Impact of the Menstrual Cycle Phases on the Movement Patterns of Sub-Elite Women Soccer Players during Competitive Matches
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Estimation of the Menstrual Cycle Phases
2.4. Contextual-Related Variables
2.5. Movement Patterns of Each Player
2.6. Statistical Analyses
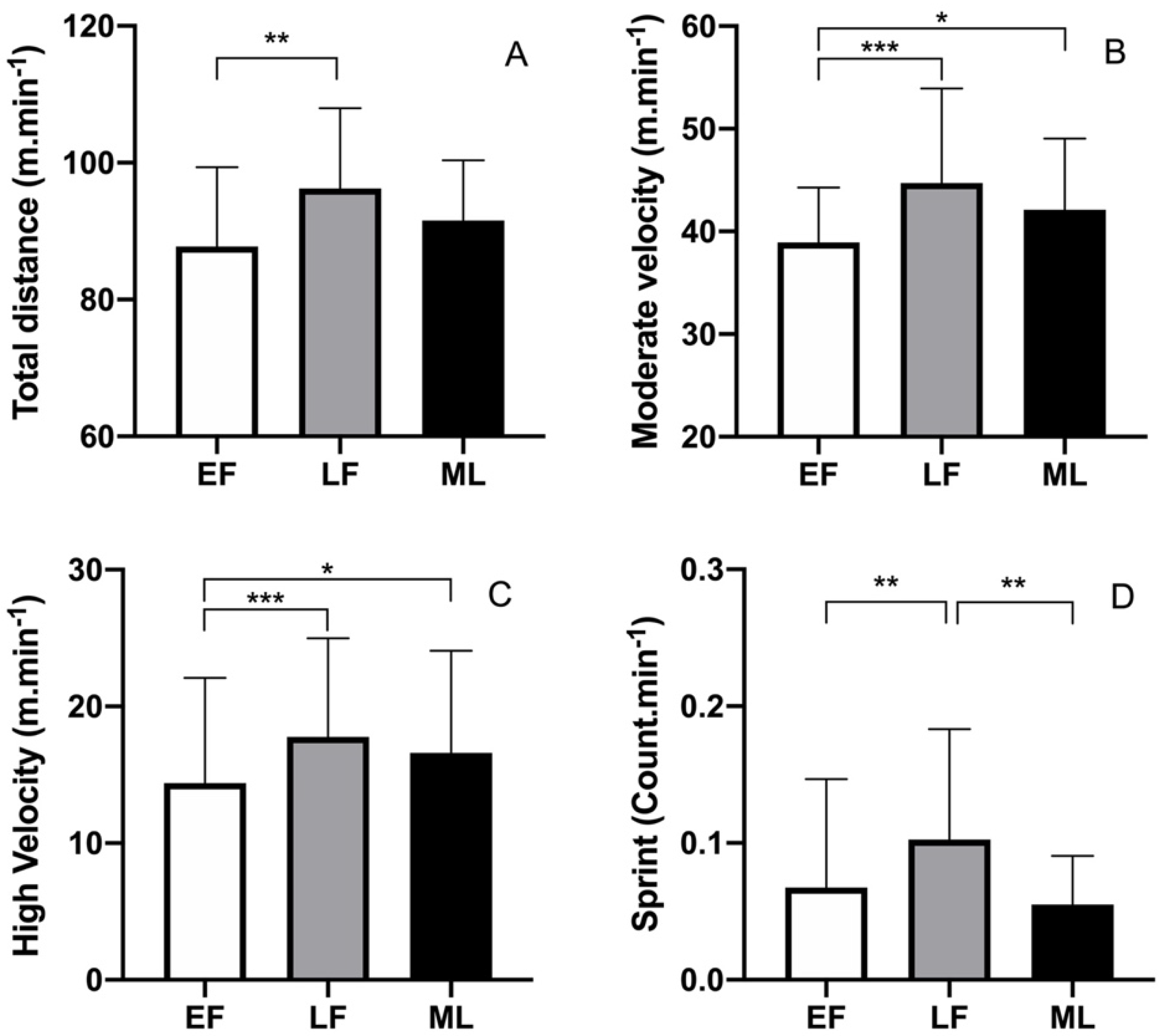
3. Results
3.1. Context of Games and Menstrual Cycle Phase
3.2. Match Physical Performance Metrics
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Larsen, B.; Morris, K.; Quinn, K.; Osborne, M.; Minahan, C. Practice does not make perfect: A brief view of athletes’ knowledge on the menstrual cycle and oral contraceptives. J. Sci. Med. Sport 2020, 23, 690–694. [Google Scholar] [CrossRef]
- Boisseau, N.; Isacco, L. Substrate metabolism during exercise: Sexual dimorphism and women’s specificities. Eur. J. Sport Sci. 2021, 17, 1–21. [Google Scholar] [CrossRef]
- Campbell, S.E.; Angus, D.J.; Febbraio, M.A. Glucose kinetics and exercise performance during phases of the menstrual cycle: Effect of glucose ingestion. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E817–E825. [Google Scholar] [CrossRef]
- Pallavi, L.C.; Souza, U.J.D.; Shivaprakash, G. Assessment of Musculoskeletal Strength and Levels of Fatigue during Different Phases of Menstrual Cycle in Young Adults. J. Clin. Diagn. Res. 2017, 11, CC11–CC13. [Google Scholar] [CrossRef]
- Tenan, M.S.; Hackney, A.C.; Griffin, L. Maximal force and tremor changes across the menstrual cycle. Eur. J. Appl. Physiol. 2016, 116, 153–160. [Google Scholar] [CrossRef]
- Bambaeichi, E.; Reilly, T.; Cable, N.T.; Giacomoni, M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol. Int. 2004, 21, 645–660. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N.; Jackson, S. Cycling time trial performance during different phases of the menstrual cycle. Eur. J. Appl. Physiol. 2005, 94, 268–276. [Google Scholar] [CrossRef]
- Ekenros, L.; Hirschberg, A.L.; Heijne, A.; Friden, C. Oral contraceptives do not affect muscle strength and hop performance in active women. Clin. J. Sport Med. 2013, 23, 202–207. [Google Scholar] [CrossRef]
- Dibrezzo, R.; Fort, I.L.; Brown, B. Dynamic strength and work variations during three stages of the menstrual cycle. J. Orthop. Sports Phys. Ther. 1988, 10, 113–116. [Google Scholar] [CrossRef]
- De Jonge, X.A.K.J. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- De Jonge, X.A.K.J.; Boot, C.R.L.; Thom, J.M.; Ruell, P.A.; Thompson, M.W. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J. Physiol. 2001, 530, 161–166. [Google Scholar] [CrossRef]
- De Janse, X.A.K.J.; Thompson, M.W.; Chuter, V.H.; Silk, L.N.; Thom, J.M. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med. Sci. Sports Exerc. 2012, 44, 2190–2198. [Google Scholar] [CrossRef]
