Efficacy of Nutritional Strategies on the Improvement of the Performance and Health of the Athlete: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Elegibility Criteria
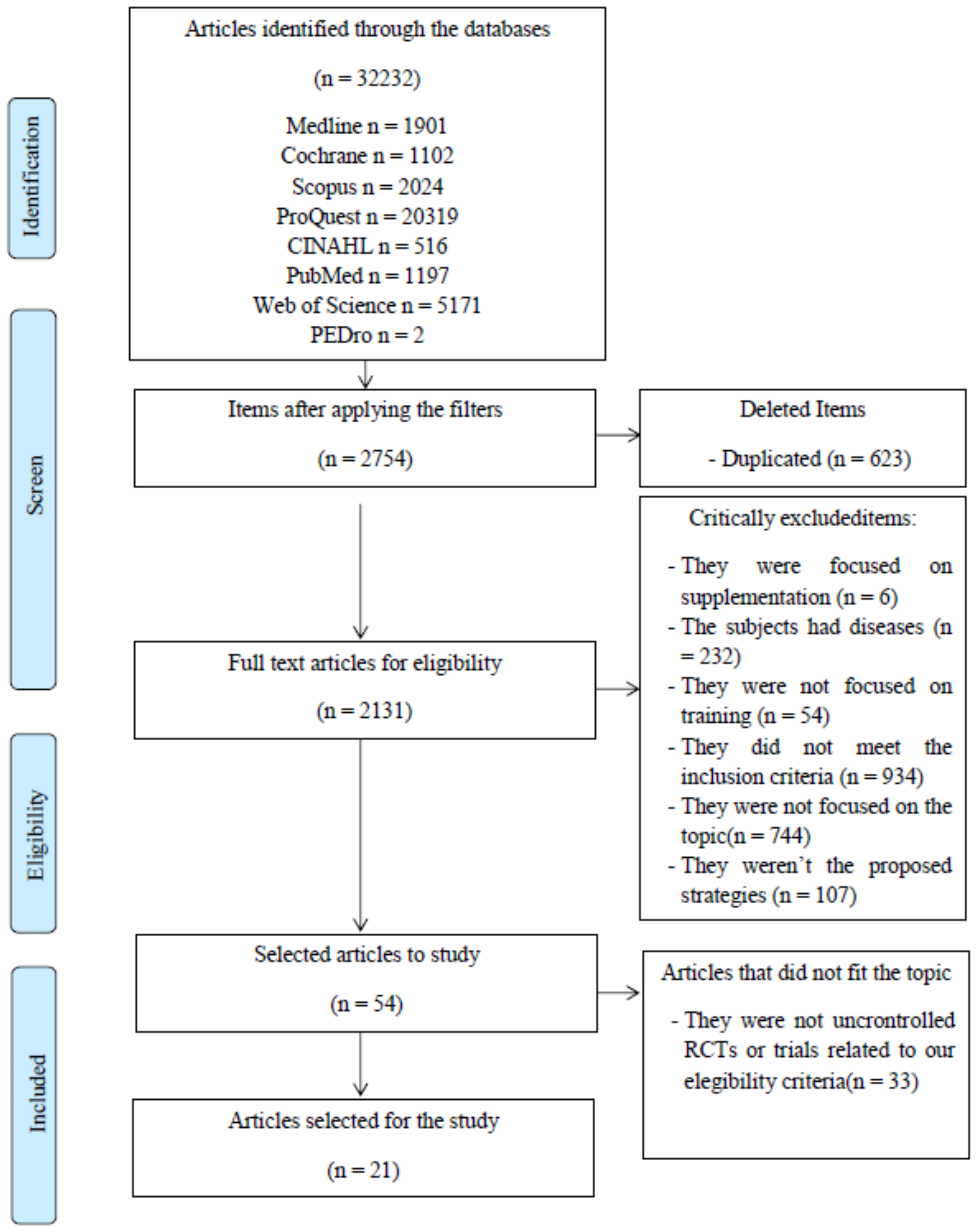
2.4. Study Selection
2.5. Data Extraction
2.6. Assessment of Methodological Quality
3. Results
3.1. Characteristics of the Selected Studies
3.1.1. Study Characteristics
3.1.2. Methodological Quality
3.2. Intermittent Fasting
3.3. Time Restricted Feeding
3.4. Ketogenic Diet
4. Discussion
4.1. Intermittent Fasting
4.2. Time-Restricted Feeding
4.3. Ketogenic Diet
4.4. Strengths and Limitations of the Study
4.5. Clinical Application of the Results
4.6. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TRF | Time-restricted feeding |
| QUALSYT | Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Varietyof fields |
| IVS | PEDro Validity Internal Scale |
| PEDro | Physiotherapy Evidence Database |
References
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Clarys, P.; Nijs, J.; Coppieters, I.; Polli, A.; Malfliet, A. Chronic Musculoskeletal Pain and Nutrition: Where Are We and Where Are We Heading? PM R 2020, 12, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalsen, A.; Li, C.; Kaiser, K.; Lüdtke, R.; Meier, L.; Stange, R.; Kessler, C. In-patient treatment of fibromyalgia: A controlled nonrandomized comparison of conventional medicine versus integrative medicine including fasting therapy. Evid.-Based Complement. Altern. Med. 2013, 2013, 908610. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Guo, J.; Chen, K.Y.; Leibel, R.L.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Ravussin, E. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am. J. Clin. Nutr. 2019, 109, 1328–1334. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Burke, L.M. Ketogenic low-CHO, high-fat diet: The future of elite endurance sport? J. Physiol. 2021, 599, 819–843. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Verma, N.; Thakkar, N.; Yeung, C.; Sung, H.K. Intermittent fasting: Physiological implications on outcomes in mice and men. Physiology 2020, 35, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Deasy, W.; Stathis, C.; Hayes, A.; Cooke, M. Intermittent Fasting with or without Exercise Prevents Weight Gain and Improves Lipids in Diet-Induced Obese Mice. Nutrients 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, S.; Rajeev, V.; Fann, D.Y.; Jo, D.; Arumugam, T.V. Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav. 2020, 10, e01444. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S.; Adamello, V.; Jolla, L. Fasting Circadian Rhythms Time resticted eating. Cell Metab. 2017, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef] [Green Version]
- Templeman, I.; Thompson, D.; Gonzalez, J.; Walhin, J.-P.; Reeves, S.; Rogers, P.J.; Brunstrom, J.M.; Karagounis, L.G.; Tsintzas, K.; Betts, J.A. Intermittent fasting, energy balance and associated health outcomes in adults: Study protocol for a randomised controlled trial. Trials 2018, 19, 86. [Google Scholar] [CrossRef] [Green Version]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, I.; Pezarat-Correia, P.; Minderico, C.; Mendonca, G.V. Effects of intermittent fasting on specific exercise performance outcomes: A systematic review including meta-analysis. Nutrients 2020, 12, 1390. [Google Scholar] [CrossRef]
- Sibille, K.T.; Bartsch, F.; Reddy, D.; Fillingim, R.B.; Keil, A. Increasing neuroplasticity to bolster chronic pain treatment: A role for intermittent fasting and glucose administration? J. Pain 2016, 17, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea Meira, I.; Romão, T.T.; Do Prado, H.J.P.; Krüger, L.T.; Pires, M.E.P.; Da Conceição, P.O. Ketogenic diet and epilepsy: What we know so far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsharairi, N.A. The role of short-chain fatty acids in mediating very low-calorie ketogenic diet-infant gut microbiota relationships and its therapeutic potential in obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Sharma, A.P.; Ross, M.L.; Welvaert, M.; Slater, G.J.; Burke, L.M. Chronic ketogenic low carbohydrate high fat diet has minimal effects on acid–base status in elite athletes. Nutrients 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Kysel, P.; Haluzíková, D.; Doležalová, R.P.; La ˇnková, I.; Lacinová, Z.; Kasperová, B.J. The Influence of Cyclical Ketogenic Reduction Diet vs. Nutritionally Balanced Reduction Diet on Body. Nutrients 2020, 12, 2832. [Google Scholar] [CrossRef]
