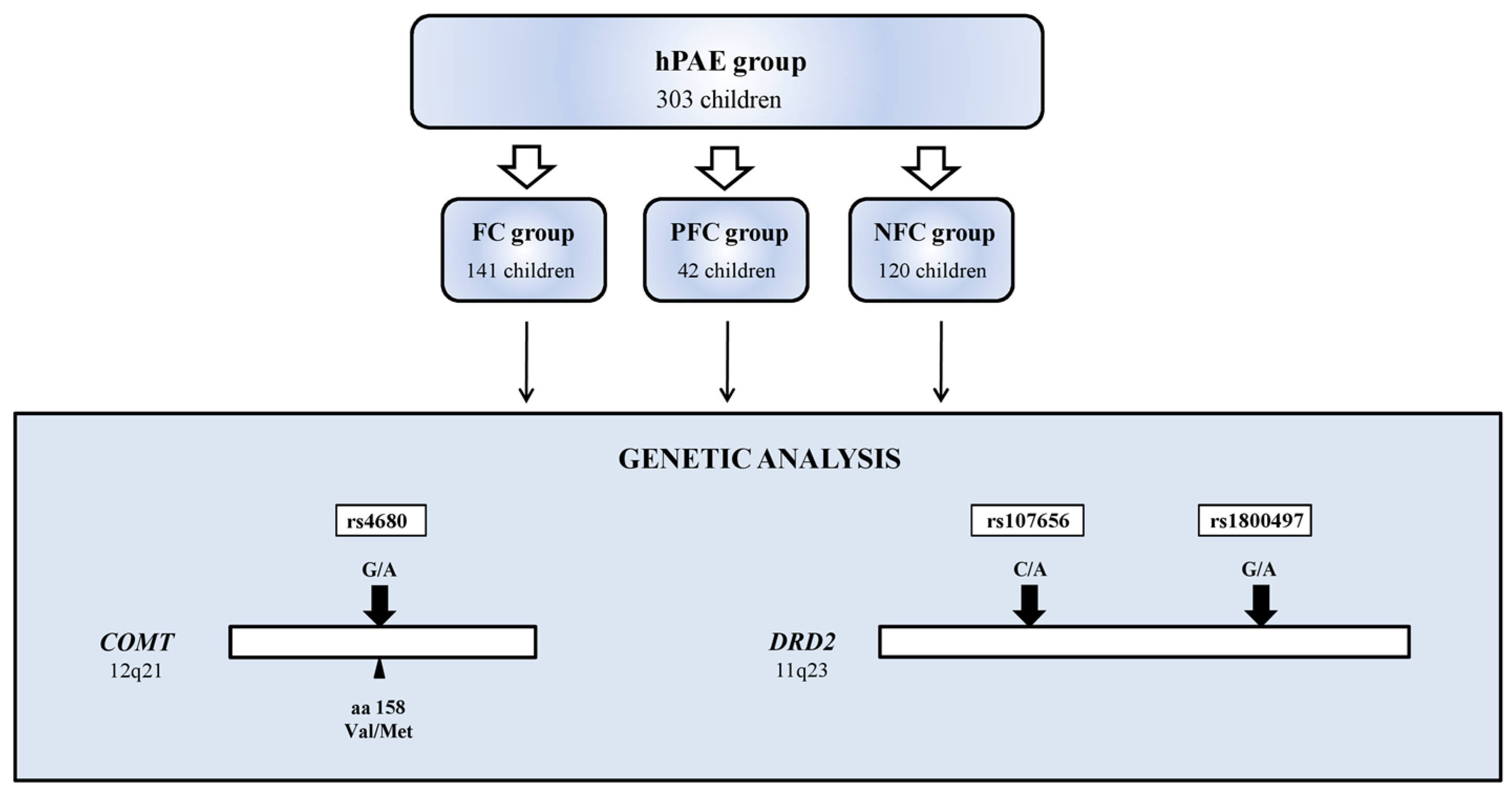
2.1. Study Group
2.2. Eligibility for MPH Treatment
2.3. Assessment of Efficacy and Safety of MPH Treatment
2.4. Genetic Analysis
2.5. Genotyping
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Price, K.J.; Miskelly, K.J. Why Ask Why? Logical Fallacies in the Diagnosis of Fetal Alcohol Spectrum Disorder. Ethic-Behav. 2014, 25, 418–426. [Google Scholar] [CrossRef]
- Ben Lovely, C.; Fernandes, Y.; Eberhart, J.K. Fishing for Fetal Alcohol Spectrum Disorders: Zebrafish as a Model for Ethanol Teratogenesis. Zebrafish 2016, 13, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidik, A.; Dixon, G.; Buckley, D.M.; Kirby, H.G.; Sun, S.; Eberhart, J.K. Exposure to ethanol leads to midfacial hypoplasia in a zebrafish model of FASD via indirect interactions with the Shh pathway. BMC Biol. 2021, 19, 134. [Google Scholar] [CrossRef]
- Spagnolo, A. Teratogenesis of alcohol. Ann. Dell’istituto Super. Sanita 1993, 29, 89–96. [Google Scholar]
- DeJong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Widder, M.; Mierzwa, L.; Schwerg, L.; Schecke, H.; Kornhuber, J.; Bouna-Pyrrou, P.; Bumb, J.M.; Richter-Schmidinger, T.; Lenz, B. Evaluation of the German biographic screening interview for fetal alcohol spectrum disorder (BSI-FASD). Sci. Rep. 2021, 11, 5233. [Google Scholar] [CrossRef] [PubMed]
- Carson, G.; Cox, L.V.; Crane, J.; Croteau, P.; Graves, L.; Kluka, S.; Koren, G.; Martel, M.-J.; Midmer, D.; Nulman, I.; et al. Alcohol Use and Pregnancy Consensus Clinical Guidelines. J. Obstet. Gynaecol. Can. 2010, 32 (Suppl. 3), S1–S2. [Google Scholar] [CrossRef]
- McGough, J.J.; McCracken, J.T.; Loo, S.K.; Manganiello, M.; Leung, M.C.; Tietjens, J.R.; Trinh, T.; Baweja, S.; Suddath, R.; Smalley, S.L.; et al. A Candidate Gene Analysis of Methylphenidate Response in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, P.; Harousseau, H.; Borteyru, J.P.; Menuet, J.C. Children of Alcoholic Parents—Observed Anomalies: Discussion of 127 Cases. Ther. Drug Monit. 2003, 25, 132–136. [Google Scholar] [CrossRef]
- Riley, E.P.; McGee, C.L. Fetal Alcohol Spectrum Disorders: An Overview with Emphasis on Changes in Brain and Behavior. Exp. Biol. Med. 2005, 230, 357–365. [Google Scholar] [CrossRef]
- Jones, K.L. The effects of alcohol on fetal development. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, D.; Cardoso, C.; McGrath, J. Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder—A meta-analysis. J. Child Psychol. Psychiatry 2015, 57, 116–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, S.N.; Riley, E.P. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J. Int. Neuropsychol. Soc. 1999, 5, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Riley, E.P. The Quest for a Neurobehavioral Profile of Heavy Prenatal Alcohol Exposure. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2011, 34, 51–55. [Google Scholar]
