Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Recruitment
2.3. Oral Health Examination
2.4. Data Collection
2.5. Exposure Variable
2.6. Outcome Variables
2.7. Data Analysis
2.8. Ethics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chrisopoulos, S.; Harford, J.E.; Ellershaw, A. Oral Health and Dental Care in Australia: Key Facts and Figures 2015; AIHW: Canberra, Australia, 2016. [Google Scholar]
- Figuero, E.; Carrillo-de-Albornoz, A.; Martín, C.; Tobías, A.; Herrera, D. Effect of pregnancy on gingival inflammation in systemically healthy women: A systematic review. J. Clin. Periodontol. 2013, 40, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Papapanou, P.N. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes—Systematic review. J. Clin. Periodontol. 2013, 84 (Suppl. 4), 181–194. [Google Scholar] [CrossRef] [PubMed]
- Boggess, K.A.; Lieff, S.; Murtha, A.P.; Moss, K.; Beck, J.; Offenbacher, S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet. Gynecol. 2003, 101, 227–231. [Google Scholar] [PubMed]
- Xiong, X.; Buekens, P.; Vastardis, S.; Yu, S.M. Periodontal disease and pregnancy outcomes: State-of-the-science. Obstet. Gynecol. Surv. 2007, 62, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.; Murtha, A.; Jared, H.; Boggess, K.; Lieff, S.; Heine, P. Post-cesarean infection in women with periodontal disease. Am. J. Obstet. Gynecol. 2002, 187, 225. [Google Scholar]
- Nibali, L.; D’Aiuto, F.; Griffiths, G.; Patel, K.; Suvan, J.; Tonetti, M.S. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: A case-control study. J. Clin. Periodontol. 2007, 34, 931–937. [Google Scholar] [CrossRef]
- Duarte, P.M.; da Rocha, M.; Sampaio, E.; Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Bastos, M.F.; Faveri, M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J. Periodontol. 2010, 81, 1056–1063. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Van Winkelhoff, A.J. Infection and inflammatory mechanisms. J. Clin. Periodontol. 2013, 40, S1–S7. [Google Scholar] [CrossRef]
- Ardila, C.M.; Lafaurie, G.I. Asociación entre porphyromona gingivalis y proteína C reactiva en enfermedades sistêmicas inflamatorias. Av. Periodoncia 2010, 22, 45–53. [Google Scholar] [CrossRef][Green Version]
- Jonakait, G.M. The effects of maternal inflammation on neuronal development: Possible mechanisms. Int. J. Dev. Neurosci. 2007, 25, 415–425. [Google Scholar] [CrossRef]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Arregoces, F.; Latorre-Uriza, C.; Velosa-Porras, J.; Roa-Molina, N.; Ruiz, A.J.; Silva, J.; Arias, E.; Echeverri, J. Inflammatory response in pregnant women with high risk of preterm delivery and its relationship with periodontal disease: A pilot study. Acta Odontol. Latinoam. 2018, 31, 53–57. [Google Scholar] [PubMed]