- Bailey, C.P.; Hennessy, E. A review of the ketogenic diet for endurance athletes: Performance enhancer or placebo effect? J. Int. Soc. Sports Nutr. 2020, 17, 33. [Google Scholar] [CrossRef]
- Welch, V.; Petticrew, M.; Tugwell, P.; Moher, D.; O’Neill, J.; Waters, E.; White, H. Extensión PRISMA-Equidad 2012: Guías para la escritura y la publicación de revisiones sistemáticas enfocadas en la equidad en salud. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2013, 34, 60–67. [Google Scholar] [CrossRef]
- Urrútia, G.; Bonfill, X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Gomez-Conesa, A.; Serrano, C.S.; Matamoros, D.C.; López-López, J.A. The Spanish translation and adaptation of the Pedro scale. Physiotherapy 2015, 101, e463–e464. [Google Scholar] [CrossRef] [Green Version]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Naharudin, M.N.B.; Yusof, A. The effect of 10 days of intermittent fasting on Wingate anaerobic power and prolonged high-intensity time-to-exhaustion cycling performance. Eur. J. Sport Sci. 2018, 18, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.A.; Richardson, J.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. Postprandial metabolism and appetite do not differ between lean adults that eat breakfast or morning fast for 6 weeks. J. Nutr. 2018, 148, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edinburgh, R.M.; Hengist, A.; Smith, H.A.; Travers, R.L.; Betts, J.A.; Thompson, D.; Walhin, J.P.; Wallis, G.A.; Hamilton, D.L.; Stevenson, E.J.; et al. Skipping breakfast before exercise creates a more negative 24-hour energy balance: A randomized controlled trial in healthy physically active young men. J. Nutr. 2019, 149, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition 2018, 51–52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; Vandusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [Green Version]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef]
- Sjödin, A.; Hellström, F.; Sehlstedt, E.; Svensson, M.; Burén, J. Effects of a ketogenic diet on muscle fatigue in healthy, young, normal-weight women: A randomized controlled feeding trial. Nutrients 2020, 12, 955. [Google Scholar] [CrossRef] [Green Version]
- Terada, T.; Toghi Eshghi, S.R.; Liubaoerjijin, Y.; Kennedy, M.; Myette-Côté, É.; Fletcher, K.; Boulé, N.G. Overnight fasting compromises exercise intensity and volume during sprint interval training but improves high-intensity aerobic endurance. J. Sports Med. Phys. Fit. 2019, 59, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidi, V.; Ili, V.; Toski, L. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin. Nutr. 2021, 40, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.; Meeusen, R.; Farooq, A.; Ryu, J.; Fenneni, M.A.; Nikolovski, Z.; Elshafie, S.; Chamari, K.; Roelands, B. Three days of intermittent fasting: Repeated-sprint performance decreased by vertical-stiffness impairment. Int. J. Sports Physiol. Perform. 2017, 12, 287–294. [Google Scholar] [CrossRef]
- Dethlefsen, M.M.; Bertholdt, L.; Gudiksen, A.; Stankiewicz, T.; Bangsbo, J.; Van Hall, G.; Plomgaard, P.; Pilegaard, H. Training state and skeletal muscle autophagy in response to 36 h of fasting. J. Appl. Physiol. 2018, 125, 1609–1619. [Google Scholar] [CrossRef]
- Gueldich, H.; Zghal, F.; Borji, R.; Chtourou, H.; Sahli, S.; Rebai, H. The effects of Ramadan intermittent fasting on the underlying mechanisms of force production capacity during maximal isometric voluntary contraction. Chronobiol. Int. 2019, 36, 698–708. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Keaney, L.; Dulson, D.K. Adaptation to a ketogenic diet modulates adaptive and mucosal immune markers in trained male endurance athletes. Scand. J. Med. Sci. Sports 2020, 31, 140–152. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Simmons, N.; Awan, S.; Chamari, K.; Ahmed, I. Intermittent fasting: Eating by the clock for health and exercise performance. BMJ Open Sport Exerc. Med. 2022, 8, 8–11. [Google Scholar] [CrossRef]
