Development and Evaluation of Integrated Chrono-Nutrition Weight Reduction Program among Overweight/Obese with Morning and Evening Chronotypes
Abstract
:1. Introduction
2. Materials and Methods
- Phase 1: Development of Integrated Chrono-Nutrition Weight Reduction Program
2.1. Needs Assessments of Chrono-Nutrition Domains
2.2. Integration of Chrono-Nutrition and SLIMSHAPE™ Weight Reduction Module
- Phase 2 Feasibility of Integrated Chrono-Nutrition Weight Reduction Program
2.3. Measurement
2.3.1. Socio-Demographic Background
2.3.2. Attendance and Satisfaction
2.3.3. Chronotypes and Sleep Parameters
2.3.4. Dietary Information
- a.
- Energy intake (kcal) during early window = the sum of energy intake before mid-point of eating. Thus, %E intake during early window = ((energy intake (kcal) during early window ÷ total energy intake) × 100). The same calculation method was applied for intake in the late window.
- b.
- For example, carbohydrate intake early window = the sum of carbohydrate intake before the midpoint of eating. Thus, %E from carbohydrate intake during early window = (((carbohydrate intake (g) during early window × 4 kcal) ÷ total energy intake) × 100). The same calculation method was applied for the intake in the late window and suited the other macronutrient (protein and fat) intake.
2.3.5. Physical Activity
2.3.6. Adiposity, Biochemical and Clinical Parameters
2.4. Statistical Method
3. Results
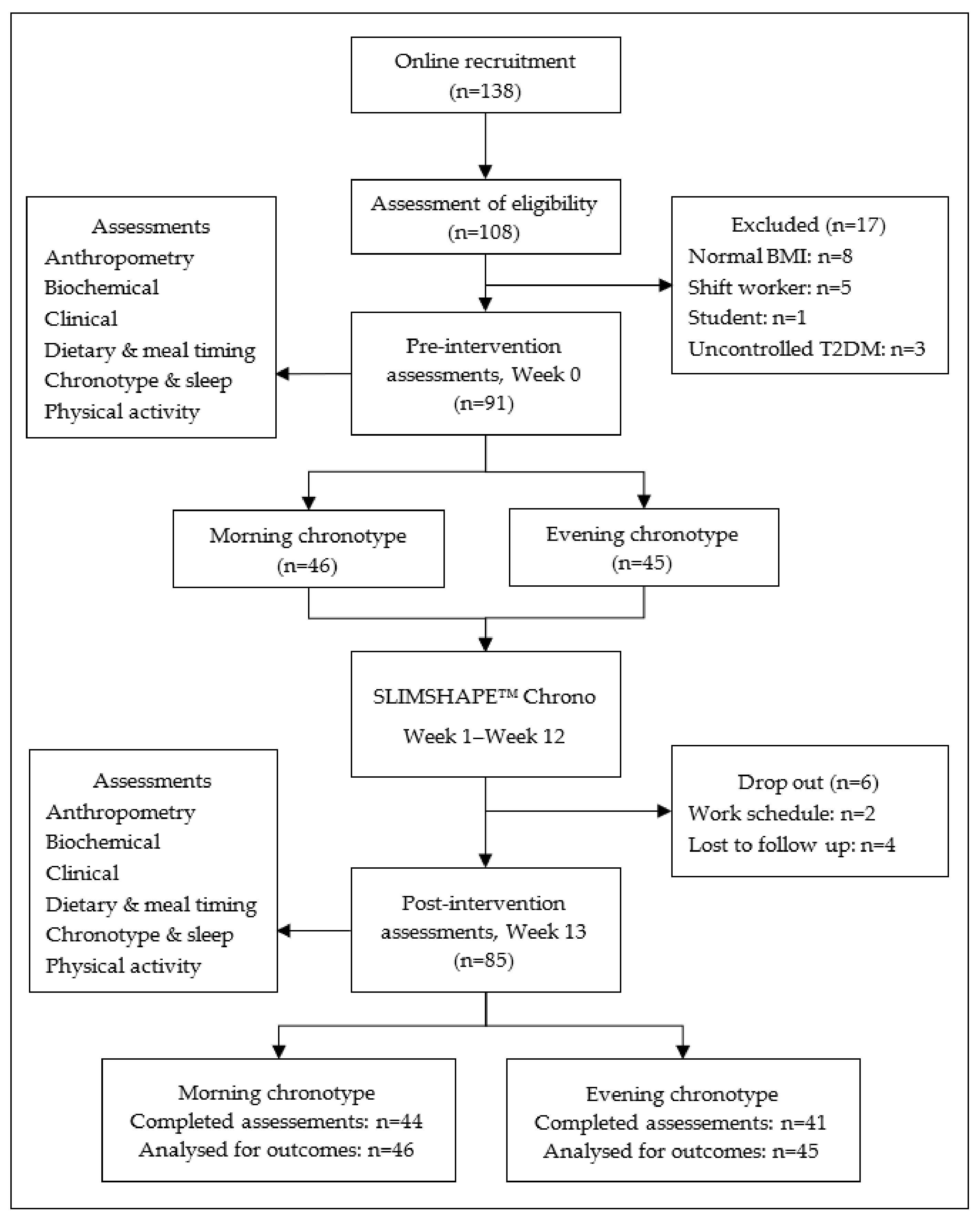
3.1. Study Participants
3.2. Attendance and Satisfaction Rate
| Parameters | Total (n = 91) | MT (n = 46) | ET (n = 45) | p-Value |
|---|---|---|---|---|
| Age a | 39.6 ± 6.3 | 40.8 ± 6.7 | 38.7 ± 5.8 | 0.257 |
| Gender b | ||||
| Women | 68 (74.7) | 37 (80.4) | 31 (68.9) | 0.205 |
| Men | 23 (25.3) | 9 (19.6) | 14 (31.1) | |
| Race b | ||||
| Malay | 89 (97.8) | 45(97.8) | 44 (97.8) | 0.987 |
| Chinese | 2 (2.2) | 1 (2.2) | 1 (2.2) | |
| Marital status b | ||||
| Married | 72 (79.1) | 41 (89.1) | 31 (68.9) | 0.018 |
| Single/divorcee/widow | 19 (20.9) | 5 (10.9) | 14 (31.1) | |
| Education level b | ||||
| Tertiary | 81 (89.0) | 41 (89.1) | 40 (88.9) | 0.971 |
| Secondary | 10 (11.0) | 5 (10.9) | 5 (11.1) | |
| Monthly household income b | ||||
| Low | 10 (11.0) | 5 (10.9) | 5 (11.1) | 0.136 |
| Middle | 61 (67.0) | 27 (58.7) | 34 (75.6) | |
| High | 20 (22.0) | 14 (30.4) | 6 (13.3) | |
| Self-reported medical history | ||||
| Hypertension, n (%) b | 11 (12.1) | 5 (10.9) | 6 (13.3) | 0.718 |
| Diabetes mellitus, n (%) b | 6 (6.6) | 2 (4.3) | 4 (8.9) | 0.434 |
| Dyslipidaemia, n (%) b | 11 (12.1) | 3 (6.5) | 8 (17.8) | 0.100 |