- McLay, R.T.; Thomson, C.D.; Williams, S.M.; Rehrer, N.J. Carbohydrate loading and female endurance athletes: Effect of menstrual-cycle phase. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 189–205. [Google Scholar] [CrossRef]
- Vaiksaar, S.; Jurimae, J.; Maestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jurimae, T. No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J. Strength Cond. Res. 2011, 25, 1571–1578. [Google Scholar] [CrossRef]
- Billaut, F.; Bishop, D. Muscle fatigue in males and females during multiple-sprint exercise. Sports Med. 2009, 39, 257–278. [Google Scholar] [CrossRef]
- Stolen, T.; Chamari, K.; Castagna, C.; Wisloff, U. Physiology of soccer: An update. Sports Med. 2005, 35, 501–536. [Google Scholar] [CrossRef]
- Archiza, B.; Andaku, D.K.; Beltrame, T.; Libardi, C.A.; Borghi-Silva, A. The Relationship Between Repeated-Sprint Ability, Aerobic Capacity, and Oxygen Uptake Recovery Kinetics in Female Soccer Athletes. J. Hum. Kinet. 2020, 75, 115–126. [Google Scholar] [CrossRef]
- Sundgot-Borgen, J.; Torstveit, M.K. The female football player, disordered eating, menstrual function and bone health. Br. J. Sports Med. 2007, 41 (Suppl. 1), i68–i72. [Google Scholar] [CrossRef][Green Version]
- Henderson, Z.J.; Scribbans, T. Intrauterine Contraception and Athletic Performance: Where is the Data? Health Fit. J. Can. 2020, 13, 37–45. [Google Scholar] [CrossRef]
- Julian, R.; Skorski, S.; Hecksteden, A.; Pfeifer, C.; Bradley, P.S.; Schulze, E.; Meyer, T. Menstrual cycle phase and elite female soccer match-play: Influence on various physical performance outputs. Sci. Med. Footb. 2020, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Durnin, J.V.; Womersley, J. The relationship between skinfold thickness and body fat in adults of middle age. J. Physiol. 1969, 200, 105P–106P. [Google Scholar] [PubMed]
- Schaumberg, M.A.; Jenkins, D.G.; de Janse, X.A.K.J.; Emmerton, L.M.; Skinner, T.L. Oral Contraceptive Use Dampens Physiological Adaptations to Sprint Interval Training. Med. Sci. Sports Exerc. 2017, 49, 717–727. [Google Scholar] [CrossRef] [PubMed]
- De Janse, X.A.K.J.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.; Ackerman, K.E.; Sipila, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]
- Diez, A.; Lozano, D.; Arjol-Serrano, J.L.; Mainer-Pardos, E.; Castillo, D.; Torrontegui-Duarte, M.; Nobari, H.; Jaen-Carrillo, D.; Lampre, M. Influence of contextual factors on physical demands and technical-tactical actions regarding playing position in professional soccer players. BMC Sports Sci. Med. Rehabil. 2021, 13, 157. [Google Scholar] [CrossRef]
- Hughes, T.; Jones, R.K.; Starbuck, C.; Sergeant, J.C.; Callaghan, M.J. The value of tibial mounted inertial measurement units to quantify running kinetics in elite football (soccer) players. A reliability and agreement study using a research orientated and a clinically orientated system. J. Electromyogr. Kinesiol. 2019, 44, 156–164. [Google Scholar] [CrossRef]