- Bowler, A.L.; Polman, R. Role of a Ketogenic Diet on Body Composition, Physical Health, Psychosocial Well-Being and Sports Performance in Athletes: A Scoping Review. Sports 2020, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lei, S.; Wang, X.; Cheng, S. The effect of a ketogenic low-carbohydrate, high-fat diet on aerobic capacity and exercise performance in endurance athletes: A systematic review and meta-analysis. Nutrients 2021, 13, 2896. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Ledesma, S.; Gijon-Nogueron, G.; Reina-Martín, I.; Ortega-Avila, A.B.; Pruimboom, L. Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2468. [Google Scholar] [CrossRef]
- Gudden, J.; Arias Vasquez, A.; Bloemendaal, M. The effects of intermittent fasting on brain and cognitive function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef]
- Seidler, K.; Barrow, M. Intermittent fasting and cognitive performance—Targeting BDNF as potential strategy to optimise brain health. Front. Neuroendocrinol. 2022, 65, 100971. [Google Scholar] [CrossRef]
- Meeusen, R.; Decroix, L. Nutritional supplements and the brain. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 200–211. [Google Scholar] [CrossRef]
| Item | BinNaharudin et al. (2018) | Carr, et al. (2018) | Cheriff et al. (2016) | Chowdhury et al. (2018) | Dethlefsen et al. (2018) | Edinburgh et al. (2019) | Gasmi et al. (2017) | Grant et al. (2017) | Grant et al. (2019) | Gueldich et al. (2019) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | X | X | X | X | X | X | ||||
| 3 | X | |||||||||
| 4 | X | X | X | X | X | X | ||||
| 5 | ||||||||||
| 6 | X | |||||||||
| 7 | X | |||||||||
| 8 | X | X | X | X | X | X | ||||
| 9 | X | X | X | X | X | |||||
| 10 | X | X | X | X | X | X | X | X | X | |
| 11 | X | X | X | X | X | X | X | X | X | X |
| TotalPEDro | 5/10 | 4/10 | 4/10 | 5/10 | 2/10 | 6/10 | 6/10 | 4/10 | 7/10 | 3/10 |
| Item | Kysel et al. (2020) | McAllister et al. (2019) | Moro et al. (2016) | Moro et al. (2020) | Shaw et al. (2020) | Sjödin et al. (2020) | Terada et al. (2019) | Vargas et al. (2018) | Vidic’ et al. (2021) | Zajac et al. (2014) | Zinn et al. (2017) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | X | X | X | X | X | X | X | X | X | X | |
| 3 | |||||||||||
| 4 | X | X | X | X | X | X | X | X | X | ||
| 5 | |||||||||||
| 6 | X | ||||||||||
| 7 | X | X | X | X | |||||||
| 8 | X | X | X | X | X | X | X | X | |||
| 9 | X | X | X | X | X | X | X | ||||
| 10 | X | X | X | X | X | X | X | X | X | ||
| 11 | X | X | X | X | X | X | X | X | X | X | X |
| TotalPEDro | 7/10 | 6/10 | 7/10 | 7/10 | 3/10 | 4/10 | 7/10 | 6/10 | 5/10 | 6/10 | 1/10 |
| Item | BinNaharudin, et al. (2018) | Carr et al. (2018) | Cheriff et al. (2016) | Chowdhury et al. (2018) | Dethlefsen et al. (2018) | Edinburgh et al. (2019) | Gasmi et al. (2017) | Grant et al. (2017) | Grant et al. (2019) | Gueldich et al. (2019) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | X | X | X | X | X | X | ||||
| 3 | X | |||||||||
| 5 | ||||||||||
| 6 | X | |||||||||
| 7 | ||||||||||
| 8 | X | X | X | X | X | X | X | |||
| 9 | X | X | X | X | X | |||||
| IVS | 3/7 | 1/7 | 2/7 | 2/7 | 0/7 | 3/7 | 2/7 | 1/7 | 4/7 | 2/7 |
| Quality | Low | Low | Low | Low | Low | Low | Low | Low | Medium | Low |
| Item | Kysel et al. (2020) | McAllister et al. (2019) | Moro et al. (2016) | Moro et al. (2020) | Shaw et al. (2020) | Sjödin et al. (2020) | Terada et al. (2019) | Vargas et al. (2018) | Vidic´ et al. (2021) | Zajac et al. (2014) | Zinn et al. (2017) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | X | X | X | X | X | X | X | X | X | X | |
| 3 | |||||||||||
| 5 | |||||||||||
| 6 | X | ||||||||||
| 7 | X | X | X | X | |||||||
| 8 | X | X | X | X | X | X | X | X | |||
| 9 | X | X | X | X | X | X | X | ||||
| IVS | 4/7 | 3/7 | 4/7 | 4/7 | 1/7 | 2/7 | 4/7 | 3/7 | 2/7 | 3/7 | 0/7 |
| Quality | Medium | Low | Medium | Medium | Low | Low | Medium | Low | Low | Low | Low |
| ITEM | Carr et al. (2018) | Cheriff et al. (2016) | Dethlefsen et al. (2018) | Gueldich et al. (2019) | Shaw et al. (2020) | Zajac et al. (2014) | Zinn et al. (2017) |
|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | Partial | Partial | Yes | Yes | Parcial |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Partial |
| 3 | Partial | Yes | Yes | Partial | Yes | Partial | Partial |
| 4 | Yes | Partial | No | Yes | No | Yes | Yes |
| 5 | No | N/A | N/A | N/A | N/A | N/A | N/A |
| 6 | No | N/A | N/A | N/A | N/A | N/A | N/A |
| 7 | No | N/A | N/A | N/A | N/A | N/A | N/A |
| 8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | Yes | Yes | Yes | Yes | Yes | Yes | Partial |
| 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11 | Yes | Yes | Yes | Yes | Yes | Yes | Partial |