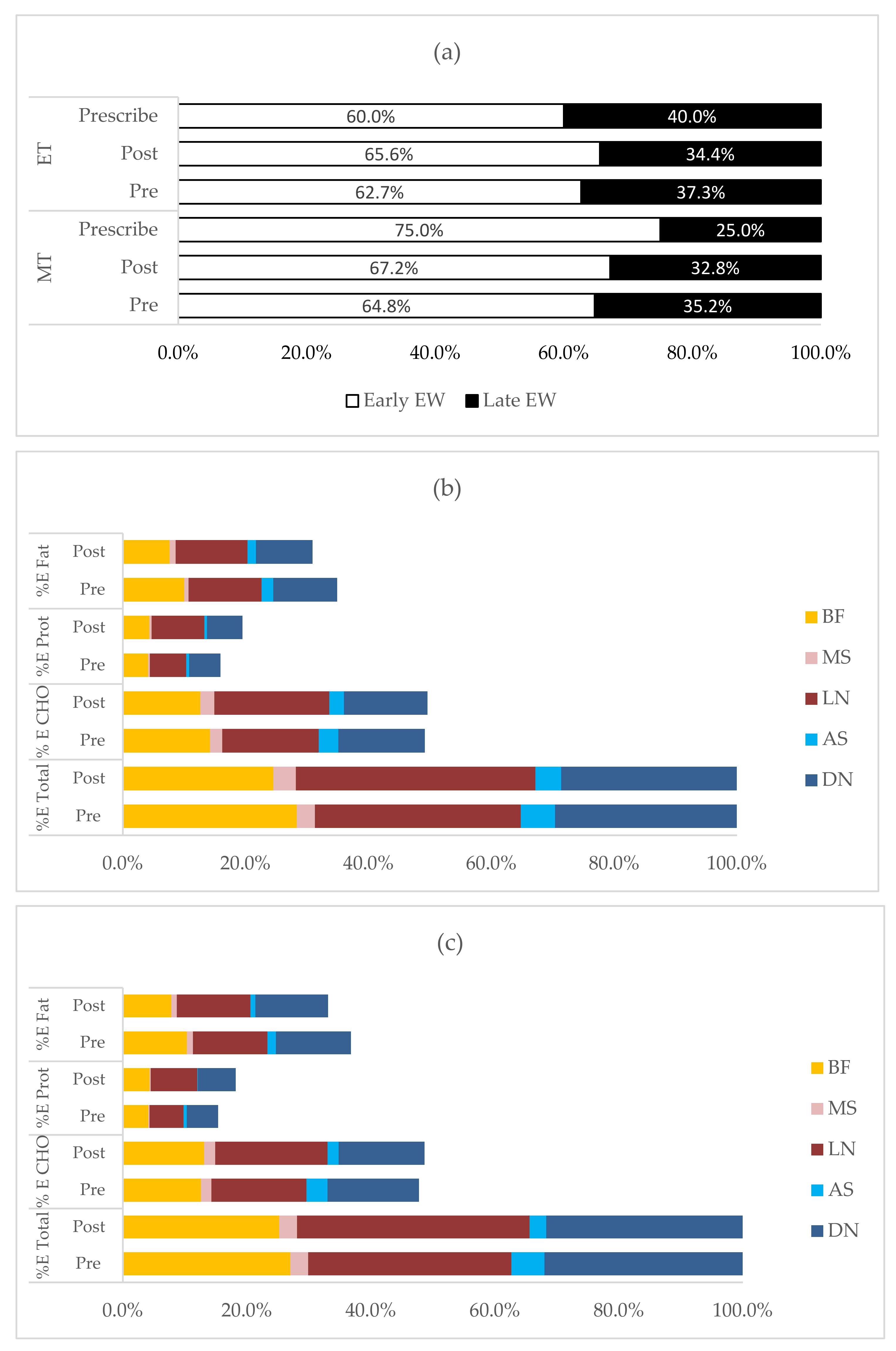
3.3. Changes in Dietary, Sleep and Physical Activity between Pre- and Post-Intervention
3.4. Changes in Temporal Pattern of Energy and Macronutrient Intake between Pre- and Post-Intervention
3.5. Changes in Adiposity and Biochemical Parameters between Pre- and Post-Intervention
3.6. Refinement of the Integrated Chrono-Nutrition Weight Reduction Intervention
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Xu, J.; Pan, X.; Song, X.; Shan, L.; Zhao, Y.; Shan, P.F. Global burden of noncommunicable disease attributable to high body mass index in 195 countries and territories, 1990–2017. Endocrine 2020, 69, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body Mass Index and Risk for COVID-19–Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March–December 2020. MMWR Surveill. Summ. 2021, 70, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Sagastume, D.; Mertens, E.; Uzhova, I.; Smith, J.; Wu, J.H.Y.; Bishop, E.; Onopa, J.; Shi, P.; Micha, R.; et al. Effectiveness of workplace wellness programmes for dietary habits, overweight, and cardiometabolic health: A systematic review and meta-analysis. Lancet Public Health 2021, 6, e648–e660. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef] [Green Version]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Galasso, L.; Montaruli, A.; Jankowski, K.S.; Bruno, E.; Castelli, L.; Mulè, A.; Chiorazzo, M.; Ricceri, A.; Erzegovesi, S.; Caumo, A.; et al. Binge eating disorder: What is the role of physical activity associated with dietary and psychological treatment? Nutrients 2020, 12, 3622. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G.K. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Pot, G.K. Chrono-nutrition—An emerging, modifiable risk factor for chronic disease? Nutr. Bull. 2021, 46, 114–119. [Google Scholar] [CrossRef]
- Makarem, N.; Sears, D.D.; St-Onge, M.P.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Mi, J.; Aggarwal, B. Habitual nightly fasting duration, eating timing, and eating frequency are associated with cardiometabolic risk in women. Nutrients 2020, 12, 3043. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Cornier, M.A.; Catenacci, V.A.; Tussey, E.J.; Grau, L.; Arbet, J.; Broussard, J.L.; Rynders, C.A. Later meal and sleep timing predicts higher percent body fat. Nutrients 2021, 13, 73. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Madjd, A.; Taylor, M.A.; Delavari, A.; Malekzadeh, R.; MacDonald, I.A.; Farshchi, H.R. Beneficial effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weight-loss program: A randomized clinical trial. Am. J. Clin. Nutr. 2016, 104, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Raynor, H.A.; Li, F.; Cardoso, C. Daily pattern of energy distribution and weight loss. Physiol. Behav. 