Multidimensional Sleep Health Is Associated with Cardiovascular Disease Prevalence and Cardiometabolic Health in US Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Sleep
2.3. Operationalization of the Multidimensional Sleep Health Score
2.4. Ascertainment of Cardiovascular Disease
2.5. Assessment of Cardiometabolic Risk Factors
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Multidimensional Sleep Health and Cardiometabolic Risk Factors
3.3. Multidimensional Sleep Health and Prevalent Cardiometabolic Outcomes
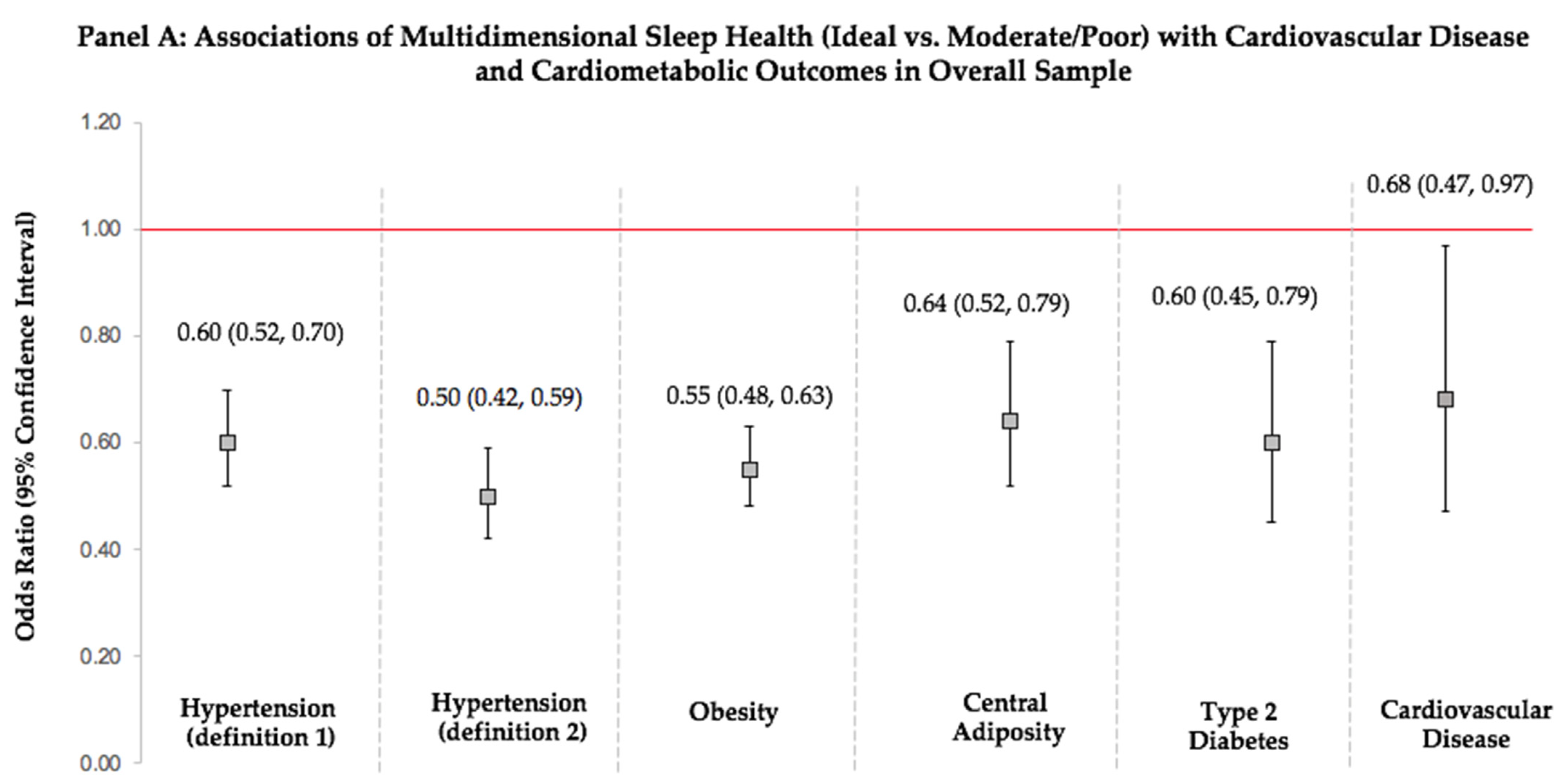
3.4. Multidimensional Sleep Health and Prevalent Cardiovascular Disease
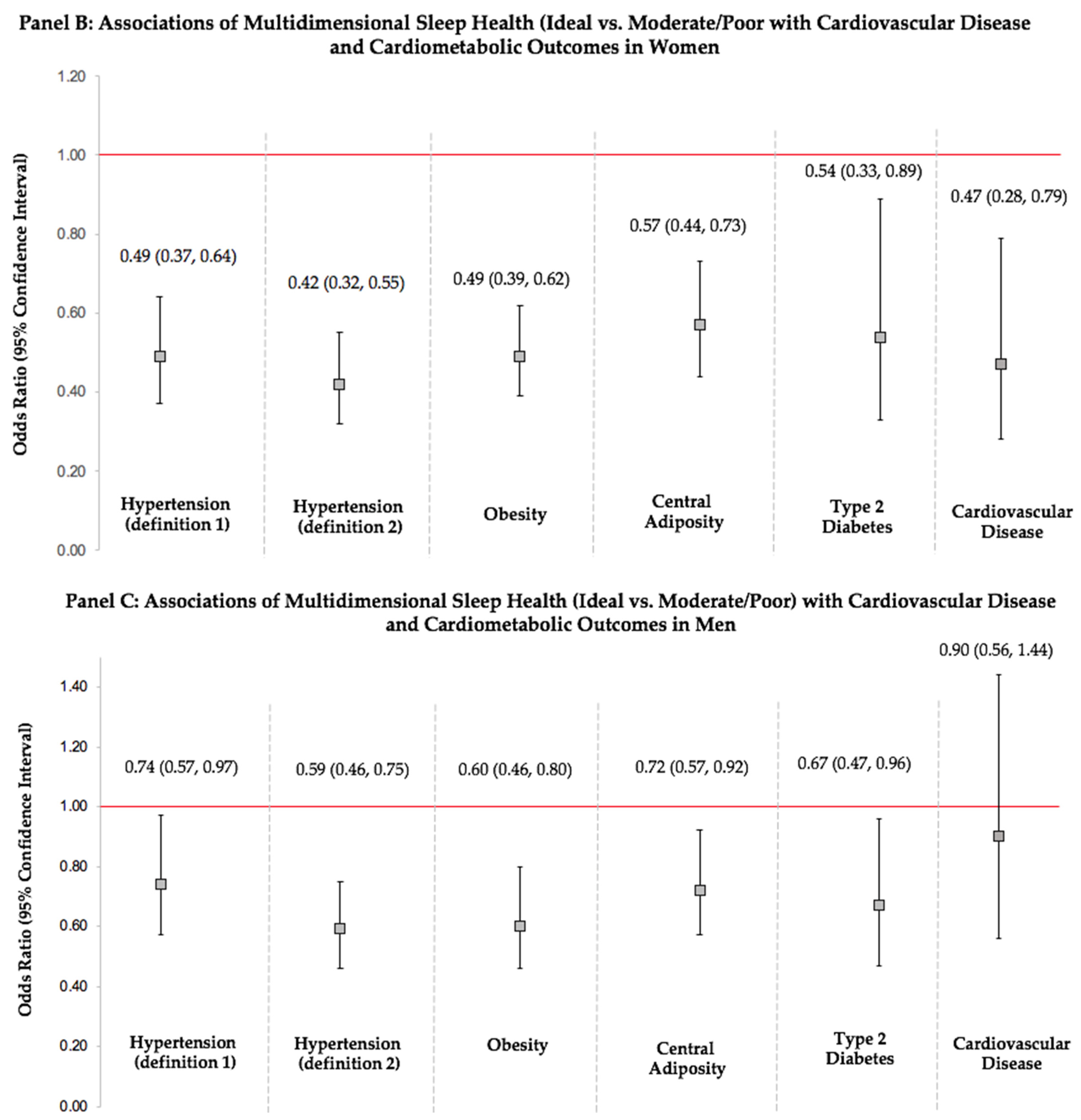
3.5. Sex Differences in Associations of Multidimensional Sleep Health with Cardiovascular Disease and Cardiometabolic Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| BMI | body mass index |
| BP | blood pressure |
| CMH | cardiometabolic health |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| HTN | hypertension |
| NHANES | National Health and Nutrition Examination Survey |
| SBP | systolic blood pressure |
| T2D | type 2 diabetes |
| WC | waist circumference |
References
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed]
- Colten, H.R.; Altevogt, B.M. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; The National Academies Press: Washington, DC, USA, 2006; pp. 1–404. [Google Scholar] [CrossRef]
- National Sleep Foundation. 2013 International Bedroom Poll, Summery of Findings. 2013. Available online: https://www.sleepfoundation.org/wp-content/uploads/2018/10/RPT495a.pdf (accessed on 7 March 2022).