| 12 | Yes | Partial | Partial | Yes | Yes | Yes | N/A |
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 0.75 | 0.90 | 0.81 | 0.90 | 0.90 | 0.95 | 0.75 |
| Name | Type of Study | Sample | Intervention | Measurements | Results |
|---|---|---|---|---|---|
| BinNaharudin et al. (2018) | RCT | N = 20 men Control: 10 Age: 20 ± 1 Intervention: 10 Age: 21 ± 1 Losses: 0 | Both groups had a trial period to get used to the program for 7 days. Every 2 days, they performed the Wingate Test and in the next session, a prolonged high-intensity cycling test, both in a period of 10 days. This period was repeated twice interspersing a 4-week break. The control 5 times a day consumed a total of 2492 ± 20 kcal. The intervention 4 times a day consumed a total of 1500 ± 55 kcal by skipping lunch. | VO2 peak. Blood and urine samples. Body mass. | From day 4 the intervention decreased their body mass (p < 0.001). Decrease in the performance of the Wingate Test in the intervention during the first days (p < 0.05). However, it recovered after the fourth day (p < 0.05). Decrease in the performance of the cycling test in the intervention during the sessions, but there was a tendency to recover performance in the later phases. Compared to Day 0, the cycling test performance in the intervention group reduced on Day 2 (p < 0.0001), Day 4 (p < 0.001), Day 6 (p < 0.001), Day 8 (p < 0.05) and Day 10 (p < 0.05). After the exercises, the intervention had higher blood glucose levels (p < 0.05) and lower blood levels of triglycerides in the last sessions (p < 0.01). |
| Carr et al. (2018) | Non-randomized parallel group study | N = 28 (17 men and 7 women) Control: 8 Intervention A: 9 Intervention B: 7 Losses: 4 | All groups followed an intensive supervised training session every day for 3 weeks. Control had a high-carb diet. Intervention A had a low-carb and high-fat diet. Intervention B had a diet of periodic carbohydrates. | Walking economy. VO2 peak tested on treadmill. Net endogenous acid production (NEAP). Blood samples. | Within each of the three groups, there was a significant increase in VO2max compared with baseline (p < 0.05). Blood pH: At 4 min and 6 min post-exercise there were no significant difference between pH of HCHO and LCHF (95% CI = (−0.10; 0.02) and (−0.08; 0.04), respectively. Bicarbonate: there were no significant differences in post-training intervention blood [HCO3-] between groups (F(1,161.15) = 0.14; p = 0.71). Lactate: Post-intervention, there were no significant differences between groups (F(14,311.07) = 1.28; p = 0.22). Post-intervention, NEAP was significantly higher in LCHF compared with HCHO control (95% CI = (10.44; 36.04)) but there was no difference for PCHO compared with HCHO (95% CI = (−19.06; 7.23)). |
| Cherif et al. (2016) | Counterbalanced test Quasi-experimental study Uncontrolled trial | N = 21 men Age: 29.8 ± 5.9 Intervention: 21 Losses: 0 | In both sessions, endurance tests were carried out based on sprint repetitions. Control session: they followed a normal diet. Intervention session: fasted 14 h for 3 days in a row. After 7 days of intervention, the diets are exchanged between groups. | Biomechanical and biochemical markers measured by blood samples and body mass index. | Speed sprint and vertical stiffness declined in the fasting session compared to the control session (p = 0.030). During the two first sprints of the second repeated sprints set, sprint speed significantly decreased in the fasting session (p = 0.003) compared to the control session (p = 0.048), while horizontal power declined in the fasting session compared to the control session only in sixth sprint (p = 0.017). Triglyceride levels improved during fasting (p = 0.037). Total cholesterol (p = 0.581) and LDL-c (p = 0.835) levels did not vary in this session. HDL-C was higher in fasting session compared to control session at post-exercise (p = 0.039). |
| Chowdhury, et al. (2018) | RCT | N = 31 (12 men and 19 women) Control: 15 (6 men and 9 women) Age: 36 ± 12 Intervention: 16 (6 men and 10 women) Age: 35 ± 10 Losses: 0 | For 6 weeks: Control group followed a diet which consisted of ingesting ≥ 700 kcal, during the first 2 h after waking up, of the 1100 kcal daily maximum Intervention group remained in fasted state until midday. Their total daily intake was 1200 kcal. | Body mass Resting metabolic rate Appetite Score | There were no differences between the levels of energy regulation, glycerin, peptides and appetite. Glucagon concentrations were lower in the intervention group compared to control group, where it went up (p = 0.06). |
