A Meta-Analysis of the Effectiveness of Telemedicine in Glycemic Management among Patients with Type 2 Diabetes in Primary Care
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Literature Screening and Data Extraction
2.4. Methodological Quality Evaluation
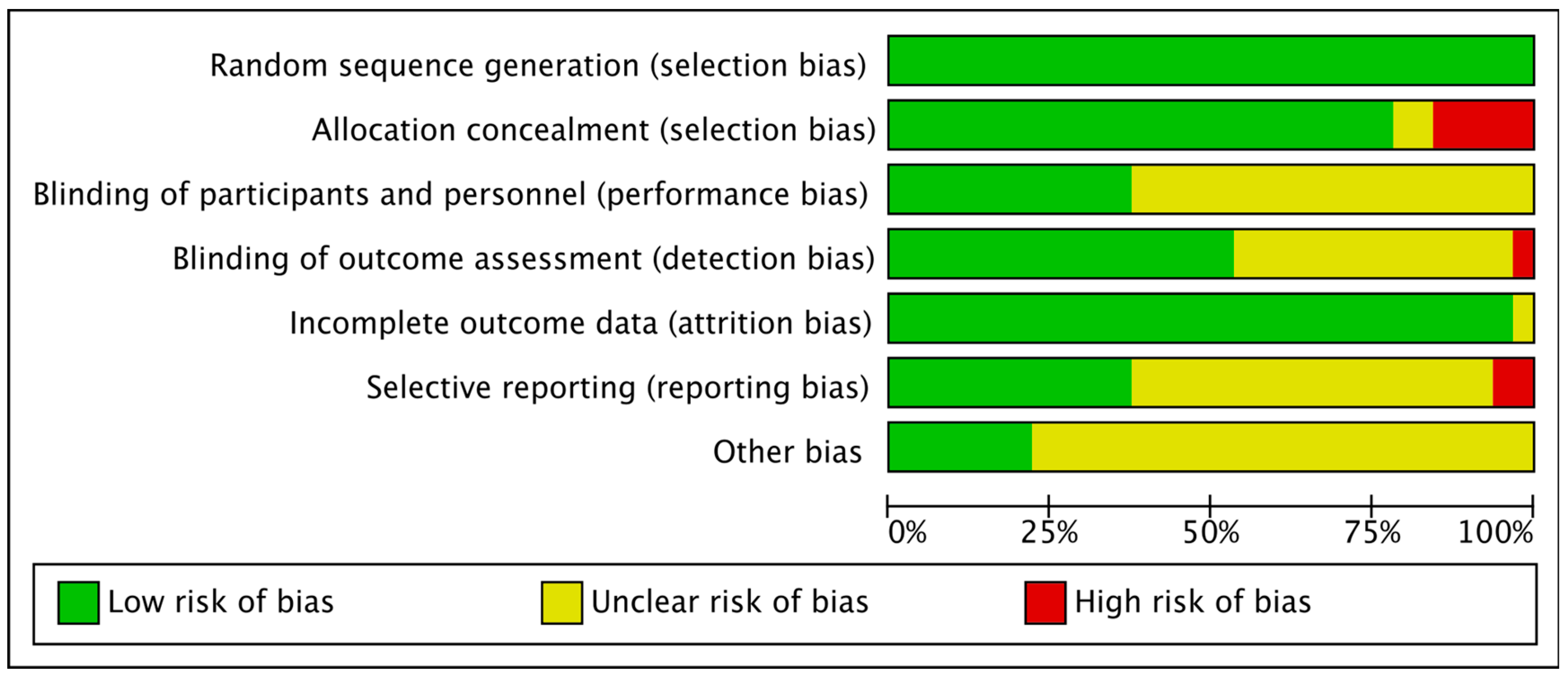
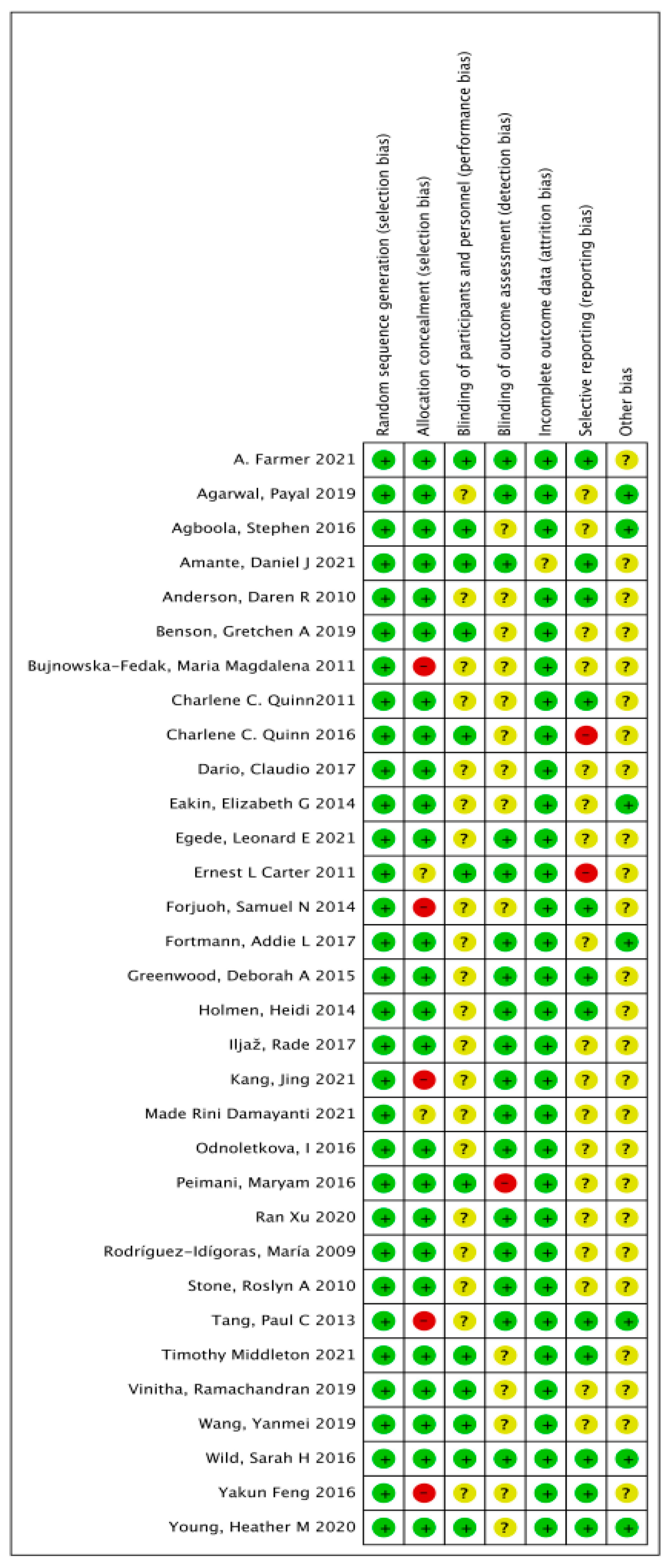
2.5. Risk Bias Evaluation
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Literature