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cunningham, T.J.; Giles, W.H.; Croft, J.B. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015, 16, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M. Determining the level of sleepiness in the American population and its correlates. J. Psychiatr. Res. 2012, 46, 422–427. [Google Scholar] [CrossRef]
- Huang, T.; Redline, S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: The multi-ethnic study of atherosclerosis. Diabetes Care 2019, 42, 1422–1429. [Google Scholar] [CrossRef]
- Huang, T.; Mariani, S.; Redline, S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2020, 75, 991–999. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Jackson, C.L.; Redline, S.; Emmons, K.M. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Ann. Rev. Public Health 2015, 36, 417. [Google Scholar] [CrossRef] [Green Version]
- Redline, S.; Foody, J. Sleep disturbances: Time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation 2011, 124, 2049–2051. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Wallace, M.L. Sleep Health: A New Paradigm for Sleep and Aging Research. Innov. Aging 2018, 2 (Suppl. 1), 595. [Google Scholar] [CrossRef]
- Hale, L.; Troxel, W.; Buysse, D.J. Sleep Health: An Opportunity for Public Health to Address Health Equity. Annu. Rev. Public Health 2020, 41, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Buysse, D.J.; Redline, S.; Stone, K.L.; Ensrud, K.; Leng, Y.; Ancoli-Israel, S.; Hall, M.H. Multidimensional Sleep and Mortality in Older Adults: A Machine-Learning Comparison with Other Risk Factors. J. Gerontol. Ser. A Biolog. Sci. Med. Sci. 2019, 74, 1903–1909. [Google Scholar] [CrossRef]
- Lee, S.; Mu, C.X.; Wallace, M.L.; Andel, R.; Almeida, D.M.; Buxton, O.M.; Patel, S.R. Sleep health composites are associated with the risk of heart disease across sex and race. Sci. Rep. 2022, 12, 2023. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2018. NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/ (accessed on 15 July 2022).
- Makarem, N.; Zuraikat, F.M.; Aggarwal, B.; Jelic, S.; St-Onge, M.P. Variability in Sleep Patterns: An Emerging Risk Factor for Hypertension. Curr. Hypertens. Rep. 2020, 22, 19. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Makarem, N.; Redline, S.; Aggarwal, B.; Jelic, S.; St-Onge, M.P. Sleep Regularity and Cardiometabolic Heath: Is Variability in Sleep Patterns a Risk Factor for Excess Adiposity and Glycemic Dysregulation? Curr. Diabetes Rep. 2020, 20, 38. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical practice guidelines. Hypertension 2018, 71, E13–E115. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5–S20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Body Mass Index—BMI. 9 March 2022. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 12 March 2022).
- National Heart, Lung and Blood Institute. Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. 2022. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm (accessed on 8 March 2022).
- Ensrud, K.E.; Kats, A.M.; Schousboe, J.T.; Langsetmo, L.; Vo, T.N.; Blackwell, T.L.; Buysse, D.J.; Ancoli-Israel, S.; Stone, K.L. Multidimensional sleep health and subsequent health-care costs and utilization in older women. Sleep 2020, 43, zsz230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, Q.; Wang, M.; Zhou, T.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408 802 UK Biobank Participants. Circulation 2021, 143, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Aggarwal, B. Gender Differences in Associations Between Insufficient Sleep and Cardiovascular Disease Risk Factors and Endpoints: A Contemporary Review. Gend. Genome 2017, 1, 80–88. [Google Scholar] [CrossRef]