| Dethlefsen et al. (2018) | Quasi-experimental study Uncontrolled trial | N = 17 men Trained: 6 Age: 27 ± 4 Untrained: 7 Age: 28 ± 3 Losses: 4 | Both groups fasted for 36 h and samples were taken 2, 12, 24 and 36 h after the last meal. The trained group had a VO2max above 55 mL·min−1·kg−1. The untrained group had a VO2max below 45 mL·min−1·kg−1. | Amino acid quantification. Lysing of muscle tissue. Polyacrylamide gel on electrophoresis with sodium dodecylsulfate followed by Western blotting. Blood samples. | Phosphorylation and protein levels of several proteins related to autophagy were higher in the trained group (p < 0.05). Skeletal muscle LC3I protein content was ~30% lower (p < 0.05) at 12, 24, and 36 h than at 2 h after the meal in untrained subjects. The LC3II protein content in skeletal muscle was ~20% lower (p < 0.05) at 12 and 24 h and tended to be lower (p = 0.059) at 36 h than at 2 h after the meal in untrained subjects. The p62 protein content in skeletal muscle was ~60% lower (p < 0.05) at 24 h than 2 h after the meal in untrained subjects, whereas fasting had no effect on p62 protein content in trained subjects. |
| Edinburgh et al. (2019) | RCT | N = 12 men Age: 23 ± 3 Intervention: 12 Losses: 0 1.1.1 | Subjects followed 3 different programs for a 24 h period with 1 week of washout between them: Breakfast followed by rest: breakfast was eaten and no type of exercise was performed. Breakfast before exercise: breakfast was eaten and 60 min of cycling at 50% peak power output was performed. Fasting before exercise: breakfast was skipped and 60 min of cycling at 50% of peak power output was performed. | Blood samples Expired gas samples Energy expenditure Energy intake | Participants who fasted before exercise strategy consumed less calories compared to the other strategies (p < 0.01). Energy expenditure was only significatively lower when breakfast was followed by rest (p < 0.01). There was a significant usage of plasmatic glucose with the fasting before exercise strategy (p = 0.03). |
| Gasmi et al. (2017) | RCT | N = 40 men Control: 20 Young men: 10 Age: 24.90 ± 1.10 Aged men: 53.90 ± 4.09 Intervention: 20 Young men: 10 Age: 26.90 ± 1.97 Aged men: 10 Age: 51.60 ± 5.87 Losses: 0 | During the months February, March and April: Intervention group followed 12 h of fasting 2 days a week with a rest stage of 48 h (Monday and Thursday) Control group kept their usual diet. | Energy (kcal/day) Fat Wmax Red cells Hemoglobin Running-based anaerobic sprint test | There was no significant difference in muscular strength in any group. Concentration levels of hemoglobin, red cells and white cells were significantly higher in young participants (p > 0.05). There was no improvement in levels of CD3, CD4+, and CD8+ (p > 0.05). |
| Grant et al. (2017) | RCT | N = 28 men Control: 8 Age: 22.0 ± 2.4 Intervention: 10 Age: 22.9 ± 4.1 Losses: 10 | Both groups performed a resistance training program 3 days/week for 8 weeks. Control continued with his usual diet. Intervention could only eat in a period of 4 h a day 4 times a week without limitation of quantity. | Body composition. Muscle performance. | The values of body composition were lean soft tissue (p = 0.30), fat mass (p = 0.14) and body fat (p = 0.37). Muscular performance increased in both groups, but the intervention group showed greater improvements in lower body strength and upper and lower body endurance. The exercises that evaluated the muscular performance were bench press 1-RM (p = 0.35), bench press endurance (p = 0.17), hip sled 1-RM (p = 0.07) and hip sled endurance (p = 0.97). There was greater increase in lean soft tissue in the control group of 2.3 Kg on average, as opposed to −0.2 Kg in intervention group, but it is not a significative difference. |