- Mattson, S.N.; Bernes, G.A.; Doyle, L.R. Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1046–1062. [Google Scholar] [CrossRef]
- Bertrand, J.; Floyd, L.L.; Weber, M.K. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR. Recomm. Rep. 2005, 54, 1–14. [Google Scholar]
- Kodituwakku, P.W.; Handmaker, N.S.; Cutler, S.K.; Weathersby, E.K.; Handmaker, S.D. Specific Impairments in Self-Regulation in Children Exposed to Alcohol Prenatally. Alcohol. Clin. Exp. Res. 1995, 19, 1558–1564. [Google Scholar] [CrossRef]
- Nanson, J.L.; Hiscock, M. Attention deficits in children exposed to alcohol prenatally. Alcohol. Clin. Exp. Res. 1990, 14, 656–661. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Sampson, P.D.; Olson, H.C.; Bookstein, F.L.; Barr, H.M.; Scott, M.; Feldman, J.; Mirsky, A.F. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring-a longitudinal prospective Study. Alcohol. Clin. Exp. Res. 1994, 18, 202–218. [Google Scholar] [CrossRef] [Green Version]
- Burd, L. FASD and ADHD: Are they related and How? BMC Psychiatry 2016, 16, 325. [Google Scholar] [CrossRef] [Green Version]
- Popova, S.; Lange, S.; Burd, L.; Rehm, J. Health care burden and cost associated with fetal alcohol syndrome: Based on official Canadian data. PLoS ONE 2012, 7, e43024. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Burd, L.; Rehm, J. The Economic Burden of Fetal Alcohol Spectrum Disorder in Canada in 2013. Alcohol Alcohol. 2015, 51, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Klug, M.G.; Martsolf, J.T.; Kerbeshian, J. Fetal alcohol syndrome: Neuropsychiatric phenomics. Neurotoxicol. Teratol. 2003, 25, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.I.; Carballo, J.J.; Riveiro-Alvarez, R.; Soto-Insuga, V.; Rodrigo, M.; Mahillo-Fernandez, I.; Abad-Santos, F.; Dal-Ré, R.; Ayuso, C. Pharmacogenetics of methylphenidate in childhood attention-deficit/hyperactivity disorder: Long-term effects. Sci. Rep. 2017, 7, 10391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, L.A.; Joshi, S.H.; O’Neill, J.; Kalender, G.; Dillon, A.; Best, K.M.; Narr, K.L.; Alger, J.R.; Levitt, J.G.; O’Connor, M.J. Cortical gyrification in children with attention deficit-hyperactivity disorder and prenatal alcohol exposure. Drug Alcohol Depend. 2021, 225, 108817. [Google Scholar] [CrossRef]
- Faraone, S.V. Using Meta-analysis to Compare the Efficacy of Medications for Attention-Defcit/Hyperactivity Disorder in Youths. Pharm. Ther. 2009, 34, 678–694. [Google Scholar]
- Maia, C.R.M.; Cortese, S.; Caye, A.; Deakin, T.K.; Polanczyk, G.V.; Polanczyk, C.A.; Rohde, L.A.P. Long-Term Efficacy of Methylphenidate Immediate-Release for the Treatment of Childhood ADHD. J. Atten. Disord. 2016, 21, 3–13. [Google Scholar] [CrossRef]