2018, 192, 167–172. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Vera, B.; Dashti, H.S.; Gómez-Abellán, P.; Hernández-Martínez, A.M.; Esteban, A.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci. Rep. 2018, 8, 945. [Google Scholar] [CrossRef] [Green Version]
- Bazzani, A.; Marantonio, S.; Andreozzi, G.; Lorenzoni, V.; Bruno, S.; Cruz-Sanabria, F.; d’Ascanio, P.; Turchetti, G.; Faraguna, U. Late chronotypes, late mealtimes. Chrononutrition and sleep habits during the COVID-19 lockdown in Italy. Appetite 2022, 172, 105951. [Google Scholar] [CrossRef]
- Maukonen, M.; Kanerva, N.; Partonen, T.; Kronholm, E.; Tapanainen, H.; Kontto, J.; Männistö, S. Chronotype differences in timing of energy and macronutrient intakes: A population-based study in adults. Obesity 2017, 25, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Fárková, E.; Šmotek, M.; Bendová, Z.; Manková, D.; Kopřivová, J. Chronotype and social jet-lag in relation to body weight, apetite, sleep quality and fatigue. Biol. Rhythm Res. 2021, 52, 1205–1216. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Chronotype and adherence to the mediterranean diet in obesity: Results from the opera prevention project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; De Hollanda, A.; Canteras, M.; Garaulet, M.; Izquierdo-Pulido, M. Evening chronotype associates with obesity in severely obese subjects: Interaction with CLOCK 3111T/C. Int. J. Obes. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Gangwar, A.; Tiwari, S.; Rawat, A.; Verma, A.; Singh, K.; Kant, S.; Garg, R.K.; Singh, P.K. Circadian Preference, Sleep Quality, and Health-impairing Lifestyles Among Undergraduates of Medical University. Cureus 2018, 10, e2856. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.S.G.; Gallego, M.G.; Soler, I.D.; Ortega, M.C.B.; Cáceres, C.M.M.; Morante, J.J.H. Effect of a chronotype-adjusted diet on weight loss effectiveness: A randomized clinical trial. Clin. Nutr. 2019, 39, 1041–1048. [Google Scholar] [CrossRef]
- Strojny, Z.; Rutkowski, R.; Kanikowska, A.; Zawada, A.; Juchacz, A.; Grzymisławski, M.; Sato, M.; Litwinowicz, M.; Korybalska, K.; Bręborowicz, A.; et al. No significant effect of the individual chronotype on the result of moderate calorie restriction for obesity—A pilot study. Nutrients 2021, 13, 4089. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Cauter, E. Van Disturbances of sleep and circadian rhythms: Novel risk factors for obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nur Syafiqa, N.S.B.; Tharumalay, R.D.; Din, N.C.; Ibrahim, N.; Amit, N.; Farah, N.M.F.; Osman, R.A.; Hamid, M.F.A.; Ibrahim, I.A.; et al. The effects of circadian rhythm disruption on mental health and physiological responses among shift workers and general population. Int. J. Environ. Res. Public Health 2020, 17, 7156. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.C.; Horgan, G.; Mikkelsen, M.L.K.; Palmeira, A.L.; Scott, S.; Duarte, C.; Santos, I.; Encantado, J.; Driscoll, R.O.; Turicchi, J.; et al. Association between objectively measured sleep duration, adiposity and weight loss history. Int. J. Obes. 2020, 44, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.; Burrows, T.L.; Skinner, J.A.; Duncan, M.J. The influence of sleep health on dietary intake: A systematic review and meta-analysis of intervention studies. J. Hum. Nutr. Diet. 2021, 34, 273–285. [Google Scholar] [CrossRef]
- Gan, W.Y.; Mohd Nasir, M.T.; Zalilah, M.S.; Hazizi, A.S. Differences in eating behaviours, dietary intake and body weight status between male and female Malaysian university students. Malays. J. Nutr. 2011, 17, 213–228. [Google Scholar]
- Manan, W.A.W.M.; Firdaus, N.I.; Safiah, M.Y.; Haslinda, S.M.D.; Poh, B.K.; Norimah, A.K.; Azmi, M.Y.; Tahir, A.; Mirnalini, K.; Zalilah, M.S.; et al. Meal patterns of malaysian adults: Findings from the Malaysian Adults Nutrition Survey (MANS). Malays. J. Nutr. 2012, 18, 221–230. [Google Scholar]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Ludin, A.F.M. The association between chronotype and dietary pattern among adults: A scoping review. Int. J. Environ. Res. Public Health 2020, 17, 68. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Circadian rhythms, food timing and obesity. Proc. Nutr. Soc. 2016, 75, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Rusali, R.; Shahar, S.; Lee, X.W.; Abdul Manaf, Z. Effectiveness of a Structured Weight Management Programme at Workplace among Employees of a Petroleum Industry in Malaysia. J. Sains Kesihat. Malays. 2016, 14, 49–56. [Google Scholar] [CrossRef]
- Rusali, R.; Manaf, Z.A.; Shahar, S.; Mazri, F.H.; Ibrahim, N.; Ludin, A.F.M.; Singh, D.K.A.; Ali, N.M. Comparison of the effectiveness of online and face-to-face weight-loss interventions in the workplace: Evidence from Malaysia. Sains Malays. 2018, 47, 2437–2445. [Google Scholar] [CrossRef]
- Glanz, K.; Rimer, B.K. Theory at a Glance A Guide for Helath Promotion in Practice; U.S. Department of Health and Human Services: Washington, DC, USA, 2005.