| Grant et al. (2019) | RCT | N = 40 women Control: 14 Age: 22.0 ± 2.4 Intervention A: 13 Age: 22.1 ± 2.1 Intervention B:13 Age:22.3 ± 3.4 Losses: 16 | All groups completed an endurance training program for 8 weeks. Control followed a normal diet. Intervention A: could consume calories between 12 am and 8 pm hours. Intervention B: could consume calories between 12 am and 8 pm in addition to consuming β-hydroxy β-methyl butyrate supplements. | Lean mass and fat rates. Body composition. Muscle performance. Resting metabolic rate and use of substrates. Brachial blood pressure. Blood and saliva samples. | Fat rate: statistically significant changes in favor of intervention (p = 0.12). Muscular performance increased in all groups with no significant differences between them. The analyzed values were resistance training volume (p > 0.05), muscular performance (p > 0.05), rate of force development (p > 0.05) vertical jump performance (p > 0.05), physical activity energy expenditure (p = 0.034), sedentary time (p = 0.048), moderate- or vigorous-intensity physical activity (p > 0.05), steps per day (p > 0.05). There are no significant differences in metabolic and physiological characteristics. The analyzed values were Urinary HMB concentrations (p < 0.01), body composition (p > 0.05), resting metabolism (p > 0.05), blood variables (p > 0.05), vascular assessments (p > 0.05) and cortisol awakening response (p > 0.05). |
| Gueldich et al. (2019) | Quasi-experimental study Uncontrolled trial | N = 10 men Age: 22.06 ± 1.98 Intervention: 10 Losses: 0 | The intervention fasted for a month following the tradition of Ramadan. The group performed 3 repetitions of a maximum voluntary isometric contraction of knee extension with electrostimulation during 4 phases: one week before Ramadan, at the end of the first week, during the fourth week and two weeks after its completion | Voluntary activation level (VAL). Electromyographic signals. Contraction potential at rest. Depression, anxiety and fatigue: Profile of Mood States questionnaire in French version (POMS-f). Maximum voluntary isometric contraction (MVIC) values. 1.1.2 | VAL (p < 0.05) and MVIC (p < 0.001) values decreased during the first week of Ramadan. Neuromuscular efficiency (p = 0.15) and the potential of contraction at rest (p = 0.07) remained stable throughout the month. The values of the POMS-f were higher during the first week. Significant difference in comparison with before Ramadan and the other measured days. p < 0.005 in week 1, p < 0.01 in week 4 and p < 0.001 after Ramadan. |
| Kysel et al. (2020) | RCT | N = 25 men Control: 12 Age: 24 ± 4 Intervention: 13 Age: 23 ± 5 Losses: 0 | During an 8-weekperiod, both groups followed a programmed diet with strength and resistance training. Muscles from chest, back and legs were trained in 3 sessions on 3 different days. Resistance training consisted of a 30 min run at constant heart rate at 70% of maximal heart rate. In addition, the total energy intake of each participant was calculated and reduced by 500kcal per day. Cyclical ketogenic reduction diet: first 5 days of the week the carbohydrate consumption was reduced to 30 g per day and on the last 2 days it was increased to 8–10 g per kg of non-fat tissue. Nutritionally balanced reduction diet: only the 500 kcal per day restriction was included. | Body composition Muscle strength Endurance performance Peak workload Peak oxygen uptake | Both groups decreased body weight, body fat mass and body mass index. Lean body mass and body water content were significantly reduced by the intervention, while they were not influenced in control group (p < 0.05). Intervention did not produce a significant improvement in muscle strength, while control group was able to improve it (p < 0.05). Respiratory exchange ratio decreased in subjects on intervention while it did not vary in control group (p < 0.05). Spiroergometric results were higher in control group (p < 0.05). |
| McAllister et al. (2019) | Pilot study Randomized and controlled | N = 26 men Age: 22 ± 2.5 Control: 12 Intervention: 10 Losses: 4 | Both groups could only eat in a period of 8 h a day for 28 days. The control (ab libitum) could consume as much as they wanted in the set time interval. The intervention (isocaloric) should be kept 300 kcal/day below a normal daily intake. | Blood pressure. Body composition. Blood samples. Hunger, satiety, concentration, mood, energy, alertness and focus: visual analogue scale. | In both groups, body fat and blood pressure decreased (p < 0.05). In both groups there were increases in HDL-c and adiponectin (p < 0.05). |