- Murray, A.M.; Varley, M.C. Technology in soccer. In Elite Soccer Players: Maximizing Performance and Safety; Curtis, R., Benjamin, C., Huggins, R., Casa, D.J., Eds.; Routledge; Taylor & Francis: New York, NY, USA, 2020; pp. 37–53. [Google Scholar]
- Boyd, L.J.; Ball, K.; Aughey, R.J. The reliability of MinimaxX accelerometers for measuring physical activity in Australian football. Int. J. Sports Physiol. Perform. 2011, 6, 311–321. [Google Scholar] [CrossRef]
- Polglaze, T.; Dawson, B.; Hiscock, D.J.; Peeling, P. A comparative analysis of accelerometer and time-motion data in elite men’s hockey training and competition. Int. J. Sports Physiol. Perform. 2015, 10, 446–451. [Google Scholar] [CrossRef]
- Carling, C.; Le Gall, F.; Dupont, G. Analysis of repeated high-intensity running performance in professional soccer. J. Sports Sci. 2012, 30, 325–336. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, J.D.; Jovanovic, M. Sprint Mechanical Characteristics of Female Soccer Players: A Retrospective Pilot Study to Examine a Novel Approach for Correction of Timing Gate Starts. Front. Sports Act. Living 2021, 3, 629694. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Engen, L.C.; Wisloff, U.; Hoff, J. Aerobic endurance training improves soccer performance. Med. Sci. Sports Exerc. 2001, 33, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Datson, N.; Hulton, A.; Andersson, H.; Lewis, T.; Weston, M.; Drust, B.; Gregson, W. Applied physiology of female soccer: An update. Sports Med. 2014, 44, 1225–1240. [Google Scholar] [CrossRef]
- Vescovi, J.D.; Favero, T.G. Motion characteristics of women’s college soccer matches: Female Athletes in Motion (FAiM) study. Int. J. Sports Physiol. Perform. 2014, 9, 405–414. [Google Scholar] [CrossRef]
- Randell, R.K.; Carter, J.M.; Jeukendrup, A.E.; Lizarraga, M.A.; Yanguas, J.I.; Rollo, I. Fat Oxidation Rates in Professional Soccer Players. Med. Sci. Sports Exerc. 2019, 51, 1677–1683. [Google Scholar] [CrossRef]
- Hackney, A.C. Effects of the menstrual cycle on resting muscle glycogen content. Horm. Metab. Res. 1990, 22, 647. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Hackney, A.C.; Sharp, R.L. The menstrual cycle and exercise: Performance, muscle glycogen, and substrate responses. Int. J. Sports Med. 1989, 10, 264–269. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef]