- Department of Statistics Malaysia (DOSM). Household Income & Basic Amenities Survey Report 2019; DOSM: Putrajaya, Malaysia, 2019.
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F.; Karim, N.A.; Ban, A.Y.L.; Osman, R.A. Modified Munich chronotype questionnaire for application to short-interval split sleep of non-shift workers. Chronobiol. Int. 2021, 38, 659–665. [Google Scholar] [CrossRef]
- Shahar, S.; Earland, J.; Rahman, S.A. Validation of a dietary history questionnaire against a 7-d weighed record for estimating nutrient intake among rural elderly Malays. Malays. J. Nutr. 2000, 6, 33–44. [Google Scholar]
- Allison, K.C.; Lundgren, J.D.; O’Reardon, J.P.; Martino, N.S.; Sarwer, D.B.; Wadden, T.A.; Crosby, R.D.; Engel, S.G.; Stunkard, A.J. The Night Eating Questionnaire (NEQ): Psychometric properties of a measure of severity of the Night Eating Syndrome. Eat. Behav. 2008, 9, 62–72. [Google Scholar] [CrossRef]
- Soo, K.L.; Wan Abdul Manan, W.M.; Wan Suriati, W.N. The Bahasa Melayu version of the global physical activity questionnaire: Reliability and validity study in Malaysia. Asia Pac. J. Public Health 2015, 27, NP184–NP193. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 312–419. [Google Scholar] [CrossRef] [Green Version]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F.; Karim, N.A.; Hazwari, N.D.; Kek, Q.W.; Abdul Basir, S.M.; Arifin, A. Do Temporal Eating Patterns Differ in Healthy versus Unhealthy Overweight/Obese Individuals? Nutrients 2021, 13, 4121. [Google Scholar] [CrossRef]
- Wellard-Cole, L.; Davies, A.; Allman-Farinelli, M. Contribution of foods prepared away from home to intakes of energy and nutrients of public health concern in adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef]
- Mitchell, E.; Yang, Q.; Behr, H.; Ho, A.; DeLuca, L.; May, C.; Michaelides, A. Long-Term Food Choice and Weight Loss on a Mobile Program with Food Color System. Curr. Dev. Nutr. 2021, 5, 982. [Google Scholar] [CrossRef]
- Molina, T.A.; Burgess, H.J. Calculating the dim light melatonin onset: The impact of threshold and sampling rate. Chronobiol. Int. 2011, 28, 714–718. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Montaruli, A.; Castelli, L.; Galasso, L.; Mulè, A.; Bruno, E.; Esposito, F.; Caumo, A.; Roveda, E. Effect of chronotype on academic achievement in a sample of Italian University students. Chronobiol. Int. 2019, 36, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Taillard, J.; Sagaspe, P.; Philip, P.; Bioulac, S. Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochem. Pharmacol. 2021, 191, 114438. [Google Scholar] [CrossRef]
- Papandreou, C.; Bulló, M.; Díaz-López, A.; Martínez-González, M.A.; Corella, D.; Castañer, O.; Vioque, J.; Romaguera, D.; Martínez, A.J.; Pérez-Farinós, N.; et al. High sleep variability predicts a blunted weight loss response and short sleep duration a reduced decrease in waist circumference in the PREDIMED-Plus Trial. Int. J. Obes. 2020, 44, 330–339. [Google Scholar] [CrossRef]
- Garcez, M.R.; de Castro, M.A.; César, C.L.G.; Goldbaum, M.; Fisberg, R.M. A chrononutrition perspective of diet quality and eating behaviors of Brazilian adolescents in associated with sleep duration. Chronobiol. Int. 2021, 38, 387–399. [Google Scholar] [CrossRef]