| Moro et al. (2016) | RCT | N = 34 men Age: 29.21 ± 3.8 Control: 17 Intervention: 17 Losses: 0 | Both trained for 8 weeks (3 sessions per week) in a resistance training program. Control: ate at 8 am, 1 pm and 8 pm. Intervention: ate at 1 pm, 4 pm and 8 pm. | Height and weight. Fat and lean mass rates. Muscle areas Aspirated oxygen and expelled carbon dioxide. Blood samples. Upper and lower limb strength. | Greater decrease in fat (p = 0.0448) and maintenance of lean mass in favor of intervention group. Increased strength in both groups without a significant difference between them ( p > 0.05). Insulin growth factor levels type 1 (p = 0.0397) and testosterone (p = 0.0476) decreased in intervention group. Decreased respiratory rate in intervention group (p = 0.0421). Decreased glucose (p = 0.0011) and insulin levels (0.0303) in intervention group. Due to that, there were an improvement in the homeostatic model assessment in intervention group. |
| Moro et al. (2020) | RCT | N = 16 men Control: 8 Age: 19.38 ± 1.60 Intervention: 8 Age: 19.38 ± 2.39 Losses: 0 | Both groups cycled 500 ± 50 km a week divided into 6 weekly cycling sessions for 4 weeks. Controls (ND) consumed a complete diet divided into 3 meals between 7 am and 9 pm. Intervention (ERT) consumed a complete diet at an interval of 8 h (10 am to 6 pm). | Body composition. Resting metabolic rate. Peak power output (PPO). Blood samples. | Reduction inbody fat in favor of the intervention group (p = 0.01). The resting metabolic rate has no significant interaction between groups (p > 0.05). There is no significant difference between groups in sports performance (p > 0.05). The intervention shows a decrease in inflammatory markers (p < 0.005). Leucocytes decreased in both groups (p = 0.001), but the difference between baseline and final values was only significant for the control group. |
| Shaw et al. (2020) | Cross design Uncontrolled trial | N = 10 men Intervention: 8 Age: 29.6 ± 5.1 Losses: 2 | For 31 days, participants were on a certain diet with a week of interspersed rest. The intervention with ketogenic diet consumed <50 g/day of carbohydrates. Diet was composed of 15–20% proteins and 80–85% fats. The intervention with his usual diet did not vary their consumption. | Blood and saliva samples. Incremental exercise test to exhaustion: days 1 and 31 ran to exhaustion at 70% of their VO2 peak. | Diets had no significant effects on VO2 peak or exercise tests until exhaustion (p > 0.05). The ketogenic diet can alter the pro- and anti-inflammatory immune response of cytokines (p < 0.05) and the segregation of immunoglobulin A (p < 0.001). |
| Sjödin et al. (2020) | RCT | N = 24 women Age:18–30 Control: 8 Intervention: 9 Losses: 7 | During a 4-week period, each group followed their programmed diet. After the first intervention was complete, a washout period of 15 weeks took place. The groups then interchanged their diets for another 4 weeks. Control group followed a program diet from the National Food Agency. Intervention group followed a ketogenic diet. | VO2 max Lactate Handgrip time to fatigue and strength Graded Incremental Ergometer Cycling Test | The first results did not show a significant change in strength and time to fatigue in the intervention group. Time to fatigue in cycling test was reduced by 2 min in intervention group (p < 0.001). Participants experienced an improvement in muscular fatigue during the exercise days. Ketogenic diet had an unfavorable effect on muscle fatigue and might affect perceived exertion during daily life activities. |
| Terada et al. (2019) | RCT | N = 25 men Control: 9 Age: 34.0 ± 8.2 Intervention: 11 Age: 33.3 ± 7.2 Losses: 5 | Both groups trained 4 weeks (3 sessions/week). Aerobic cycling of maximum intensity. Control: they consumed exogenous carbohydrates. Intervention: night fasting. | High intensity aerobic endurance: T85%. Aerobic capacity: (VO2 peak). Mechanical work.Peak power output (PPO). Fatigue rate. | Mean of T85% was longer in the intervention (p = 0.038). Aerobic capacity did not vary between groups (p > 0.05). Mechanical work (p = 0.010) and Peak Power Output (p = 0.021) were lower in the intervention group, but still can have a greater impact on the ability to sustain high-intensity aerobic endurance exercise compared to control group. Fatigue rate did not vary between groups (p > 0.05). |