- Makarem, N.; Alcántara, C.; Williams, N.; Bello, N.A.; Abdalla, M. Effect of Sleep Disturbances on Blood Pressure. Hypertension 2021, 77, 1036–1046. [Google Scholar] [CrossRef]
- Brindle, R.C.; Yu, L.; Buysse, D.J.; Hall, M.H. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: Results from the Midlife in the United States (MIDUS) study. Sleep 2019, 42, zsz116. [Google Scholar] [CrossRef]
- Bowman, M.A.; Brindle, R.C.; Joffe, H.; Kline, C.E.; Buysse, D.J.; Appelhans, B.M.; Kravitz, H.M.; Matthews, K.A.; Neal-Perry, G.S.; Krafty, R.T.; et al. Multidimensional sleep health is not cross-sectionally or longitudinally associated with adiposity in the Study of Women’s Health Across the Nation (SWAN). Sleep Health 2020, 6, 790–796. [Google Scholar] [CrossRef]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Buxton, O.M.; Lee, S.; Marino, M.; Beverly, C.; Almeida, D.M.; Berkman, L. Sleep Health and Predicted Cardiometabolic Risk Scores in Employed Adults From Two Industries. J. Clin. Sleep Med. 2018, 14, 371. [Google Scholar] [CrossRef] [Green Version]
- Makarem, N.; St-Onge, M.P.; Liao, M.; Lloyd-Jones, D.M.; Aggarwal, B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: Findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health 2019, 5, 501–508. [Google Scholar] [CrossRef]
- Bai, J.; He, B.; Wang, N.; Chen, Y.; Liu, J.; Wang, H.; Liu, D. Snoring Is Associated with Increased Risk of Stroke: A Cumulative Meta-Analysis. Front. Neurol. 2021, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lunsford-Avery, J.R.; Engelhard, M.M.; Navar, A.M.; Kollins, S.H. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci. Rep. 2018, 8, 14158. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Makarem, N.; Liao, M.; St-Onge, M.P.; Aggarwal, B. Measures of Poor Sleep Quality Are Associated with Higher Energy Intake and Poor Diet Quality in a Diverse Sample of Women from the Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2020, 9, e014587. [Google Scholar] [CrossRef]
- Mossavar-Rahmani, Y.; Weng, J.; Wang, R.; Shaw, P.A.; Jung, M.; Sotres-Alvarez, D.; Castaneda, S.; Gallo, L.C.; Gellman, M.D.; Qi, Q.; et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. J. Sleep Res. 2017, 26, 739–746. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Cardinal, B.J. Association between objectively-measured physical activity and sleep, NHANES 2005–2006. Ment. Health Phys. Act. 2011, 4, 65–69. [Google Scholar] [CrossRef]
- McClain, J.J.; Lewin, D.S.; Laposky, A.D.; Kahle, L.; Berrigan, D. Associations between physical activity, sedentary time, sleep duration and daytime sleepiness in US adults. Prev. Med. 2014, 66, 68–73. [Google Scholar] [CrossRef]
- Reid, M.; Maras, J.E.; Shea, S.; Wood, A.C.; Castro-Diehl, C.; Johnson, D.A.; Huang, T.; Jacobs, D.R.; Crawford, A.; St-Onge, M.-P.; et al. Association between diet quality and sleep apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep 2019, 42, zsy194. [Google Scholar] [CrossRef]
- Billings, M.E.; Johnson, D.A.; Simonelli, G.; Moore, K.; Patel, S.; Roux, A.V.D.; Redline, S. Neighborhood Walking Environment and Activity Level Are Associated With OSA: The Multi-Ethnic Study of Atherosclerosis. Chest 2016, 150, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.H.; Brindle, R.C.; Buysse, D.J. Sleep and Cardiovascular Disease: Emerging Opportunities for Psychology. Am. Psychol. 2018, 73, 994. [Google Scholar] [CrossRef]
