Effects of Iron Powder Addition and Thermal Hydrolysis on Methane Production and the Archaeal Community during the Anaerobic Digestion of Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Feedstock
2.2. Determination of Physiochemical Index
2.3. Reactor Setup and Experimental Design
2.4. Microbiological Analysis
2.5. Calculations and Statistics
3. Results
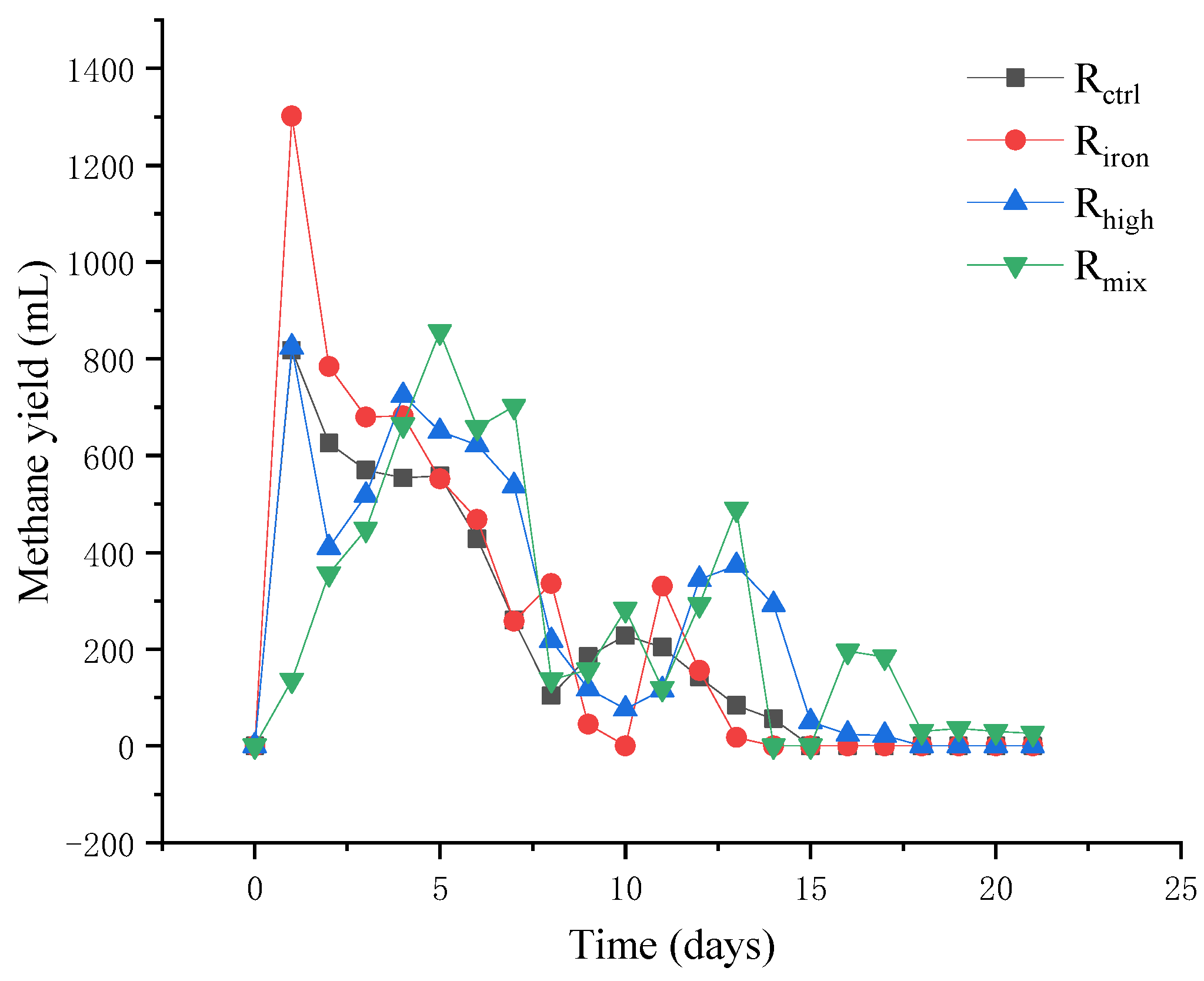
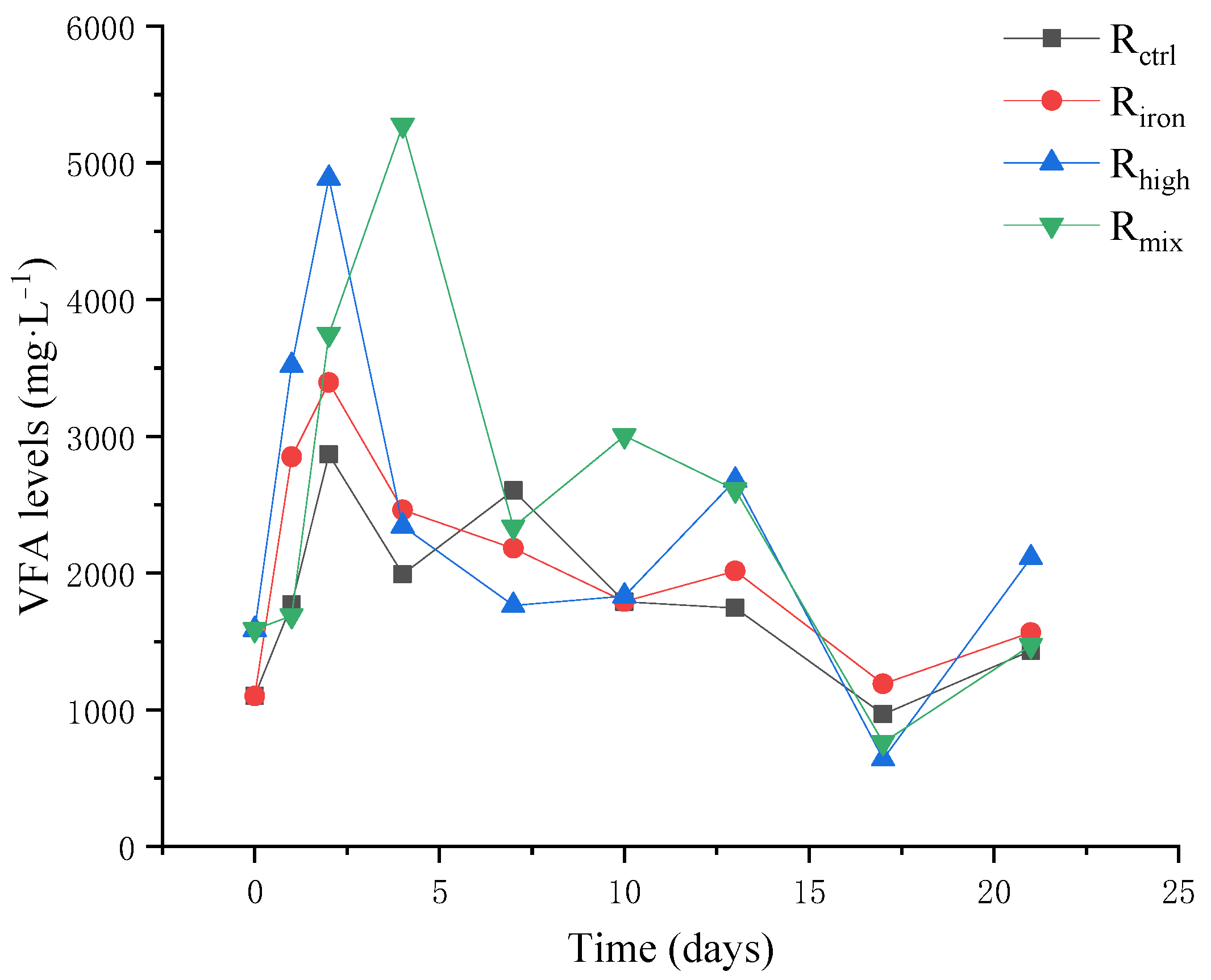
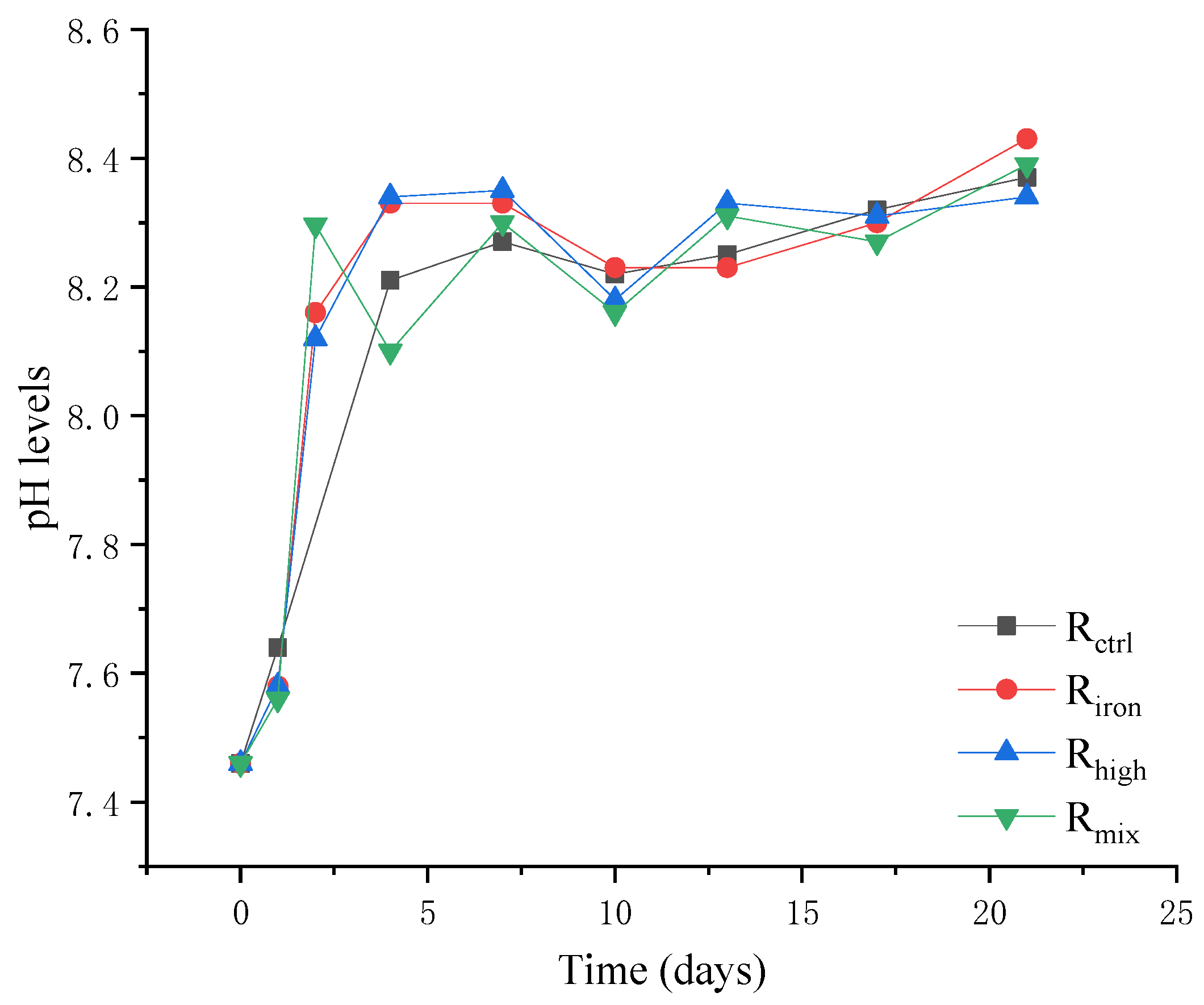
3.1. Performance of Anaerobic Digestion Reactors
3.1.1. Methane Production Efficiency
3.1.2. VFA Accumulation and pH Fluctuation
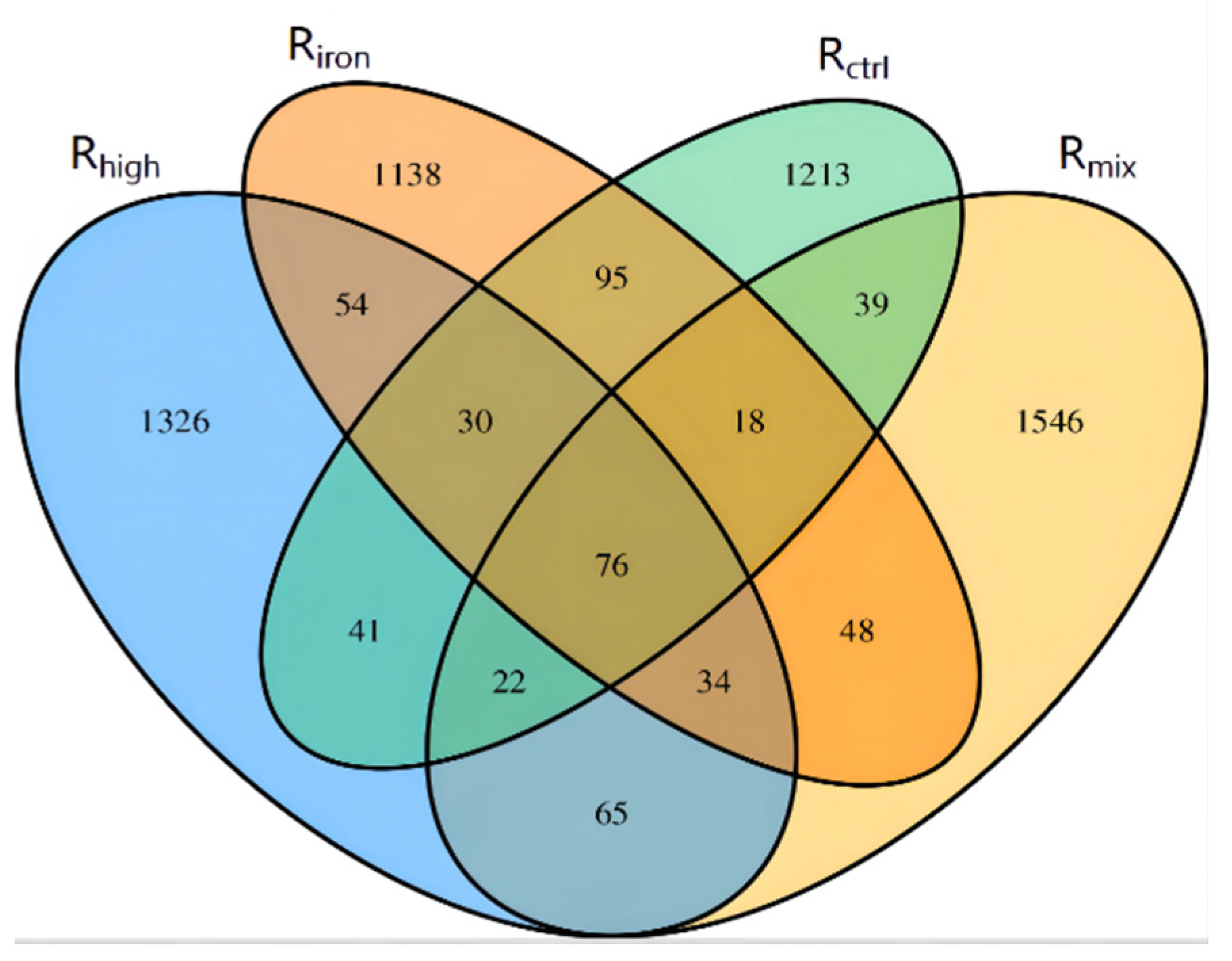
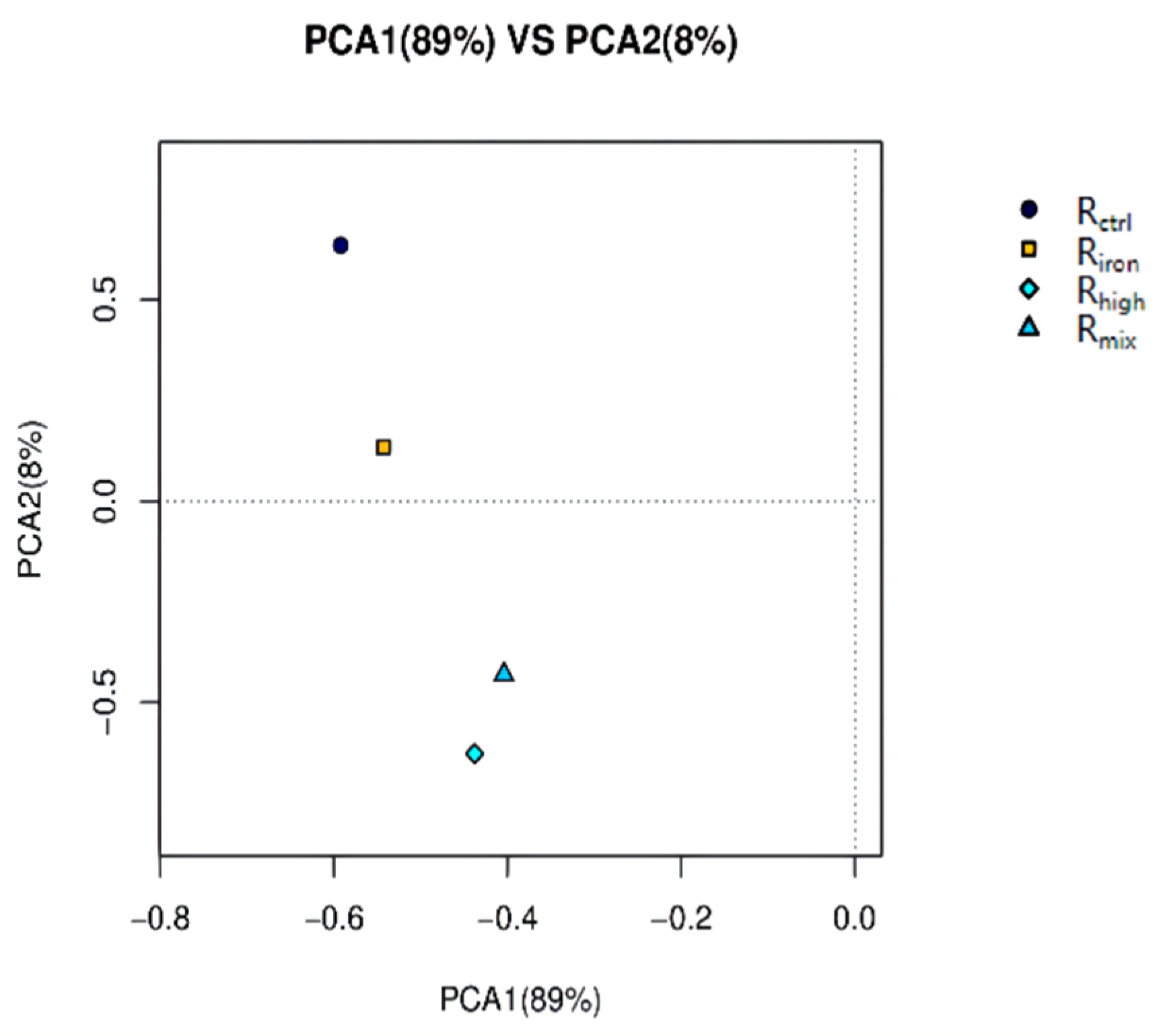
3.2. Microbial Community Composition and Dynamics
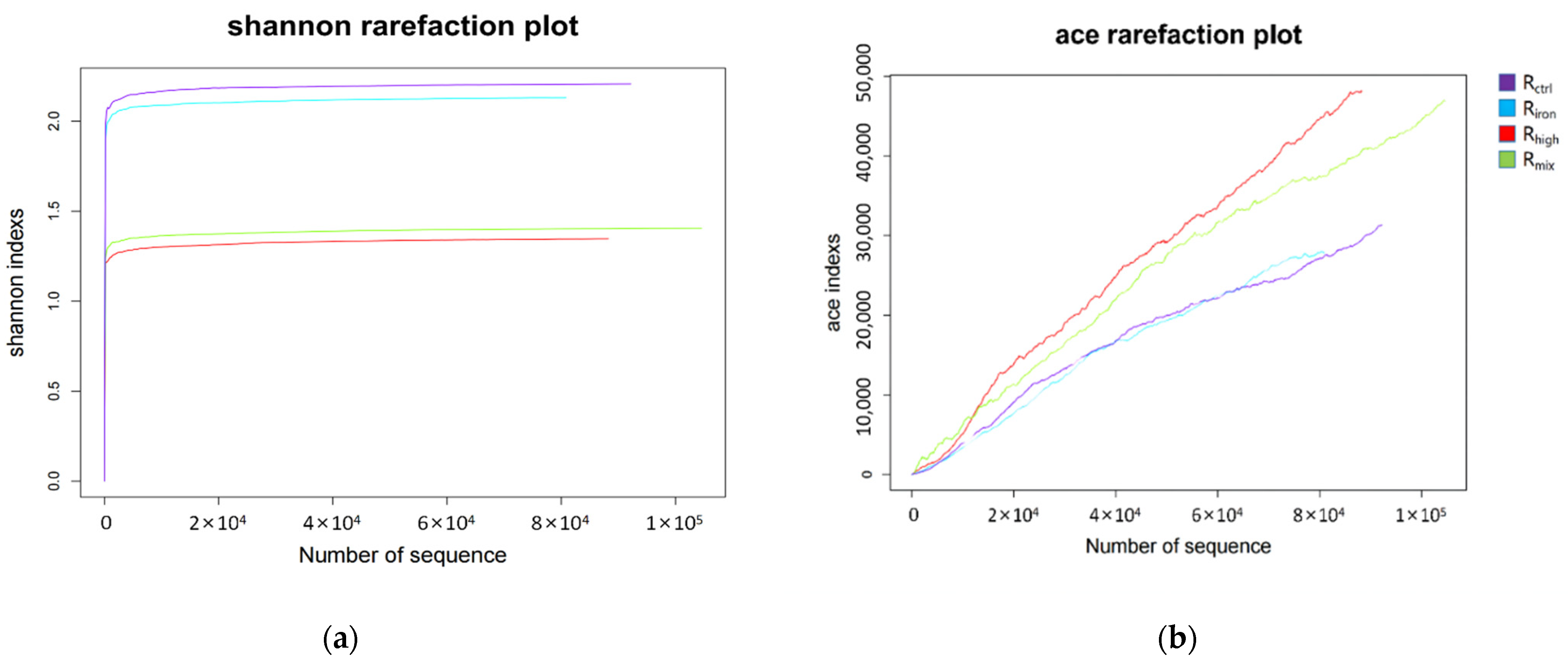
3.2.1. Richness and Diversity Analysis of Archaeal Community
3.2.2. Methanogenic Archaeal Community for Different Pretreatments
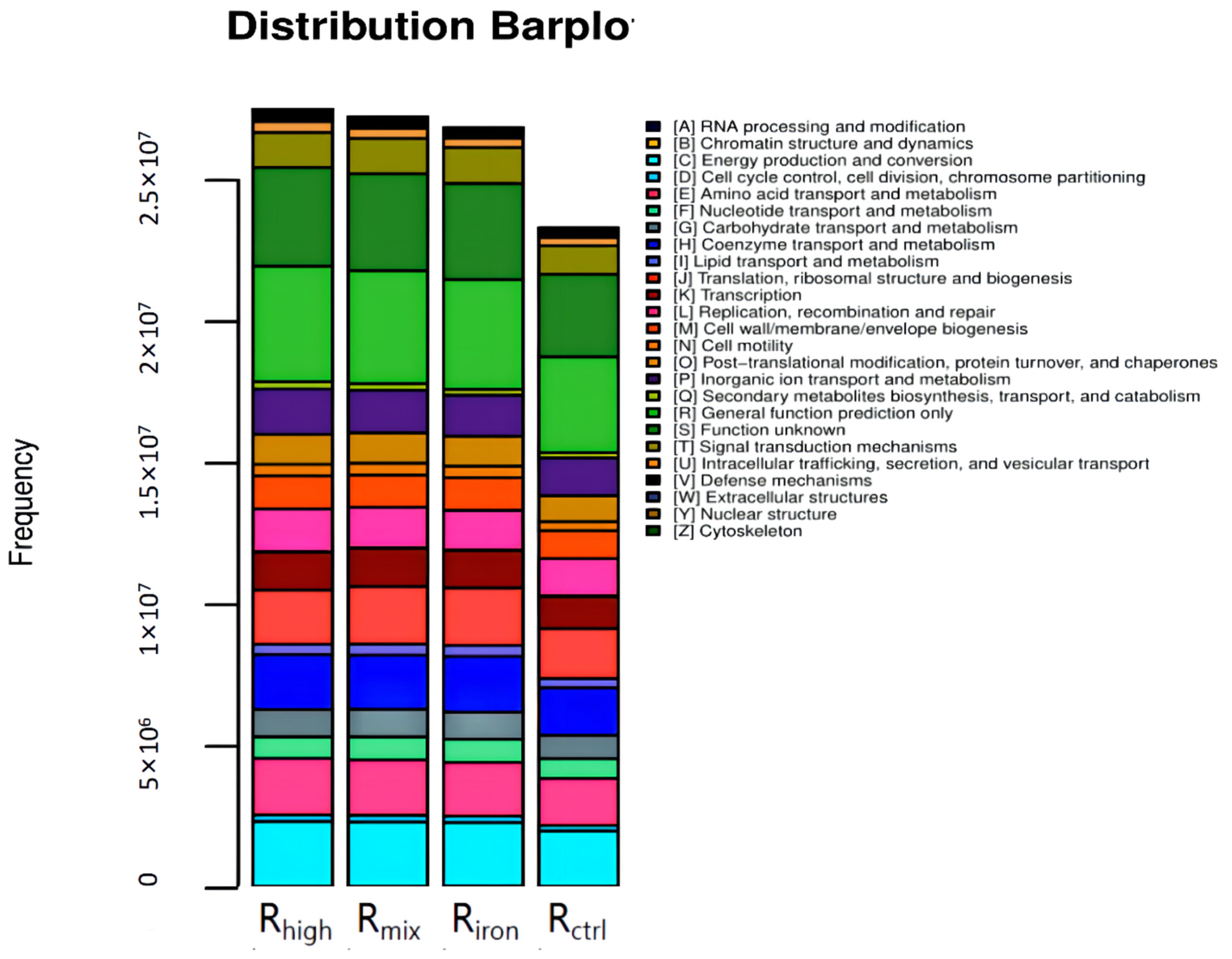
3.2.3. Functional Abundance of Archaeal in Different Reactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamali, M.; Gameiro, T.; Costa, M.E.V.; Capela, I. Anaerobic digestion of pulp and paper mill wastes–An overview of the developments and improvement opportunities. Chem. Eng. J. 2016, 298, 162–182. [Google Scholar] [CrossRef]