Quality Evaluation and Risk Bias
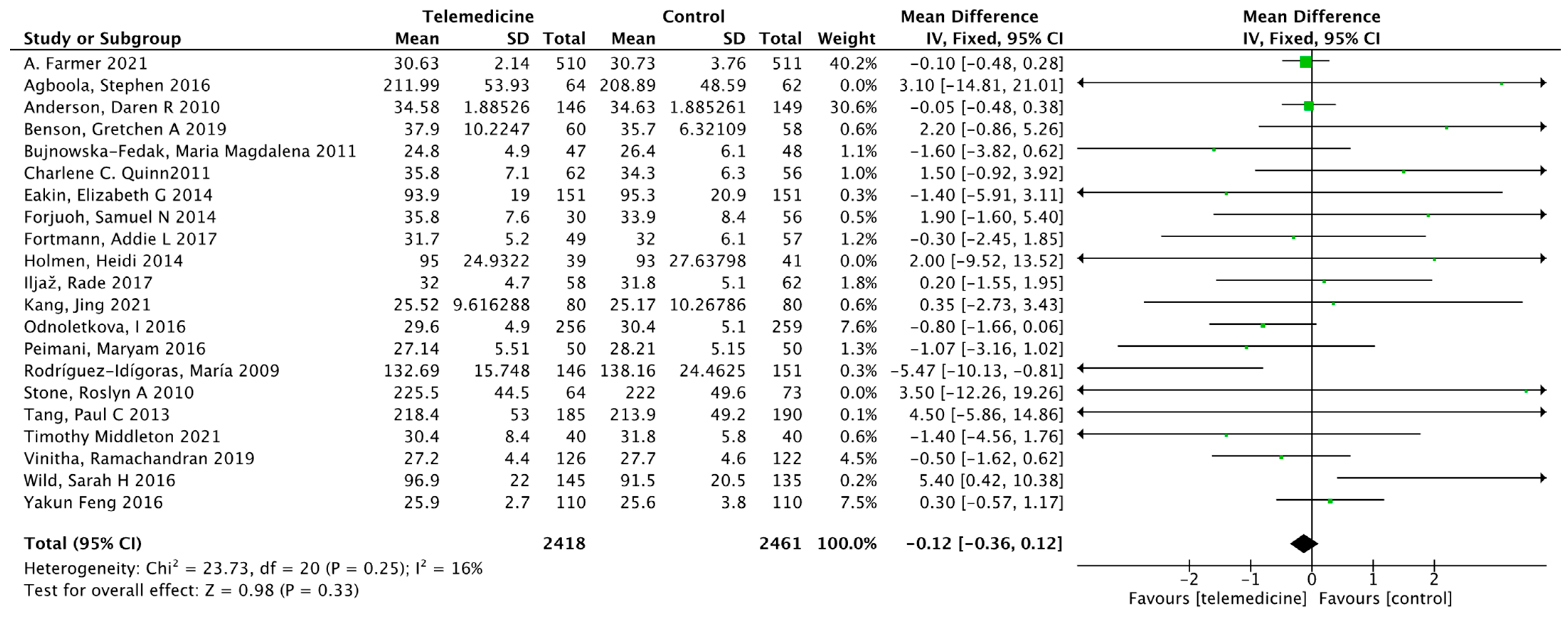
3.3. Results of the Meta-Analysis