- Makarem, N.; Shechter, A.; Carnethon, M.R.; Mullington, J.M.; Hall, M.H.; Abdalla, M. Sleep Duration and Blood Pressure: Recent Advances and Future Directions. Curr. Hypertens. Rep. 2019, 21, 33. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic Factors | % | Sleep Factors * | % | Cardiometabolic Risk Factors | Mean ± SD | Cardiovascular Disease and Cardiometabolic Outcomes | % |
|---|---|---|---|---|---|---|---|
| Sex | Sleep Health Categories * | Body Mass Index (kg/m2) | 29.8 ± 7.4 | Obesity‡ | 41.8% | ||
| Women | 51.1% | Poor (0–1) | 8.5% | ||||
| Men | 48.9% | Moderate (2–3) | 50.3% | ||||
| Ideal (4–5) | 41.2% | ||||||
| Age Group | Sleep Duration * | Waist Circumference (cm) | 100.7 ± 17.2 | Central Adiposity‡ | 57.1% | ||
| 20–44 y | 44.7% | Short or Long Sleeper | 40.8% | ||||
| 45–80 y | 55.3% | Sufficient Sleeper | 59.2% | ||||
| Nativity | Sleep Regularity * | Fasting Glucose (mg/dL) | 110.3 ± 37.1 | Type 2 Diabetes‡ | 14.7% | ||
| Born in US Mainland | 81.1% | Variable sleep duration and/or timing | 40.2% | ||||
| Born Outside of US | 18.9% | Regular sleep duration and/or timing | 59.8% | ||||
| Marital Status | Disordered Sleep * | Systolic Blood Pressure (mmHg) | 122.8 ± 19.3 | Hypertension (2017 ACC/AHA Definition)† | 43.8% | ||
| Married/Living with Partner | 64.3% | Frequent snoring/snorting | 47.9% | ||||
| Single/Widowed/Divorced | 35.7% | Never/Rare snoring/snorting | 52.1% | ||||
| Race/Ethnicity | Sleep Latency * | Diastolic Blood Pressure (mmHg) | 73.4 ± 12.2 | Hypertension (JNC 7 Definition)† | 31.2% | ||
| NH White | 62.9% | Difficulty Falling Asleep | 30.1% | ||||
| NH Black | 10.9% | No Difficulty Falling Asleep | 69.9% | ||||
| NH Asian | 6.0% | ||||||
| Hispanic | 15.6% | ||||||
| Other Race/Multi-Racial | 4.6% | ||||||
| Education | Daytime Sleepiness (Alertness) * | - | - | Cardiovascular Disease§ | 11.6% | ||
| Less than College | 37.3% | Often/Frequent Daytime Sleepiness | 27.3% | ||||
| Some College and Above | 62.7% | Never/Rare Daytime Sleepiness | 72.7% |
| Sleep Health Scores * | Body Mass Index (kg/m2) | Waist Circumference (cm) | Fasting Glucose (mg/dL) | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) |
|---|---|---|---|---|---|
| β (95% CI) p-Value | β (95% CI) p-Value | β (95% CI) p-Value | β (95% CI) p-Value | β (95% CI) p-Value | |
| MDSH Score * | |||||
| Poor (0–1) (ref) | ref | ref | ref | ref | ref |
| Moderate (2–3) | −3.26 (−4.51, −2.01) p < 0.001 | −8.44 (−10.81, −6.07) p < 0.001 | −4.21 (−9.53, 1.11) p = 0.113 | −2.60 (−5.06, −0.14) p = 0.040 | −2.61 (−4.33, −0.90) p = 0.005 |
| Ideal (4–5) | −5.34 (−6.50, −4.17) p < 0.001 | −13.32 (−15.42, −11.23) p < 0.001 | −6.40 (−11.24, −1.56) p = 0.013 | −4.22 (−6.95, −1.49) p = 0.005 | −3.42 (−5.38, −1.47) p = 0.002 |
| MDSH Score * | |||||
| Ideal vs. Moderate/Poor | −2.53 (−2.98, −2.09) p < 0.001 | −6.06 (−7.47, −4.65) p < 0.001 | −2.76 (−4.98, −0.54) p = 0.018 | −1.97 (−3.67, −0.26) p = 0.026 | −1.16 (−1.82, −0.50) p = 0.002 |
| Sleep Duration Score (1 vs. 0) * | −0.29 (−1.06, 0.48) p = 0.438 | −0.53 (−2.11, 1.05) p = 0.487 | −3.22 (−6.86, 0.42) p = 0.079 | −0.34 (−2.00, 1.32) p = 0.673 | 0.49 (−0.52, 1.49) p = 0.318 |
| Sleep Regularity Score (1 vs. 0) * | −0.29 (−1.15, 0.57) p = 0.479 | −0.63 (−2.64, 1.38) p = 0.514 | 0.90 (−2.11, 3.91) p = 0.535 | −1.56 (−3.23, 0.11) p = 0.064 | −1.26 (−2.53, 0.01) p = 0.052 |