- De Souza, M.J.; Maguire, M.S.; Rubin, K.R.; Maresh, C.M. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med. Sci. Sports Exerc. 1990, 22, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Ekman, M.; Morgan, G.; Johansson, O.; Jansson, E.; Esbjornsson, M. Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem. Cell Biol. 2005, 124, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Cabelka, C.A.; Baumann, C.W.; Collins, B.C.; Nash, N.; Le, G.; Lindsay, A.; Spangenburg, E.E.; Lowe, D.A. Effects of ovarian hormones and estrogen receptor alpha on physical activity and skeletal muscle fatigue in female mice. Exp. Gerontol. 2019, 115, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ansdell, P.; Brownstein, C.G.; Skarabot, J.; Hicks, K.M.; Simoes, D.C.M.; Thomas, K.; Howatson, G.; Hunter, S.K.; Goodall, S. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J. Appl. Physiol. 2019, 126, 1701–1712. [Google Scholar] [CrossRef]
- Cesar, G.M.; Pereira, V.S.; Santiago, P.R.; Benze, B.G.; da Costa, P.H.; Amorim, C.F.; Serrao, F.V. Variations in dynamic knee valgus and gluteus medius onset timing in non-athletic females related to hormonal changes during the menstrual cycle. Knee 2011, 18, 224–230. [Google Scholar] [CrossRef]
- Pereira, H.M.; Larson, R.D.; Bemben, D.A. Menstrual Cycle Effects on Exercise-Induced Fatigability. Front. Physiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Findlay, R.J.; Macrae, E.H.R.; Whyte, I.Y.; Easton, C.; Forrest, L.J. How the menstrual cycle and menstruation affect sporting performance: Experiences and perceptions of elite female rugby players. Br. J. Sports Med. 2020, 54, 1108–1113. [Google Scholar] [CrossRef]
- Armour, M.; Hyman, M.S.; Al-Dabbas, M.; Parry, K.; Ferfolja, T.; Curry, C.; MacMillan, F.; Smith, C.A.; Holmes, K. Menstrual Health Literacy and Management Strategies in Young Women in Australia: A National Online Survey of Young Women Aged 13–25 Years. J. Pediatr. Adolesc. Gynecol. 2021, 34, 135–143. [Google Scholar] [CrossRef]
- Chrisler, J.C.; Marvan, M.L.; Gorman, J.A.; Rossini, M. Body appreciation and attitudes toward menstruation. Body Image 2015, 12, 78–81. [Google Scholar] [CrossRef]
- Schooler, D.; Ward, L.M.; Merriwether, A.; Caruthers, A.S. Cycles of shame: Menstrual shame, body shame, and sexual decision-making. J. Sex. Res. 2005, 42, 324–334. [Google Scholar] [CrossRef]
- Krustrup, P.; Mohr, M.; Nybo, L.; Draganidis, D.; Randers, M.B.; Ermidis, G.; Orntoft, C.; Roddik, L.; Batsilas, D.; Poulios, A.; et al. Muscle metabolism and impaired sprint performance in an elite women’s football game. Scand. J. Med. Sci. Sports 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Speroff, L.; Glass, R.H.; Kase, N.G. Clinical Gynecologic Endocrinology and Infertility; LWW: Philadelphia, PA, USA, 1999. [Google Scholar]
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Swinton, P.A.; Dolan, E. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD (CV%) | |
|---|---|
| Age (years) | 25.7 ± 3.3 (12.8%) |
| Height (cm) | 167.3 ± 7.2 (4.3%) |
| Body mass (kg) | 58.9 ± 6.3 (10.7%) |
| Body fat (%) a | 23.0 ± 2.0 (8.7%) |
| Menstrual cycle length (days) | 30.0 ± 2.6 (8.7%) |
| EF | LF | ML | Statistics | ||