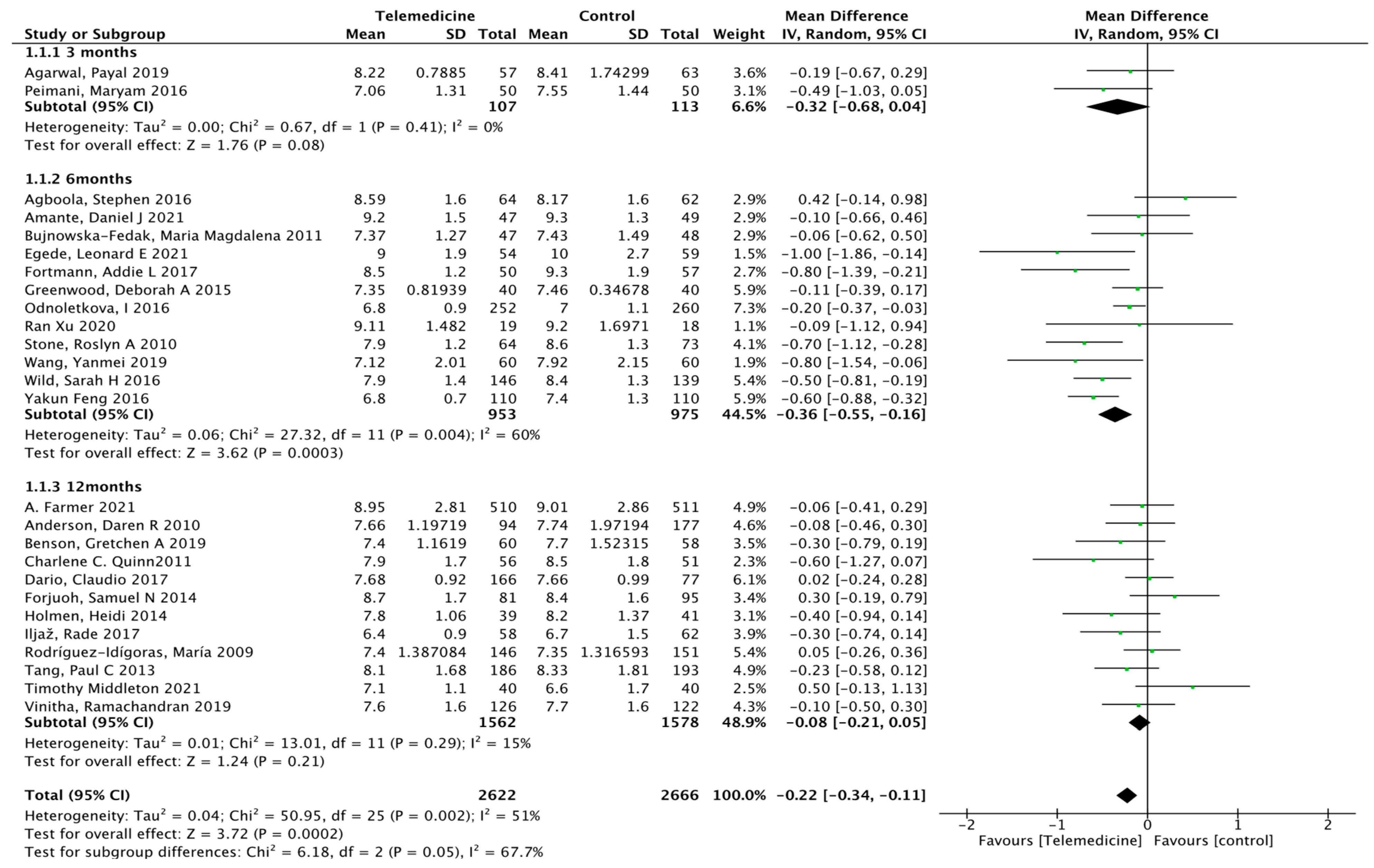
3.3.1. Primary Results (Effect on HbA1c)
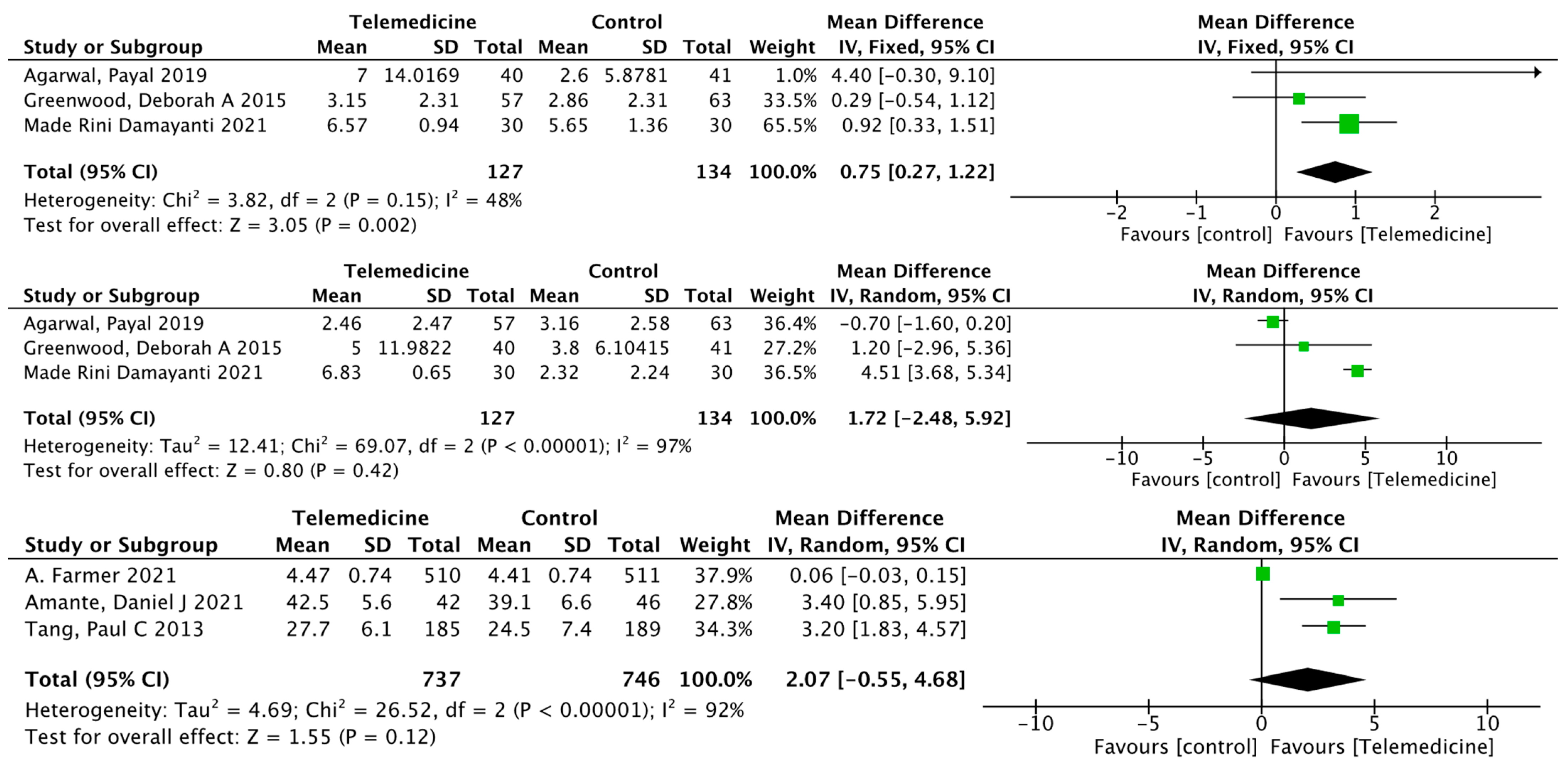
3.3.2. Secondary Results