- Peña, I.D.; Gevorkiana, R.; Shi, W.-X. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur. J. Pharmacol. 2015, 764, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Barr, C.L.; Feng, Y.; Wigg, K.G.; Schachar, R.; Tannock, R.; Roberts, W.; Malone, M.; Kennedy, J.L. 5?-Untranslated region of the dopamine D4 receptor gene and attention-deficit hyperactivity disorder. Am. J. Med Genet. 2001, 105, 84–90. [Google Scholar] [CrossRef]
- Bédard, A.-C.; Schulz, K.P.; Cook, E.H.; Fan, J.; Clerkin, S.M.; Ivanov, I.; Halperin, J.M.; Newcorn, J.H. Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. NeuroImage 2010, 53, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Shook, D.; Brady, C.; Lee, P.S.; Kenealy, L.; Murphy, E.; Gaillard, W.D.; VanMeter, J.; Cook, E.H.; Stein, M.; Vaidya, C.J. Effect of dopamine transporter genotype on caudate volume in childhood ADHD and controls. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2010, 156, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, A. Biological and social influences on cognitive control processes dependent on prefrontal cortex. Prog. Brain Res. 2011, 189, 319–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funahashi, Y.; Yoshino, Y.; Yamazaki, K.; Ozaki, Y.; Mori, Y.; Mori, T.; Ochi, S.; Iga, J.-I.; Ueno, S.-I. Analysis of methylation and -141C Ins/Del polymorphisms of the dopamine receptor D2 gene in patients with schizophrenia. Psychiatry Res. 2019, 278, 135–140. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, H.; Yang, L.; Gao, F.; Fan, Y.; Feng, J.; Ma, X.-C. Associations between dopamine D2 receptor gene polymorphisms and schizophrenia risk: A PRISMA compliant meta-analysis. Neuropsychiatr. Dis. Treat. 2016, 12, 3129–3144. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wang, Y.; Wang, L.; Li, J.; Ma, Q.; Tao, J.; Zhou, X.; Zhou, H.; Jiang, Y.; Pan, G.; et al. Polymorphisms of DRD2 and DRD3 genes and Parkinson’s disease: A meta-analysis. Biomed. Rep. 2014, 2, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voisey, J.; Swagell, C.D.; Hughes, I.P.; van Daal, A.; Noble, E.P.; Lawford, B.R.; Young, R.M.; Morris, C.P. A DRD2 and ANKK1 haplotype is associated with nicotine dependence. Psychiatry Res. 2012, 196, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Montel, R.A.; Shen, P.-H.; Mash, D.C.; Goldman, D. Assessment of the Association of D2 Dopamine Receptor Gene and Reported Allele Frequencies With Alcohol Use Disorders: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1914940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.-Q.; Qiao, L.; Xue, X.-D.; Fu, J.-H. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: A meta-analysis. Neurosci. Lett. 2015, 590, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.-Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef]