- Ding, H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. A review of methane production from agricultural residues in China. Renew. Sustain. Energy Rev. 2016, 54, 857–865. [Google Scholar] [CrossRef]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J. Biomethanation and its potential. Methods Enzymol. 2011, 494, 327–351. [Google Scholar]
- Han, Y.; Zhuo, Y.; Peng, D.; Yao, Q.; Li, H.; Qu, Q. Influence of thermal hydrolysis pretreatment on organic transformation characteristics of high solid anaerobic digestion. Bioresour. Technol. 2017, 244, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, C.; Kalamdhad, A.S. Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: A review. Bioresour. Technol. 2017, 245, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, B.; Chen, Z. Simultaneous recovery of vivianite and produce short-chain fatty acids from waste activated sludge using potassium ferrate as pre-oxidation treatment. Environ. Res. 2022, 208, 112661. [Google Scholar] [CrossRef] [PubMed]
- Zubrowska-Sudol, M.; Podedworna, J.; Sytek-Szmeichel, K.; Bisak, A.; Krawczyk, P.; Garlicka, A. The effects of mechanical sludge disintegration to enhance full-scale anaerobic digestion of municipal sludge. Therm. Sci. Eng. Prog. 2018, 5, 289–295. [Google Scholar] [CrossRef]
- Neumann, P.; González, Z.; Vidal, G. Sequential ultrasound and low-temperature thermal pretreatment: Process optimization and influence on sewage sludge solubilization, enzyme activity and anaerobic digestion. Bioresour. Technol. 2017, 234, 178–187. [Google Scholar] [CrossRef]
- Kavitha, S.; Jayashree, C.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 2014, 168, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yan, M.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Hydrogenotrophic methanogens are the key for a successful bioaugmentation to alleviate ammonia inhibition in thermophilic anaerobic digesters. Bioresour. Technol. 2019, 293, 122070. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yi, H.; Li, H.; Guo, X.; Xiao, B. Effects of thermal and thermal-alkaline pretreatments on continuous anaerobic sludge digestion: Performance, energy balance and, enhancement mechanism. Renew. Energy 2020, 147, 2409–2416. [Google Scholar] [CrossRef]
- Jin, X.; Ai, W.; Dong, W. Lignocellulose degradation, biogas production and characteristics of the microbial community in solid-state anaerobic digestion of wheat straw waste. Life Sci. Space Res. 2022, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Han, S.K.; Lee, C.Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Chi, Y.; Yan, J.H.; Ni, M. Experimental Study on Thermal Hydrolysis and Dewatering Characteristics of Mechanically Dewatered Sewage Sludge. Dry. Technol. 2011, 29, 1741–1747. [Google Scholar] [CrossRef]