|---|---|---|---|---|---|
| Game location | Home | 14 | 14 | 7 | χ2 (2.72) = 4.71, p = 0.10 |
| Away | 9 | 12 | 16 | ||
| Match Outcome | Won | 16 | 18 | 14 | χ2 (4.72) = 3.16, p = 0.53 |
| Drawn | 7 | 6 | 9 | ||
| Lost | 0 | 2 | 0 | ||
| Ranking difference | −4.9 ± 3.9 | −5.08 ± 4.6 | −5.1 + 4.5 | p = 0.88 | |
| (−79%) | (−90%) | (−88%) | |||
| Point difference | 10.5 ± 9.9 | 11 ± 10.2 | 10.1 ± 10.1 | p = 0.73 | |
| (94%) | (93%) | (99%) |
| 1st H | 2nd H | Linear Mixed Effect: p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EF | LF | ML | EF | LF | ML | MC | GP | MC * GP | |
| DTOT (m.min−1) | 89.1 ± 13.7 (15.4%) | 98.5 ± 11.9 | 92.5 ± 8.3 | 85.3 ± 10.3 | 91.8 ± 12.3 | 89.4 ± 9.7 | 0.01 | 0.03 | 0.73 |
| (12.10%) | (9.00%) | (12.10%) | (13.30%) | (10.90%) | |||||
| LowV (m.min−1) | 34.6 ± 3.1 | 34.3 ± 3.4 | 33.1 ± 3.5 | 33.2 ± 1.8 | 31.9 ± 2.0 | 31.9 ± 2.9 | 0.13 | 0.004 | 0.61 |
| (9.00%) | (9.90%) | (10.60%) | (5.40%) | (6.30%) | (9.10%) | ||||
| ModV (m.min−1) | 39.5 ± 6.2 | 45.7 ± 9.2 | 42.4 ± 6.8 | 37.9 ± 5.0 | 42.6 ± 9.3 | 41.0 ± 7.0 | 0.001 | 0.053 | 0.77 |
| −15.70% | (20.10%) | (16.00%) | (13.20%) | (21.80%) | (17.10% | ||||
| HighV (m.min−1) | 14.0 ± 7.4 | 17.0 ± 6.5 | 15.8 ± 6.7 | 13.3 ± 6.8 | 15.9 ± 6.1 | 15.3 ± 6.9 | 0.005 | 0.26 | 0.93 |
| −52.90% | (38.20%) | (42.40%) | (51.10%) | (38.40%) | (45.10%) | ||||
| SprintV (m.min−1) | 0.64 ± 0.86 | 1.12 ± 1.31 | 0.95 ± 1.35 | 0.60 ± 0.82 | 1.06 ± 1.27 | 0.92 ± 1.31 | 0.21 | 0.85 | 0.99 |
| −134.40% | (117.00%) | (142.10%) | (136.70%) | (120.00%) | (142.40%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igonin, P.-H.; Rogowski, I.; Boisseau, N.; Martin, C. Impact of the Menstrual Cycle Phases on the Movement Patterns of Sub-Elite Women Soccer Players during Competitive Matches. Int. J. Environ. Res. Public Health 2022, 19, 4465. https://doi.org/10.3390/ijerph19084465
Igonin P-H, Rogowski I, Boisseau N, Martin C. Impact of the Menstrual Cycle Phases on the Movement Patterns of Sub-Elite Women Soccer Players during Competitive Matches. International Journal of Environmental Research and Public Health. 2022; 19(8):4465. https://doi.org/10.3390/ijerph19084465
Chicago/Turabian StyleIgonin, Pierre-Hugues, Isabelle Rogowski, Nathalie Boisseau, and Cyril Martin. 2022. "Impact of the Menstrual Cycle Phases on the Movement Patterns of Sub-Elite Women Soccer Players during Competitive Matches" International Journal of Environmental Research and Public Health 19, no. 8: 4465. https://doi.org/10.3390/ijerph19084465
APA StyleIgonin, P.-H., Rogowski, I., Boisseau, N., & Martin, C. (2022). Impact of the Menstrual Cycle Phases on the Movement Patterns of Sub-Elite Women Soccer Players during Competitive Matches. International Journal of Environmental Research and Public Health, 19(8), 4465. https://doi.org/10.3390/ijerph19084465