- Zai, C.C.; Ehtesham, S.; Choi, E.; Nowrouzi, B.; De Luca, V.; Stankovich, L.; Davidge, K.; Freeman, N.; King, N.; Kennedy, J.L.; et al. Dopaminergic system genes in childhood aggression: Possible role for DRD2. World J. Biol. Psychiatry 2011, 13, 65–74. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Yamamoto, K.M.; Froehlich, T.E. Genetic Influence on Efficacy of Pharmacotherapy for Pediatric Attention-Deficit/Hyperactivity Disorder: Overview and Current Status of Research. CNS Drugs 2020, 34, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Białecka, M.; Kurzawski, M.; Roszmann, A.; Robowski, P.; Sitek, E.J.; Honczarenko, K.; Gorzkowska, A.; Budrewicz, S.; Mak, M.; Jarosz, M.; et al. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenetics Genom. 2012, 22, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mizuno, Y.; Fujisawa, T.X.; Takiguchi, S.; Kong, J.; Kosaka, H.; Tomoda, A. The Effects of COMT Polymorphism on Cortical Thickness and Surface Area Abnormalities in Children with ADHD. Cereb. Cortex 2018, 29, 3902–3911. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Jung, M.; Fujisawa, T.X.; Takiguchi, S.; Shimada, K.; Saito, D.N.; Kosaka, H.; Tomoda, A. Catechol-O-methyltransferase polymorphism is associated with the cortico-cerebellar functional connectivity of executive function in children with attention-deficit/hyperactivity disorder. Sci. Rep. 2017, 7, 4850. [Google Scholar] [CrossRef]
- Myer, N.M.; Boland, J.R.; Faraone, S.V. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol. Psychiatry 2017, 23, 1929–1936. [Google Scholar] [CrossRef]
- Cheon, K.-A.; Jun, J.-Y.; Cho, D.-Y. Association of the catechol-O-methyltransferase polymorphism with methylphenidate response in a classroom setting in children with attention-deficit hyperactivity disorder. Int. Clin. Psychopharmacol. 2008, 23, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Gizer, I.R.; Ficks, C.; Waldman, I.D. Candidate gene studies of ADHD: A meta-analytic review. Qual. Life Res. 2009, 126, 51–90. [Google Scholar] [CrossRef]
- Klecka, M.; Janas-Kozik, M.; Jelonek, I.; Siwiec, A.; Rybakowski, J. Validation of the Polish version of the Washington 4-digit code for the assessment of Fetal Alcohol Spectrum Disorders. Psychiatr. Polska 2017, 51, 335–347. [Google Scholar] [CrossRef]
- Kutchins, H.; Kirk, S.A. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994; ISBN 0-89042-061-0. [Google Scholar]
- WHO Collaborating Centre for Research and Training in Mental Health. The Classification of Mental and Behaviour in ICD-10, 2nd ed.; Pużyński, S., Wciórka, J., Eds.; University Medical Publishing “Vesalius”: Cracow, Poland, 2000; ISBN 83-85688-25-0. [Google Scholar]
- Ter-Stepanian, M.; Grizenko, N.; Zappitelli, M.; Joober, R. Clinical Response to Methylphenidate in Children Diagnosed with Attention-Deficit Hyperactivity Disorder and Comorbid Psychiatric Disorders. Can. J. Psychiatry 2010, 55, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Snyder, J.; Nanson, J.; Snyder, R.; Block, G. A study of stimulant medication in children with FAS. In Overcoming and Preventing Secondary Disabilities in Fetal Alcohol Syndrome and Fetal Alcohol Effects; Streissguth, A., Kanter, J., Eds.; University of Washington Press: Seattle, WA, USA, 1997; ISBN 9780295976501. [Google Scholar]