- Tiuganji, N.M.; Nehme, P.; Marqueze, E.C.; Isherwood, C.M.; Martins, A.J.; Vasconcelos, S.; Cipolla-Neto, J.; Lowden, A.; Skene, D.J.; Moreno, C.R.C. Eating behavior (Duration, content, and timing) among workers living under different levels of urbanization. Nutrients 2020, 12, 375. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.M.H.; Siau, C.S.; Wong, J.E.; Wee, L.H.; Jamil, N.A.; Hoe, V.C.W. Prevalence of insufficient sleep and its associated factors among working adults in Malaysia. Nat. Sci. Sleep 2021, 13, 1109–1116. [Google Scholar] [CrossRef]
- Ross, K.M.; Graham Thomas, J.; Wing, R.R. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J. Behav. Med. 2016, 39, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Kühnle, T.; Grupe, G.; Foitzik, S.; Cremer, T.; Haszprunar, G.; Roennenberg, T. Quantitative Analysis of Human Chronotypes. Ph.D. Thesis, Ludwig-Maximilian University, Munchen, Germany, 2006. [Google Scholar]
| Components | Recommendation | Reference |
|---|---|---|
| Temporal pattern of energy intake | MT: BF—30%, MS—10%, LN—35%, AS—5% and DN—20% ET: BF—25%, MS—5%, LN—35%, AS—10% and DN—30%. | [26] |
| Mealtime | To eat main meal before 15:00. To eat dinner at least 2 ½ h before sleep onset. To avoid night eating. | [35] |
| Sleep | Sleep hours: ● Minimum: 6 h ● Optimum: 7–9 h | [36] |
| SLIMSHAPE™ | SLIMSHAPE™ Chrono |
|---|---|
| Similarities | |
| Dietary Calorie prescription: Men: 1600–1800 kcal/day Women: 1200–1500 kcal/day Macronutrient distribution: 50% CHO, 20% protein and 20% fat. Meal plan guide. Healthy eating guide through calorie counting, healthy cooking method and demonstration, understanding food labels, identify sugar-sweetened beverages and fats in cooked food. | |
| Physical activity and exercise Weekly group exercise lead by exercise physiologist, including resistance tube exercise, aerobic exercise, high-intensity interval training and yoga. Encouragement to be physically active. | |
| Behavioral therapy Group social support. Weekly weight monitoring. Session with psychologist on identifying barriers in weight reduction journey and motivational talk delivered by successful weight loss maintainers. | |
| Differences | |
| Duration 16 weeks/weekly session 2 h each session | Duration 12 weeks/weekly session 2-1/2 h each session |
| Chronotype Not assessed. | Chronotype Assessed. |
| Temporal pattern of energy intake No recommendation. | Temporal pattern of energy intake Prescription according to chronotype MT: Early window: 75% EI (BF: 30%, MS: 10%, LN: 35%) Late window: 25% EI (AS: 5% and DN: 20%) ET: Early window: 60% EI (BF: 20%, MS: 5%, LN: 35%) Late window: 40% EI (AS: 10% and DN: 30%) |
| Mealtime No recommendation. | Mealtime Midpoint of eating before 15:00. Elapsed time between last meal and sleep onset ≥ 2-1/2 h. To avoid/reduce night eating. |
| Sleep No recommendation. | Sleep Sleep hours: Minimum: 6 h Optimum: 7–9 h To maintain regular sleep–wake timing |
| MT (n = 46) | ET (n = 45) | Mean Difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | CT | Time × CT | |
| Total dietary intake | |||||||
| EI (kcal/day) | 1660 ± 445 | 1228 ± 326 | 1878 ± 466 | 1373 ± 407 | −471 (−558, −384) *** | −180 (−330, −32) * | 68 (−106, 242) |
| CHO (g/day) | 204.0 ± 64.5 | 152.1 ± 45.1 | 224.0 ± 65.3 | 167.1 ± 52.5 | −55.3 (−67.5, −43.0) *** | −17.4 (−3.1, 37.9) | 6.5 (−18.1, 31.1) |
| % E CHO | 49.2 ± 5.8 | 49.5 ± 6.3 | 47.7 ± 5.7 | 48.7 ± 6.0 | 0.7 (−0.7, 2.1) | 1.1 (−1.0, 3.0) | −0.4 (−3.2, 2.5) |