- Lian, Y.Y.; He, H.H.; Zhang, C.Z.; Li, X.C.; Chen, Y.H. Functional characterization of a matrix metalloproteinase 2 gene in Litopenaeus vannamei. Fish Shellfish. Immunol. 2019, 84, 404–413. [Google Scholar] [CrossRef]
- Yu, A.P.; Tam, B.T.; Yau, W.Y.; Chan, K.S.; Yu, S.S.; Chung, T.L.; Siu, P.M. Association of endothelin-1 and matrix metallopeptidase-9 with metabolic syndrome in middle-aged and older adults. Diabetol. Metab. Syndr. 2015, 7, 111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orange, S.; Rasko, J.E.; Thompson, J.F.; Vaughan, J.; Olive, E.; Pedler, M.; Horvath, J.S.; Hennessy, A. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine 2005, 29, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Nevers, T.; Norris, W.E.; Sharma, S. Vascular IL-10: A protective role in preeclampsia. J. Reprod. Immunol. 2011, 88, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Mesa, F.; Pozo, E.; O’Valle, F.; Puertas, A.; Magan-Fernandez, A.; Rosel, E.; Bravo, M. Relationship between periodontal parameters and plasma cytokine profiles in pregnant woman with preterm birth or low birth weight. Clin. Oral Investig. 2016, 20, 669–674. [Google Scholar] [CrossRef]
- Mahapatra, A.; Nayak, R.; Satpathy, A.; Pati, B.K.; Mohanty, R.; Mohanty, G.; Beura, R. Maternal periodontal status, oral inflammatory load, and systemic inflammation are associated with low infant birth weight. J. Periodontol. 2020, 92, 1107–1116. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Franco-Sena, A.B.; Farias, D.R.; Rebelo, F.; Kac, G. Maternal CRP concentrations during pregnancy and birth weight in a prospective cohort in Rio de Janeiro, Brazil. J. Matern. Fetal Neonatal Med. 2017, 30, 2346–2453. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Franco-Sena, A.B.; Rebelo, F.; Farias, D.R.; Lepsch, J.; Lima, N.S.; Kac, G. Factors associated with maternal serum CRP throughout pregnancy: A longitudinal study in women of Rio de Janeiro, Brazil. Nutrition 2015, 31, 1103–1108. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), 5–78. [Google Scholar] [CrossRef] [PubMed]
- Cocate, P.G.; Kac, G.; Heitmann, B.L.; Nadanovsky, P.; da Veiga, M.C.; Benaim, C.; Schlüssel, M.M.; de Castro, M.B.T.; Alves-Santos, N.H.; Baptista, A.F.; et al. Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: Protocol of a feasibility randomized controlled trial (the IMPROVE trial). Pilot Feasibility Stud. 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- IBGE (Instituto Brasileiro de Geografia e Estatística). Estimativas da População Residente Com Data de Referência 1º de Julho De 2017; IBGE: Rio de Janeiro, Brasil, 2017. [Google Scholar]
- DESANS (Departamento Geral de Segurança Alimentar e Nutricional Sustentável). Diagnóstico Situacional do Município de Duque de Caxias; DESANS: Duque de Caxias, Brasil, 2012. [Google Scholar]