- Oesterheld, J.R.; Kofoed, L.; Tervo, R.; Fogas, B.; Wilson, A.; Fiechtner, H. Effectiveness of methylphenidate in native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: A controlled pilot study. J. Child Adolesc. Psychopharmacol. 1998, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, K.D.; Koplin, B.; Dohner, V.A. Psychostimulant clinical response in fetal alcohol syndrome. Can. J. Psychiatry 2000, 45, 90–91. [Google Scholar] [PubMed]
- Attention Deficit Hyperactivity Disorder: Diagnosis and Management. National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/ng87/resources/attention-deficit-hyperactivi-ty-disorder-diagnosis-and-management-pdf-1837699732933 (accessed on 4 February 2022).
- Johnston, B.A.; Steele, J.D.; Coghill, D.; Matthews, K. Predicting methylphenidate response in attention deficit hyperactivity disorder: A preliminary study. J. Psychopharmacol. 2014, 29, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Peadon, E. Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: Clinical guidelines. Neuropsychiatr. Dis. Treat. 2010, 6, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Bhat, A.A.; Hashem, S.; Nisar, S.; Kamal, M.; Syed, N.; Temanni, M.-R.; Gupta, R.K.; Kamran, S.; Azeem, M.W.; et al. Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry 2021, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kooij, J.S.; Boonstra, A.M.; Vermeulen, S.H.; Heister, A.G.; Burger, H.; Buitelaar, J.K.; Franke, B. Response to methylphenidate in adults with ADHD is associated with a polymorphism inSLC6A3 (DAT1). Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2008, 147, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Contini, V.; Victor, M.M.; Bertuzzi, G.P.; Salgado, C.A.; Picon, F.A.; Grevet, E.H.; Rohde, L.A.; Belmonte-De-Abreu, P.; Bau, C.H. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J. Clin. Psychopharmacol. 2012, 32, 820–823. [Google Scholar] [CrossRef]
- Samochowiec, J.; Pełka-Wysiecka, J.; Rottmann, M.; Horodnicki, J. New perspectives in the treatment of psychiatric diseases-pharmacogenetic studies of dopaminergic and serotonergic neurotransmission systems. Psychiatr. Pol. 2000, 4, 561–576. [Google Scholar]