| Protein (g/day) | 66.0 ± 16.8 | 59.8 ± 11.3 | 72.8 ± 16.9 | 62.5 ± 16.9 | −8.6 (−12.0, −5.3) *** | −4.9 (−10.5, 0.7) | 3.94 (−2.8, 10.6) |
| % E protein | 15.9 ± 3.3 | 19.5 ± 3.8 | 15.5 ± 2.0 | 18.2 ± 4.1 | 3.3 (2.3, 4.2) *** | 0.9 (−0.2, 1.9) | 0.9 (−1.0, 2.8) |
| Fat (g/day) | 64.4 ± 19.4 | 42.3 ± 16.6 | 76.9 ± 22.5 | 50.5 ± 20.4 | −23.7 (−28.3, −19.1) *** | −10.3 (−17.2, −3.4) * | 3.29 (−5.9, 12.5) |
| % E fat | 34.9 ± 4.9 | 31.0 ± 6.2 | 36.8 ± 5.0 | 33.1 ± 6.3 | −3.9 (−5.3, −2.4) *** | −2.1 (−3.9, −0.2) * | −0.3 (−3.2, 2.6) |
| Meal timing | |||||||
| First meal (hh:mm) | 08:12 ± 0:40 | 08:04 ± 0:46 | 08:24 ± 0:43 | 08:22 ± 0:43 | −0.1 (−0.2, 0.03) | −0.3 (−0.5, 0.03) | 0.1 (−0.3, 0.1) |
| Last meal (hh:mm) | 19:52 ± 1:16 | 19:33 ± 1:08 | 20:29 ± 1:45 | 20:14 ± 1:37 | −0.3 (−0.6, 0.04) | −0.7 (−1.2, −0.1) * | −0.1 (−0.7, 0.6) |
| Total eating window (h) | 11.7 ± 1.6 | 11.5 ± 1.4 | 12.1 ± 1.7 | 11.9 ± 1.6 | −0.2 (−0.6, 0.2) | −0.4 (−1.0, 0.2) | 0.0 (−0.7, 0.7) |
| Midpoint eating (hh:mm) | 14:02 ± 0:38 | 13:49 ± 0:42 | 14:27 ± 1:01 | 14:18 ± 0:57 | −0.2 (−0.4, −0.02) * | −0.5 (−0.8, −0.1) ** | −0.1 (−0.4, 0.2) |
| Elapse SOn-last meal (h) | 3.1 ± 1.5 | 3.5 ± 1.3 | 3.5 ± 1.8 | 3.2 ± 1.6 | 0.1 (−0.3, 0.4) | 0.0 (−0.5, 0.5) | 0.8 (0.1, 1.5) * |
| NES score | 10.0 ± 5.1 | 8.9 ±4.3 | 10.7 ±5.4 | 8.9 ± 5.0 | −1.5 (−2.5, −0.5) ** | −0.4 (−2.2, 1.5) | 0.7 (−1.3, 2.7) |
| Sleep | |||||||
| Work SD (hour) | 6.6 ± 1.0 | 6.7 ± 0.9 | 6.1 ± 0.9 | 7.4 ± 1.2 | 0.7 (0.4, 1.0) *** | −0.1 (−0.4, 0.2) | −1.1 (−1.6, −0.6) *** |
| Free SD (hour) | 7.3 ± 1.2 | 6.5 ± 0.9 | 6.8 ± 1.5 | 6.7 ± 0.8 | −0.5 (−0.8, −0.1) *** | 0.2 (−0.2, 0.5) | −0.7 (−1.3, −0.1) * |
| Average SOn (hh:mm) | 22:57 ± 0:48 | 23:05 ± 0:50 | 23:56 ± 0:53 | 23:23 ± 0:57 | −0.2 (−0.4, −0.03) * | −0.6 (−1.0, −0.3) *** | 0.7 (0.3, 1.0) *** |
| Average SOff (hh:mm) | 05:46 ± 0:31 | 05:38 ± 0:29 | 06:17 ± 0:41 | 06:18 ± 0:47 | −0.1 (−0.1, 0.2) | −0.6 (−0.8, −0.4) *** | −0.2 (−0.4, 0.1) |
| Average SD (hour) | 6.8 ± 0.9 | 6.6 ± 0.8 | 6.3 ± 0.9 | 6.9 ± 0.7 | 0.2 (−0.0, 0.4) | 0.1 (−0.2, 0.4) | −0.8 (−1.2, −0.4) *** |
| Social jetlag (min) | 18.2 ± 30.0 | 23.6 ± 23.6 | 53.1 ± 45.0 | 39.5 ± 42.6 | −4.1 (−12.7, 4.6) | −25.4 (−37.8, −13.0) *** | 19.0 (1.7, 36.3) * |
| Physical activity (MET) | 1693.7 ± 303.8 | 3509.4 ± 387.0 | 1611.5 ± 303.8 | 3462.3 ± 387.0 | 1700.0 (1150.0, 2250.0) *** | −124.9 (−865.4, 615.5) | 48.1 (−1051.9, 1148.1) |
| MT (n = 46) | ET (n = 45) | Mean Difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Time × CT | |
| Adiposity | ||||||
| Weight (kg) | 79.8 ± 16.4 | 75.6 ± 15.6 | 81.5 ± 14.1 | 77.7 ± 13.2 | −4.0 (−4.9, −3.1) *** | −0.5 (−2.4, 1.3) |
| BMI (kg/m2) | 31.3 ± 4.3 | 29.6 ± 4.5 | 31.2 ± 4.7 | 29.8 ± 4.7 | −1.5 (−1.9, −1.2) *** | −0.2 (−0.9, 0.5) |
| Body fat (%) | 41.1 ± 7.4 | 39.0 ± 8.5 | 39.7 ± 8.1 | 37.8 ± 8.5 | −2.0 (−2.5, −1.4) *** | −0.3 (−1.4, 0.8) |
| WC (cm) | 94.0 ± 11.8 | 90.3 ± 11.7 | 94.1 ± 10.1 | 90.0 ± 9.7 | −3.9 (−4.9, −2.9) *** | 0.4 (−1.6, 2.5) |
| Biochemical | ||||||
| FBG (mmol/L) | 5.0 ± 0.5 | 4.9 ± 0.6 | 5.0 ± 0.7 | 5.0 ± 0.7 | −0.1 (−0.2, 0.03) | −0.01 (−0.2, 0.2) |
| Insulin (μIU/mL) | 13.9 ± 7.4 | 10.3 ± 6.7 | 12.7 ± 9.3 | 9.9 ± 5.8 | −3.2 (−4.5, −1.8) *** | −0.8 (−3.5, 1.9) |
| HbA1c (%) | 5.8 ± 0.5 | 5.8 ± 0.7 | 5.8 ± 0.6 | 5.8 ± 0.5 | 0.01 (−0.1, 0.1) | 0.1 (−0.1, 0.2) |