- Palmeira, P.A.; Bem-Lignani, J.; Maresi, V.A.; Mattos, R.A.; Interlenghi, G.S.; Salles-Costa, R. Temporal Changes in the Association Between Food Insecurity and Socioeconomic Status in Two Population-Based Surveys in Rio de Janeiro, Brazil. Soc. Indic. Res. 2019, 144, 1349–1365. [Google Scholar] [CrossRef]
- Adegboye, A.; Santana, D.; Cocate, P.G.; Benaim, C.; Santos, P.; Heitmann, B.; Carvalho, M.C.D.V.S.; Schlüssel, M.M.; De Castro, M.B.T.; Kac, G. Vitamin D and Calcium Milk Fortification in Pregnant Women with Periodontitis: A Feasibility Trial. Int. J. Environ. Res. Public Health 2020, 17, 8023. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.A. Book review: Oral health surveys: Basic methods, 5th ed. Br. Dent. J. 2014, 217, 333. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; Bodnar, L.M.; Joseph, K.S.; Abrams, B.; Simhan, H.N.; Platt, R.W. The bias in current measures of gestational weight gain. Paediatr. Perinat. Epidemiol. 2012, 26, 109–116. [Google Scholar] [CrossRef]
- Barbieri, P.; Nishimura, R.; Crivellenti, L.; Sartorelli, D. Relative validation of a quantitative FFQ for use in Brazilian pregnant women. Public Health Nutr. 2013, 16, 1419–1426. [Google Scholar] [CrossRef]
- Vilela, A.A.; Farias, D.R.; Eshriqui, I.; Vaz, J.S.; Franco-Sena, A.B.; Castro, M.B.; Olinto, M.T.A.; Machado, S.P.; Da Silva, A.A.M.; Kac, G. Prepregnancy healthy dietary pattern is inversely associated with depressive symptoms among pregnant Brazilian women. J. Nutr. 2014, 144, 1612–1618. [Google Scholar] [CrossRef]
- NEPA (Núcleo de Estudos e Pesquisas em Alimentação). Tabela Brasileira de Composição de Alimentos (TACO), 4th ed.; NEPA/UNICAMP: Campinas, Brasil, 2011. [Google Scholar]
- IBGE (Instituto Brasileiro de Geografia e Estatística). Pesquisa de Orçamentos Familiares. Tabela de Composição Nutricional dos Alimentos Consumidos no Brasil; IBGE: Rio de Janeiro, Brasil, 2011. [Google Scholar]
- USDA (United States Department of Agriculture). USDA National Nutrient Database for Standard Reference; USDA Agricultural Research Service: Washington, DC, USA, 2011. [Google Scholar]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in high-sensitivity c-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.T.; Júnior, E.A.; Santana, E.F.M.; Lima, J.W.O.; Cecchino, G.N.; Da Silva Costa, F. Translation and cross-cultural adaptation of the Pregnancy Physical Activity Questionnaire (PPAQ) to the Brazilian population. Čes. Gynek. 2015, 80, 290–298. [Google Scholar]
- Carrilho, T.; Rangel, T.; Rasmussen, K.; Farias, D.R.; Costa, N.; Batalha, M.; Reichenheim, M.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar]