- Frank, M.J.; Fossella, J.A. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology 2010, 36, 133–152. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Comer, S.D. A review of pharmacogenetic studies of substance-related disorders. Drug Alcohol Depend. 2015, 152, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Siever, L.J. Neurobiology of Aggression and Violence. Am. J. Psychiatry 2008, 165, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Iyegbe, C.; Powell, J.; Ursini, G.; Porcelli, A.; Bonvino, A.; Taurisano, P.; Romano, R.; Masellis, R.; Blasi, G.; et al. Interaction Between Functional Genetic Variation of DRD2 and Cannabis Use on Risk of Psychosis. Schizophr. Bull. 2015, 41, 1171–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.; Thomas, N.; Singleton, A.; Piggott, M.; Lloyd, S.; Perry, E.K.; Morris, C.; Perry, R.H.; Ferrier, I.N.; A Court, J. D2 dopamine receptor gene (DRD2) Taql A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997, 7, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Waxmonsky, J.G.; Salis, R.J.; Paluch, R.A.; Gnagy, E.M.; Mahaney, P.; Erbe, R.; Pelham, W.E.; Epstein, L.H. Dopamine-Related Genotypes and the Dose–Response Effect of Methylphenidate on Eating in Attention-Deficit/Hyperactivity Disorder Youths. J. Child Adolesc. Psychopharmacol. 2009, 19, 127–136. [Google Scholar] [CrossRef]
- McCracken, J.T.; Badashova, K.K.; Posey, D.J.; Aman, M.G.; Scahill, L.; Tierney, E.; Arnold, L.E.; Vitiello, B.; Whelan, F.; Chuang, S.Z.; et al. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J. 2013, 14, 295–302. [Google Scholar] [CrossRef]
- Lachman, H.M.; Papolos, D.F.; Saito, T.; Yu, Y.-M.; Szumlanski, C.L.; Weinshilboum, R.M. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996, 6, 243–250. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Epstein, J.N.; Nick, T.G.; Castro, M.S.M.; Stein, M.A.; Brinkman, W.B.; Graham, A.J.; Langberg, J.M.; Kahn, R.S. Pharmacogenetic Predictors of Methylphenidate Dose-Response in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 1129–1139. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.; Coghill, D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: Epidemiology, pre-vention and management. CNS Drugs 2008, 22, 213–237. [Google Scholar] [CrossRef]
- Castells, X.; Cunill, R.; Capellà, D. Treatment discontinuation with methylphenidate in adults with attention deficit hyperactivity disorder: A meta-analysis of randomized clinical trials. Eur. J. Clin. Pharmacol. 2012, 69, 347–356. [Google Scholar] [CrossRef]
- Hervas, A.; Serra-Llovich, A.; Rueda, I.; Targa, I.; Guijarro, S.; Bigorra, A.; Cancino, M.; Bote, V.; Cárcel, M.; Amasi-Hartoonian, N.; et al. Pharmacogenetic influences on the response to pharmacological treatment in autism spectrum disorders. J. Transl. Genet. Genom. 2021, 5, 278–287. [Google Scholar] [CrossRef]
- Swanson, J.; Volkow, N. Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behav. Brain Res. 2001, 130, 73–78. [Google Scholar] [CrossRef]
- Jakubowska-Dogru, E.; Elibol, B.; Dursun, I.; Yürüker, S. Effects of prenatal binge-like ethanol exposure and maternal stress on postnatal morphological development of hippocampal neurons in rats. Int. J. Dev. Neurosci. 2017, 61, 40–50. [Google Scholar] [CrossRef]