- Abelleria-Pereira, J.M.; Pérez-Elvira, S.I.; Sánchez-Oneto, J.; de la Cruz, R.; Portela, J.R.; Nebot, E. Enhancement of methane production in mesophilic anaerobic digestion of secondary sewage sludge by advanced thermal hydrolysis pretreatment. Water Res. 2015, 71, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Ferrer, I. Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Liu, T.; Jiang, K.; Qiu, F.; Fu, K.; Liu, J. Effect of low-temperature thermal hydrolysis on sludge thixotropy and dewaterability. Chin. J. Environ. Eng. 2019, 13, 977–983. [Google Scholar]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Suárez-Iglesias, O.; Urrea, J.L.; Oulego, P.; Collado, S.; Díaz, M. Valuable compounds from sewage sludge by thermal hydrolysis and wet oxidation. A review. Sci. Total Environ. 2017, 584, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Zhang, Y.; Liu, C.; Chen, Q. Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Int. Biodeterior. Biodegrad. 2016, 106, 141–149. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenes, J.P.; Carrère, H. Impacts of thermal pre-treatments on the semi-continuous anaerobic digestion of waste activated sludge. Biochem. Eng. J. 2007, 34, 20–27. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.M.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Niu, Y.T.; Liu, J.B.; Ma, S.; Li, Y.M.; Xie, L.P.; Wei, Y.S.; Meng, X.S. Enhancement for anaerobic digestion of waste activated sludge based on microwave pretreatment combined with zero valent iron. Environ. Sci. 2019, 40, 1431–1438. (In Chinese) [Google Scholar]
- Wei, W.; Cai, Z.; Fu, J.; Xie, G.J.; Li, A.; Zhou, X.; Ni, B.-J.; Wang, D.; Wang, Q. Zero valent iron enhances methane production from primary sludge in anaerobic digestion. Chem. Eng. J. 2018, 351, 1159–1165. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Wu, S.; Zhou, L. Consolidation of hydrogenotrophic methanogenesis by sulfidated nanoscale zero-valent iron in the anaerobic digestion of food waste upon ammonia stress. Sci. Total Environ. 2022, 822, 153531. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, J.T.E.; Zhang, L.; Tian, H.; Ong, K.C.; Tio, Z.K.; Zhang, J.; Tong, Y.W. Abrogating the inhibitory effects of volatile fatty acids and ammonia in overloaded food waste anaerobic digesters via the supplementation of nano-zero valent iron modified biochar. Sci. Total Environ. 2022, 817, 152968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Y.; Eshtiaghi, N.; Dai, X.; Tao, W.; Zhuo, L. Evaluation of thermal hydrolysis efficiency of mechanically dewatered sewage sludge via rheological measurement. Water Res. 2017, 116, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, Y.; Han, H.; Li, K.; Xu, C.; Zhuang, H. New insights into enhanced anaerobic degradation of Fischer-Tropsch wastewater with the assistance of magnetite. Bioresour. Technol. 