- Adegboye, A.; Santana, D.; Santos, P.; Cocate, P.G.; Benaim, C.; Castro, M.B.; Schlüssel, M.M.; Kac, G.; Heitmann, B.L. Exploratory Efficacy of Calcium-Vitamin D Milk Fortification and Periodontal Therapy on Maternal Oral Health and Metabolic and Inflammatory Profile. Nutrients 2021, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- McDade, T.W.; Borja, J.B.; Largado, F.; Adair, L.S.; Kuzawa, C.W. Adiposity and Chronic Inflammation in Young Women Predict Inflammation during Normal Pregnancy in the Philippines. J. Nutr. 2016, 146, 353–357. [Google Scholar] [CrossRef]
- Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Uriza, C.L.; Velosa-Porras, J.; Roa, N.S.; Lara, S.M.Q.; Silva, J.; Ruiz, A.J.; Arregoces, F.M.E. Periodontal Disease, Inflammatory Cytokines, and PGE2 in Pregnant Patients at Risk of Preterm Delivery: A Pilot Study. Infect. Dis. Obstet. Gynecol. 2018, 2018, 7027683. [Google Scholar]
- Sacks, G.; Seyani, L.; Lavery, S.; Trew, G. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum. Reprod. 2004, 19, 1025–1030. [Google Scholar] [CrossRef]
- Witteveen, A.B.; Henrichs, J.; Bellers, M.; van Oenen, E.; Verhoeven, C.J.; Vrijkotte, T. Mediating role of C-reactive protein in associations between pre-pregnancy BMI and adverse maternal and neonatal outcomes: The ABCD-study cohort. J. Matern. Fetal Neonatal Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Teran, E.; Escudero, C.; Moya, W.; Flores, M.; Vallance, P.; Lopez-Jaramillo, P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int. J. Gynaecol. Obstet. 2001, 75, 243–249. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jun, J.K.; Park, K.H.; Syn, H.C.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1040. [Google Scholar] [PubMed]
- Hvilsom, G.B.; Thorsen, P.; Jeune, B.; Bakketeig, L.S. C-reactive protein: A serological marker for preterm delivery? Acta. Obstet. Gynecol. Scand. 2002, 81, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, I.; Carvalho-Guerra, F.; Pereira-Leite, L.; Quintanilha, A. Lactoferrin as a sensitive blood marker of neutrophil activation in normal pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 62, 189–194. [Google Scholar] [CrossRef]
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003, 26, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Kettyle, E.; Sandler, L.; Ecker, J.L.; Roberts, J.; Thadhani, R. Obesity and preeclampsia: The potential role of inflammation. Obstet. Gynecol. 2001, 98, 757–762. [Google Scholar] [CrossRef]
- Nakishbandy, B.M.; Barawi, S.A.M. Level of C-reactive protein as an indicator for prognosis of premature uterine contractions. J. Prenat. Med. 2014, 8, 25–30. [Google Scholar]