- Gorzkowska, I.; Gorzkowski, G.; Samochowiec, A.; Suchanecka, A.; Samochowiec, J. An interaction between a polymorphism of the serotonin transporter (5HTT) gene and the clinical picture of adolescents with combined type of ADHD (hyper-kinetic disorder) and youth drinking. Psychiatr. Pol. 2014, 48, 541–551. [Google Scholar] [PubMed]
- Murawski, N.J.; Moore, E.M.; Thomas, J.D.; Riley, E.P. Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Dis-orders From Animal Models to Human Studies. Alcohol Res. 2015, 37, 97–108. [Google Scholar] [PubMed]
- Lovallo, W.R.; Enoch, M.A.; Sorocco, K.H.; Vincent, A.S.; Acheson, A.; Cohoon, A.J.; Hodgkinson, C.A.; Goldman, D. Joint impact of early life adversity and COMT Val158Met (rs4680) genotypes on the adult cortisol response to psychological stress. Psychsom. Med. 2017, 79, 631–637. [Google Scholar] [CrossRef] [PubMed]
| hPAE Group | n | Age [Years] | Female Sex [n] | Female Sex [%] |
|---|---|---|---|---|
| total | 303 | 8.94 ± 4.9 | 213 | 70.30% |
| FC | 141 | 8.96 ± 5.07 | 84 | 59.57% |
| PFC | 42 | 8.60 ± 3.87 | 37 | 88.07% |
| NFC | 120 | 9.03 ± 5.06 | 92 | 76.67% |
| SNP | Gene Name | Gene Symbol | SNP Location | Nucleotide Change | TaqMan Assay ID |
|---|---|---|---|---|---|
| rs4680 | catechol-O-methyltransferase | COMT | 22q11, coding region | G > A | C__25746809_50 |
| rs1076560 | dopamine receptor D2 | DRD2 | 11q23, intergenic | C > A | C___2278888_10 |
| rs1800497 | dopamine receptor D2 | DRD2 | 11q23, coding region | G > A | C___7486676_10 |
| Symptoms | FASD (n = 71) | NFC (n = 43) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | ±SD | AT | ±SD | AFC * | BT | ±SD | AT | ±SD | NFC * | AFC/NFC * | |
| I attention deficit | 26.3 | 7.4 | 24.6 | 7.0 | 0.2024 | 23.7 | 4.6 | 15.2 | 3.5 | <0.001 | <0.001 |
| II impulsivity | 10.3 | 2.7 | 7.6 | 2.2 | <0.0001 | 4.5 | 0.9 | 4.2 | 1.1 | 0.1274 | <0.001 |
| III overactivity | 12.0 | 3.7 | 11.1 | 3.7 | <0.0001 | 10.4 | 2.9 | 9.0 | 3.0 | 0.0163 | <0.001 |
| total | 48.6 | 12.0 | 43.3 | 10.3 | 0.001 | 38.6 | 11.2 | 28.4 | 8.1 | <0.0001 | <0.001 |
| Genotype | FASD n = 71 | NFC n = 43 | p1 | p2 | p3 | p4 | ||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||||
| COMT rs4680:G > A | ||||||||
| GG | 17 | (24.0) | 6 | (14.0) | 0.223 | 0.530 | 0.235 | 1.000 |
| AG | 40 | (56.3) | 28 | (65.1) | ||||
| AA | 14 | (19.7) | 9 | (20.9) | ||||
| DRD2 rs1076560:C > A | ||||||||
| CC | 46 | (64.8) | 27 | (62.8) | 0.688 | 0.533 | 0.843 | 0.526 |
| CA | 23 | (32.4) | 16 | (37.2) | ||||
| AA | 2 | (2.8) | 0 | (0.0) | ||||
| DRD2 rs1800497:G > A | ||||||||
| GG | 46 | (64.8) | 27 | (62.8) | 1.000 | 1.000 | 0.843 | 1.000 |
| GA | 24 | (33.8) | 15 | (34.9) | ||||
| AA | 1 | (1.4) | 1 | (2.3) | ||||
| Gene | SNP id | Risk Allele | FASD # | NFC # | p * | OR (95% CI) Heterozygotes for a Minor Allele ** | OR (95% CI) Homozygotes for a Minor Allele *** | OR (95% CI) Minor Allele Carriers **** |