2018, 257, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Dong, B.; Wu, B.; Dai, X. High-solid anaerobic digestion of sewage sludge under mesophilic conditions: Feasibility study. Bioresour. Technol. 2012, 104, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jiang, H.C.; Ma, Y.M.; Liu, X.H.; Wang, H.C. The effect of Fe on microbe and process property in wastewater and sludge treatment process. Environ. Eng. 2016, 34, 1–6. [Google Scholar]
- Nissilä, M.E.; Li, Y.C.; Wu, S.Y.; Lin, C.Y.; Puhakka, J.A. Hydrogenic and methanogenic fermentation of birch and conifer pulps. Appl. Energy 2012, 100, 58–65. [Google Scholar] [CrossRef]
- Trüper, H.G.; Dworkin, M.; Harder, W.; Schleifer, K.H. The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications; Springer: New York, NY, USA, 1992. [Google Scholar]
- Płaza, G.; Jałowiecki, Ł.; Głowacka, D.; Hubeny, J.; Harnisz, M.; Korzeniewska, E. Insights into the microbial diversity and structure in a full-scale municipal wastewater treatment plant with particular regard to Archaea. PLoS ONE 2021, 16, e0250514. [Google Scholar] [CrossRef] [PubMed]
- Palù, M.; Peprah, M.; Tsapekos, P.; Kougias, P.; Campanaro, S.; Angelidaki, I.; Treu, L. In-situ biogas upgrading assisted by bioaugmentation with hydrogenotrophic methanogens during mesophilic and thermophilic co-digestion. Bioresour. Technol. 2022, 348, 126754. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Chen, S.; Li, N.; Dong, B.; Zhao, W.; Dai, L.; Dai, X. New insights into the enhanced performance of high solid anaerobic digestion with dewatered sludge by thermal hydrolysis: Organic matter degradation and methanogenic pathways. J. Hazard. Mater. 2018, 342, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Rctrl | Riron | Rhigh | Rmix |
|---|---|---|---|---|
| TS (%) | 6.73 ± 0.05 | 6.73 ± 0.05 | 7.00 ± 0.07 | 7.00 ± 0.07 |
| VS (%) | 3.77 ± 0.04 | 3.77 ± 0.04 | 3.75 ± 0.05 | 3.75 ± 0.05 |
| SCOD (mg·L−1) | 3616.00 ± 50.34 | 3616.00 ± 50.34 | 18,720.00 ± 100.89 | 18,720.00 ± 100.89 |
| VFA (mg·L−1) | 1101.30 ± 12.45 | 1101.30 ± 12.45 | 1586.76 ± 23.61 | 1586.76 ± 23.61 |
| pH | 7.46 ± 0.10 | 7.46 ± 0.10 | 7.46 ± 0.10 | 7.46 ± 0.10 |
| Parameter | Rctrl | Riron | Rhigh | Rmix |
|---|---|---|---|---|
| TS (%) | 4.83 ± 0.01 | 5.91 ± 0.05 | 6.07 ± 0.03 | 6.09 ± 0.08 |
| VS (%) | 3.20 ± 0.01 | 3.07 ± 0.06 | 2.79 ± 0.02 | 2.85 ± 0.03 |
| SCOD (mg·L−1) | 5950.00 ± 84.48 | 7460.00 ± 56.63 | 14,120.00 ± 184.45 | 12,830.00 ± 147.86 |