- Dodds, W.G.; Lams, J.D. Maternal C-reactive protein and preterm labor. J. Repord. Med. 2003, 32, 527–530. [Google Scholar]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Arboleda, S.; Vargas, M.; Losada, S.; Pinto, A. Review of obesity and periodontitis: An epidemiological view. Br. Dent. J. 2019, 227, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Baharuddin, N.A.; Javed, F.; Vohra, F. Efficacy of non-surgical periodontal therapy in the management of chronic periodontitis among obese and non-obese patients: A systematic review and meta-analysis. Clin. Oral Investig. 2016, 20, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Spyrides, M.A.C.; Struchiner, C.J.; Barbosa, M.T.; Kac, G. Data Analysis with Repeated Measures. In Nutritional Epidemiology; Kac, G., Sichieri, R., Gigante, D.P., Eds.; Fiocruz e Atheneu: Rio de Janeiro, Brasil, 2007; pp. 245–260. [Google Scholar]
- Peres, M.A.; Macpherson, L.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
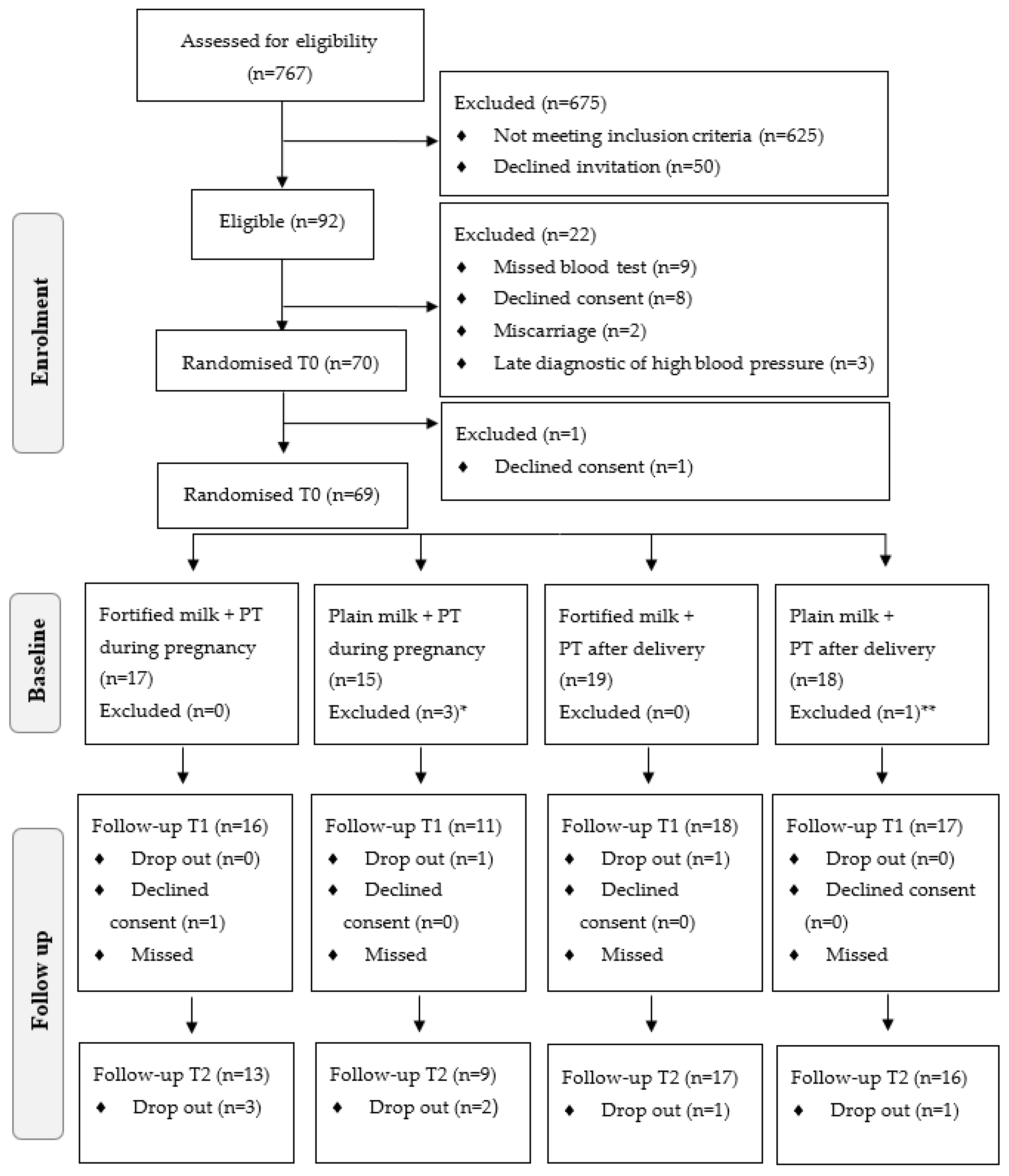
| Maternal Characteristics | Total 2 | Pre-Pregnancy BMI Categories | p * | |
|---|---|---|---|---|
| No Excess Weight (≤24.9 kg/m2) | Excess Weight (≥25 kg/m2) | |||
| Age (years) | 28.5 (7.0) | 25.0 (8.0) | 30.0 (8.0) | 0.009 |
| Gestational age (weeks) | 16.4 (4.1) | 17.0 (4.6) | 16.4 (3.7) | 0.896 |
| Years of education (years) | 12.0 (3.0) | 12.0 (3.0) | 12.0 (3.0) | 0.926 |
| Monthly per capita income (US$) 3 | 130.0 (104.1) | 120.0 (78.3) | 151.1 (152.3) | 0.185 |
| Pre-pregnancy BMI (kg/m2) | 26.3 (9.5) | 21.4 (2.2) | 30.5 (6.2) | <0.001 |
| Gestational weight gain (kg) | 10.1 (8.7) | 11.0 (6.1) | 6.9 (12.7) | 0.033 |
| Insulin (µU/mL) | 7.6 (5.2) | 4.9 (3.8) | 9.6 (4.9) | <0.001 |
| Fasting glucose (mg/dL) | 72.5 (9.5) | 68.0 (7.0) | 75.0 (8.0) | 0.001 |
| Energy intake (kcal/d) | 4054 (2334) | 4216 (2549) | 3927 (2458) | 0.278 |
| Calcium intake (mg/d) | 1037 (636) | 953 (478) | 1045 (944) | 0.867 |
| Vitamin D intake (mcg/d) | 5.3 (4.4) | 5.6 (4.2) | 5.0 (3.2) | 0.995 |
| Inflammatory diet index 4 | 0.8 (2.1) | 1.1 (2.0) | 0.8 (1.9) | 0.724 |
| Pocket depth (mm) | 4.2 (0.3) | 4.2 (0.2) | 4.3 (0.2) | 0.406 |
| CAL (mm) | 4.2 (0.3) | 4.2 (0.3) | 4.3 (0.3) | 0.360 |
| BOP (%) | 16.0 (21.0) | 15.0 (21.0) | 16.0 (22.0) | 0.823 |
| Living with a partner | ||||
| Yes | 59 (86.8) | 24 (82.8) | 35 (89.7) | 0.401 |
| No 5 | 9 (13.2) | 5 (17.2) | 4 (10.3) | |
| Parity (number of parturitions) | ||||
| 0 | 24 (35.3) | 12 (41.4) | 12 (30.8) | 0.344 |
| ≥1 | 44 (64.7) | 17 (58.6) | 27 (69.2) | |
| Self-reported skin colour | ||||
| White | 8 (11.8) | 4 (13.8) | 4 (10.3) | 0.654 |
| Mixed or black | 60 (88.2) | 25 (86.2) | 35 (89.7) | |
| Current smoking | ||||
| No | 60 (88.2) | 27 (93.1) | 33 (84.6) | 0.283 |
| Yes | 8 (11.8) | 2 (6.9) | 6 (15.4) | |
| Alcohol intake | ||||
| No | 57 (83.8) | 25 (86.2) | 32 (82.0) | 0.645 |
| Yes | 11 (16.2) | 4 (13.8) | 7 (18.0) | |