| Vargas et al. (2018) | RCT | N = 26 men Age: 30 ± 4.5 Control: 5 Intervention A: 9 Intervention B: 10 Losses: 2 | Participants were organized into 3 groups to participate in the 8-week-long study: Control group: they did not follow any programmed diet or training during the study. Intervention A: followed a ketogenic diet Intervention B: followed a non-ketogenic diet The exercise training consisted of 4 sessions per week (1 session/day) and 3 days of rest. Upper and lower limbs were trained separately in 2 sessions for each one. | Body composition | There was significant reduction in fat mass and visceral adipose tissue in the ketogenic diet (p < 0.05). Total body weight and muscle mass were significantly higher in the non-ketogenic group compared to the ketogenic group, which stayed neutral (p < 0.05). |
| Vidic et al. (2021) | RCT | N = 20 men Age: 42.7 ± 1.5 Control: 9 Intervention: 9 Losses: 2 | Subjects participated for 4 weeks in a familiarization program before starting the intervention. Participants were organized into 2 groups: Control group: a non-ketogenic diet was followed. Intervention group: a ketogenic diet was followed. Both groups carried out 4 strength training sessions per week for8 weeks. Upper and lower limbs strength were trained every week. | Bloodsamples Body composition Maximal strength | Both groups lost a similar amount of lean body mass and fat mass (p = 0.001) but preserved their maximal upper and lower limbs strength (p > 0.05). Basal and free testosterone increased in both groups (p < 0.01). Insulin levels decreased significantly in both groups (p < 0.01). |
| Zajac et al. (2014) | Cross design Uncontrolled study | N = 8 men Age: 28.3 ± 3.9 Intervention A: 4 Intervention B: 4 Losses: 0 | Both groups performed moderate and intense cycling exercises for 3 days preceded by 4 weeks of carrying out the assigned diet. Intervention A had a mixed diet. Intervention B had a low-carb ketogenic diet. After a month with the assigned diet and a week of rest, the groups interchange their diets. | Biochemical analysis. VO2 peak. Body composition. | High volume training in a ketogenic diet increases fat metabolism during exercise, observable by low triglyceride levels during max effort training and resting (p = 0.001). The ketogenic diet reduces body mass (p = 0.011), fat content (p = 0.001) and post-exercise muscle damage, which was observed by lower rest and exercise plasma creatine kinase and lactate dehydrogenase. The ketogenic diet reduces physical performance at high intensities evidenced by a lower lactate concentration (p = 0.001). |
| Zinn et al. (2017) | Pilot study Uncontrolled or randomized study | N = 5 (4 women and 1 man) Intervention: 5 Age: 49 to 55 Losses: 0 | The group continued with their usual training and also performed endurance events for 10 weeks. The intervention consumed <50 g of carbohydrates per day, 1.5 g of protein per kilo and freedom to consume the desired amount of fat. | Sports performance test. Body composition. | Decreased sports performance, visible by a decrease in time to exhaustion (p = 0.004). Reduction in body fat, measured by skin folds (p = 0.001). Improvements in the well-being of the subjects (any p-value was calculated). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Montilla, J.J.; Cuevas-Cervera, M.; Gonzalez-Muñoz, A.; Garcia-Rios, M.C.; Navarro-Ledesma, S. Efficacy of Nutritional Strategies on the Improvement of the Performance and Health of the Athlete: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4240. https://doi.org/10.3390/ijerph19074240
Perez-Montilla JJ, Cuevas-Cervera M, Gonzalez-Muñoz A, Garcia-Rios MC, Navarro-Ledesma S. Efficacy of Nutritional Strategies on the Improvement of the Performance and Health of the Athlete: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(7):4240. https://doi.org/10.3390/ijerph19074240
Chicago/Turabian StylePerez-Montilla, J. Javier, Maria Cuevas-Cervera, Ana Gonzalez-Muñoz, Maria Carmen Garcia-Rios, and Santiago Navarro-Ledesma. 2022. "Efficacy of Nutritional Strategies on the Improvement of the Performance and Health of the Athlete: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 7: 4240. https://doi.org/10.3390/ijerph19074240
APA StylePerez-Montilla, J. J., Cuevas-Cervera, M., Gonzalez-Muñoz, A., Garcia-Rios, M. C., & Navarro-Ledesma, S. (2022). Efficacy of Nutritional Strategies on the Improvement of the Performance and Health of the Athlete: A Systematic Review. International Journal of Environmental Research and Public Health, 19(7), 4240. https://doi.org/10.3390/ijerph19074240