| VFA (mg·L−1) | 1432.52 ± 18.56 | 1564.64 ± 20.84 | 2111.83 ± 15.74 | 1471.58 ± 121.87 |
| pH | 8.37 ± 0.12 | 8.43 ± 0.15 | 8.34 ± 0.10 | 8.39 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Wang, Y.; Liu, T. Effects of Iron Powder Addition and Thermal Hydrolysis on Methane Production and the Archaeal Community during the Anaerobic Digestion of Sludge. Int. J. Environ. Res. Public Health 2022, 19, 4470. https://doi.org/10.3390/ijerph19084470
Cao X, Wang Y, Liu T. Effects of Iron Powder Addition and Thermal Hydrolysis on Methane Production and the Archaeal Community during the Anaerobic Digestion of Sludge. International Journal of Environmental Research and Public Health. 2022; 19(8):4470. https://doi.org/10.3390/ijerph19084470
Chicago/Turabian StyleCao, Xiuqin, Yibin Wang, and Ting Liu. 2022. "Effects of Iron Powder Addition and Thermal Hydrolysis on Methane Production and the Archaeal Community during the Anaerobic Digestion of Sludge" International Journal of Environmental Research and Public Health 19, no. 8: 4470. https://doi.org/10.3390/ijerph19084470
APA StyleCao, X., Wang, Y., & Liu, T. (2022). Effects of Iron Powder Addition and Thermal Hydrolysis on Methane Production and the Archaeal Community during the Anaerobic Digestion of Sludge. International Journal of Environmental Research and Public Health, 19(8), 4470. https://doi.org/10.3390/ijerph19084470