| Physical Activity | ||||
| Sedentary and light | 42 (61.8) | 20 (69.0) | 22 (56.4) | 0.292 |
| Moderate | 26 (38.2) | 9 (31.0) | 17 (43.6) | |
| Sun exposure | ||||
| <30 min/day | 48 (81.4) | 18 (81.8) | 30 (81.1) | 0.944 |
| >30 min/day | 11 (18.6) | 4 (18.2) | 7 (18.9) | |
| Periodontal Parameters | Total 2 | Pre-Pregnancy BMI Categories | p | |
|---|---|---|---|---|
| No Excess Weight (≤24.9kg/m2) | Excess Weight (≥25 kg/m2) | |||
| Median (IQR) | ||||
| Δ Pocket depth (mm) 1 | 0.0 (0.3) | −0.1 (0.2) | 0.0 (0.3) | 0.171 |
| Δ CAL (mm) 1 | 0.0 (0.3) | −0.1 (0.2) | 0.0 (0.3) | 0.139 |
| Δ BOP (%) 1 | 0.0 (0.2) | −0.1 (0.2) | 0.0 (0.2) | 0.080 |
| Variables | |||
|---|---|---|---|
| CRP Change | β | 95% CI | p-Value |
| Pre-pregnancy excess of weight (no/yes) 1 | 4.39 | 2.12–6.65 | <0.01 |
| Insulin (µU/mL) | 0.35 | 0.11–0.58 | <0.01 |
| CAL (mm) | 3.08 | −1.93–8.08 | 0.23 |
| Restricted Maximum Likelihood Estimation (REML) at the convergence | 1095.4 | ||
| Variance components | Variance | SD | 95% CI |
| Woman identifier (ID) | 15.37 | 3.92 | 0–7.89 |
| Gestational age (wk) | 0.003 | 0.06 | 0–0.18 |
| Residual | 37.36 | 6.11 | 5.33–7.00 |
| Lilliefors (Kolmogorov-Smirnov) normality test | D | p-Value | |
| for residuals of fixed effects | 0.12 | <0.01 | |
| for random effects | 0.31 | <0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, D.D.; Kac, G.; dos Santos, P.P.T.; da Silva, T.C.; Benaim, C.; Cocate, P.G.; Trindade de Castro, M.B.; Heitmann, B.L.; Adegboye, A.R.A. Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial. Int. J. Environ. Res. Public Health 2022, 19, 2705. https://doi.org/10.3390/ijerph19052705
Santana DD, Kac G, dos Santos PPT, da Silva TC, Benaim C, Cocate PG, Trindade de Castro MB, Heitmann BL, Adegboye ARA. Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial. International Journal of Environmental Research and Public Health. 2022; 19(5):2705. https://doi.org/10.3390/ijerph19052705
Chicago/Turabian StyleSantana, Danilo Dias, Gilberto Kac, Pedro Paulo Teixeira dos Santos, Thainá Castro da Silva, Camila Benaim, Paula Guedes Cocate, Maria Beatriz Trindade de Castro, Berit Lilienthal Heitmann, and Amanda Rodrigues Amorim Adegboye. 2022. "Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial" International Journal of Environmental Research and Public Health 19, no. 5: 2705. https://doi.org/10.3390/ijerph19052705
APA StyleSantana, D. D., Kac, G., dos Santos, P. P. T., da Silva, T. C., Benaim, C., Cocate, P. G., Trindade de Castro, M. B., Heitmann, B. L., & Adegboye, A. R. A. (2022). Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial. International Journal of Environmental Research and Public Health, 19(5), 2705. https://doi.org/10.3390/ijerph19052705