|---|---|---|---|---|---|---|---|---|
| COMT | rs4680:G > A | G | 0.493 | 0.535 | 0.58 | 0.57 (0.20–1.65) | 0.62 (0.18–2.20) | 0.58 (0.21–1.64) |
| DRD2 | rs1076560:C > A | A | 0.196 | 0.186 | 1.00 | 0.88 (0.40–1.96) | - | 0.96 (0.44–2.11) |
| DRD2 | rs1800497:G > A | A | 0.188 | 0.198 | 0.86 | 0.98 (0.44–2.19) | 0.61 (0.04–10.22) | 0.96 (0.44–2.11) |
| MPH Treatment Group | n | Age [Years] | Female Sex [n] | Female Sex [%] |
| total | 114 | 10.08 ± 3.43 | 77 | 67.64% |
| FC | 49 | 10.55 ± 3.38 | 27 | 55.10% |
| PFC | 22 | 9.91 ± 3.37 | 18 | 81.81% |
| NFC | 43 | 9.65 ± 3.53 | 32 | 74.42% |
| n | TE (n = 104) | NTE (n = 4) | AE (n = 7) | |
| FC | 49 | 42 | 2 | 5 |
| PFC | 22 | 20 | 1 | 1 |
| NFC | 43 | 42 | 0 | 1 |
| TE | NTE | AE | p * | ||
|---|---|---|---|---|---|
| n = 114 | 104 | 3 | 7 | ||
| MAF/MAC | n alleles | ||||
| COMT rs4680 G > A | 0.500/118 | 107 | 0 | 11 | 0.04994 |
| DRD2 rs1076560 C > A | 0.1920/43 | 43 | 1 | 0 | 0.360 |
| DRD2 rs1800497 G > A | 0.1920/43 | 43 | 1 | 0 | 0.360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmiarowska, M.; Brzuchalski, B.; Grzywacz, E.; Malinowski, D.; Machoy-Mokrzyńska, A.; Pierzchlińska, A.; Białecka, M. Influence of COMT (rs4680) and DRD2 (rs1076560, rs1800497) Gene Polymorphisms on Safety and Efficacy of Methylphenidate Treatment in Children with Fetal Alcohol Spectrum Disorders. Int. J. Environ. Res. Public Health 2022, 19, 4479. https://doi.org/10.3390/ijerph19084479
Śmiarowska M, Brzuchalski B, Grzywacz E, Malinowski D, Machoy-Mokrzyńska A, Pierzchlińska A, Białecka M. Influence of COMT (rs4680) and DRD2 (rs1076560, rs1800497) Gene Polymorphisms on Safety and Efficacy of Methylphenidate Treatment in Children with Fetal Alcohol Spectrum Disorders. International Journal of Environmental Research and Public Health. 2022; 19(8):4479. https://doi.org/10.3390/ijerph19084479
Chicago/Turabian StyleŚmiarowska, Małgorzata, Bogusław Brzuchalski, Elżbieta Grzywacz, Damian Malinowski, Anna Machoy-Mokrzyńska, Anna Pierzchlińska, and Monika Białecka. 2022. "Influence of COMT (rs4680) and DRD2 (rs1076560, rs1800497) Gene Polymorphisms on Safety and Efficacy of Methylphenidate Treatment in Children with Fetal Alcohol Spectrum Disorders" International Journal of Environmental Research and Public Health 19, no. 8: 4479. https://doi.org/10.3390/ijerph19084479
APA StyleŚmiarowska, M., Brzuchalski, B., Grzywacz, E., Malinowski, D., Machoy-Mokrzyńska, A., Pierzchlińska, A., & Białecka, M. (2022). Influence of COMT (rs4680) and DRD2 (rs1076560, rs1800497) Gene Polymorphisms on Safety and Efficacy of Methylphenidate Treatment in Children with Fetal Alcohol Spectrum Disorders. International Journal of Environmental Research and Public Health, 19(8), 4479. https://doi.org/10.3390/ijerph19084479