| HOMA-IR | 3.1 ± 1.8 | 2.3 ± 2.0 | 2.9 ± 2.4 | 2.3 ± 1.6 | −0.7 (−1.1, −0.4) *** | −0.1 (−0.8, 0.6) |
| TC (mmol/L) | 5.2 ± 1.0 | 5.1 ± 1.1 | 5.1 ± 0.9 | 5.0 ± 0.8 | −0.1 (−0.2, 0.1) | 0.1 (−0.2, 0.4) |
| TG (mmol/L) | 1.3 ± 0.8 | 1.2 ± 0.7 | 1.4 ± 0.8 | 1.3 ± 0.9 | −0.1 (−0.2, −0.01) * | −0.01 (−0.2, 0.2) |
| LDL-C (mmol/L) | 3.3 ± 0.94 | 3.3 ± 1.0 | 3.1 ± 0.9 | 3.1 ± 0.8 | 0.04 (−0.1, 0.2) | 0.1 (−0.2, 0.3) |
| HDL-C (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.3 | −0.04 (−0.1, −0.01) * | 0.03 (−0.1, 0.1) |
| Uric acid (mmol/L) | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | −0.01 (−0.03, −0.00) * | 0.00 (−0.02, 0.02) |
| Blood pressure | ||||||
| Systolic (mmHg) | 128.5 ± 10.8 | 118.9 ± 12.2 | 135.6 ± 12.0 | 128.2 ± 14.2 | −8.5 (−10.7, −6.2) *** | −2.2 (−6.7, 2.3) |
| Diastolic (mmHg) | 80.4 ± 9.6 | 75.3 ± 12.4 | 81.4 ± 9.0 | 77.7 ± 9.6 | −4.3 (−6.7, −2.0) *** | −1.4 (−6.0, 3.2) |
| Components | Refinement in Interventions | Rationale |
|---|---|---|
| Temporal pattern of energy intake | Temporal energy prescription is the same for morning and evening chronotypes: Early window: 65–70% EI BF: 25–30% MS: 0–5% LN: 40% Late window: 30–35% EI AS: 0–5% DN: 25–30% Provide a complete set of menus, including the dishes for 12 weeks (intervention period). | There was no significant difference in % energy intake during early and late window between morning and evening chronotypes during pre- and post-intervention. Greater energy intake towards earlier part of the day and smaller intake towards later part of the day is beneficial for both chronotypes [48]. |
| Meal timing | No changes in meal timing recommendations. | The participants adapt well to the recommendation. |
| Sleep | No changes in sleep hours recommendation. Increase the sleep session to improve understanding on the importance of adequate sleep. | Morning chronotypes had reduced sleep duration on free days. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F.; Abdul Basir, S.M. Development and Evaluation of Integrated Chrono-Nutrition Weight Reduction Program among Overweight/Obese with Morning and Evening Chronotypes. Int. J. Environ. Res. Public Health 2022, 19, 4469. https://doi.org/10.3390/ijerph19084469
Mazri FH, Manaf ZA, Shahar S, Mat Ludin AF, Abdul Basir SM. Development and Evaluation of Integrated Chrono-Nutrition Weight Reduction Program among Overweight/Obese with Morning and Evening Chronotypes. International Journal of Environmental Research and Public Health. 2022; 19(8):4469. https://doi.org/10.3390/ijerph19084469
Chicago/Turabian StyleMazri, Fatin Hanani, Zahara Abdul Manaf, Suzana Shahar, Arimi Fitri Mat Ludin, and Siti Munirah Abdul Basir. 2022. "Development and Evaluation of Integrated Chrono-Nutrition Weight Reduction Program among Overweight/Obese with Morning and Evening Chronotypes" International Journal of Environmental Research and Public Health 19, no. 8: 4469. https://doi.org/10.3390/ijerph19084469
APA StyleMazri, F. H., Manaf, Z. A., Shahar, S., Mat Ludin, A. F., & Abdul Basir, S. M. (2022). Development and Evaluation of Integrated Chrono-Nutrition Weight Reduction Program among Overweight/Obese with Morning and Evening Chronotypes. International Journal of Environmental Research and Public Health, 19(8), 4469. https://doi.org/10.3390/ijerph19084469