| Symptoms of Sleep Disorders Score (1 vs. 0) * | −3.55 (−4.13, −2.98) p < 0.001 | −8.50 (−9.76, −7.25) p < 0.001 | −2.69 (−5.93, 0.54) p = 0.096 | −1.83 (−3.25, −0.41) p = 0.015 | −2.03 (−2.82, −1.25) p < 0.001 |
| Sleep Latency Score (1 vs. 0) * | −2.25 (−3.03, −1.47) p < 0.001 | −5.50 (−7.74, −3.25) p < 0.001 | −3.12 (−7.16, 0.92) p = 0.120 | −1.20 (−2.96, 0.56) p = 0.167 | −1.84 (−2.74, −0.94) p < 0.001 |
| Daytime Sleepiness (Alertness) Score (1 vs. 0) * | −2.23 (−3.09, −1.36) p < 0.001 | −5.77 (−7.42, −4.12) p < 0.001 | −1.78 (−5.68, 2.13) p = 0.348 | −0.37 (−1.58, 0.85) p = 0.527 | 0.50 (−0.86, 1.86) p = 0.442 |
| Sleep Health Scores * | Obesity † | Central Adiposity † | Type 2 Diabetes† | Hypertension (Definition 1) ‡ | Hypertension (Definition 2) ‡ | Cardiovascular Disease § |
|---|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Multidimensional Sleep Health Score * | ||||||
| Poor (0–1) (ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate (2–3) | 0.44 (0.35, 0.57) | 0.45 (0.33, 0.63) | 0.90 (0.48, 1.67) | 0.59 (0.42, 0.82) | 0.47 (0.35, 0.64) | 0.82 (0.56, 1.19) |
| Ideal (4–5) | 0.27 (0.20, 0.37) p-trend < 0.001 | 0.32 (0.23, 0.45) p-trend < 0.001 | 0.55 (0.27, 1.09) p-trend = 0.588 | 0.38 (0.28, 0.52) p-trend < 0.001 | 0.26 (0.18, 0.36) p-trend < 0.001 | 0.57 (0.32–1.01) p-trend = 0.625 |
| Sleep Duration Score (1 vs. 0) * | 0.95 (0.80, 1.12) | 0.99 (0.83, 1.18) | 1.02 (0.74, 1.42) | 0.90 (0.71, 1.12) | 0.67 (0.53, 0.83) | 0.66 (0.47, 0.92) |
| Sleep Regularity Score (1 vs. 0) * | 1.06 (0.85, 1.33) | 1.03 (0.88, 1.20) | 1.34 (0.99, 1.81) | 1.19 (0.93, 1.51) | 1.24 (0.97, 1.60) | 1.86 (1.37, 2.54) |
| Disordered Sleep Score (1 vs. 0) * | 0.42 (0.35, 0.50) | 0.45 (0.38, 0.54) | 0.63 (0.48, 0.84) | 0.60 (0.50, 0.72) | 0.62 (0.49, 0.78) | 1.01 (0.70, 1.45) |
| Sleep Latency Score (1 vs. 0) * | 0.57 (0.47, 0.68) | 0.68 (0.54, 0.87) | 0.65 (0.45, 0.94) | 0.53 (0.44, 0.65) | 0.51 (0.41, 0.62) | 0.59 (0.45, 0.78) |
| Daytime Sleepiness (Alertness) Score (1 vs. 0) * | 0.57 (0.48, 0.67) | 0.61 (0.51, 0.74) | 0.54 (0.41, 0.71) | 0.69 (0.55, 0.88) | 0.56 (0.44, 0.70) | 0.48 (0.35, 0.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarem, N.; Alcantara, C.; Musick, S.; Quesada, O.; Sears, D.D.; Chen, Z.; Tehranifar, P. Multidimensional Sleep Health Is Associated with Cardiovascular Disease Prevalence and Cardiometabolic Health in US Adults. Int. J. Environ. Res. Public Health 2022, 19, 10749. https://doi.org/10.3390/ijerph191710749
Makarem N, Alcantara C, Musick S, Quesada O, Sears DD, Chen Z, Tehranifar P. Multidimensional Sleep Health Is Associated with Cardiovascular Disease Prevalence and Cardiometabolic Health in US Adults. International Journal of Environmental Research and Public Health. 2022; 19(17):10749. https://doi.org/10.3390/ijerph191710749
Chicago/Turabian StyleMakarem, Nour, Carmela Alcantara, Sydney Musick, Odayme Quesada, Dorothy D. Sears, Ziyu Chen, and Parisa Tehranifar. 2022. "Multidimensional Sleep Health Is Associated with Cardiovascular Disease Prevalence and Cardiometabolic Health in US Adults" International Journal of Environmental Research and Public Health 19, no. 17: 10749. https://doi.org/10.3390/ijerph191710749
APA StyleMakarem, N., Alcantara, C., Musick, S., Quesada, O., Sears, D. D., Chen, Z., & Tehranifar, P. (2022). Multidimensional Sleep Health Is Associated with Cardiovascular Disease Prevalence and Cardiometabolic Health in US Adults. International Journal of Environmental Research and Public Health, 19(17), 10749. https://doi.org/10.3390/ijerph191710749