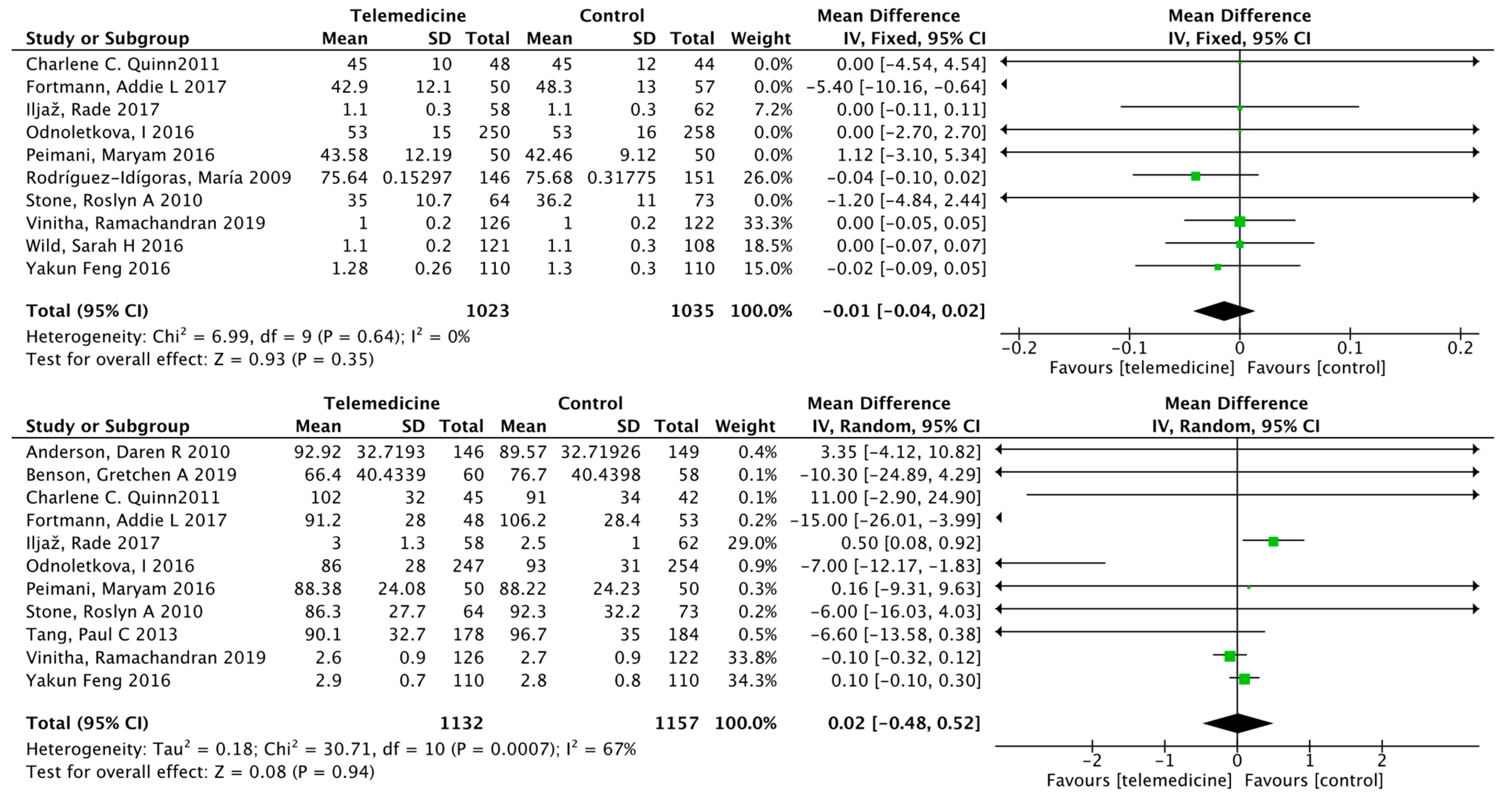
Impact on Fasting Blood Glucose
Effects on Postprandial Blood Glucose
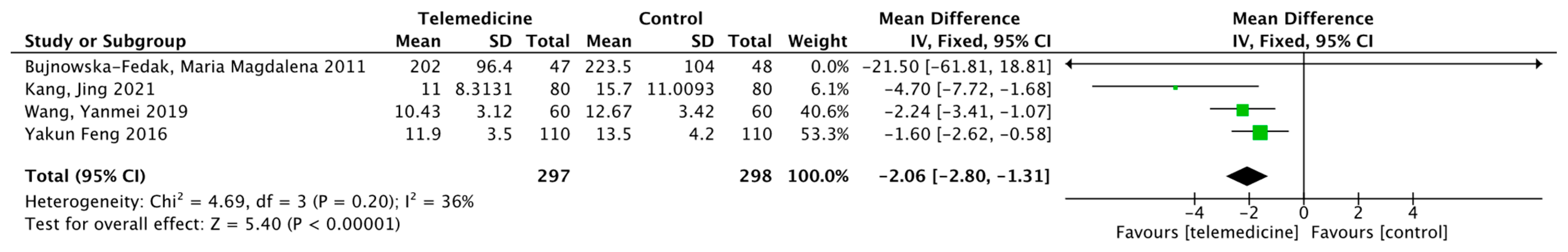
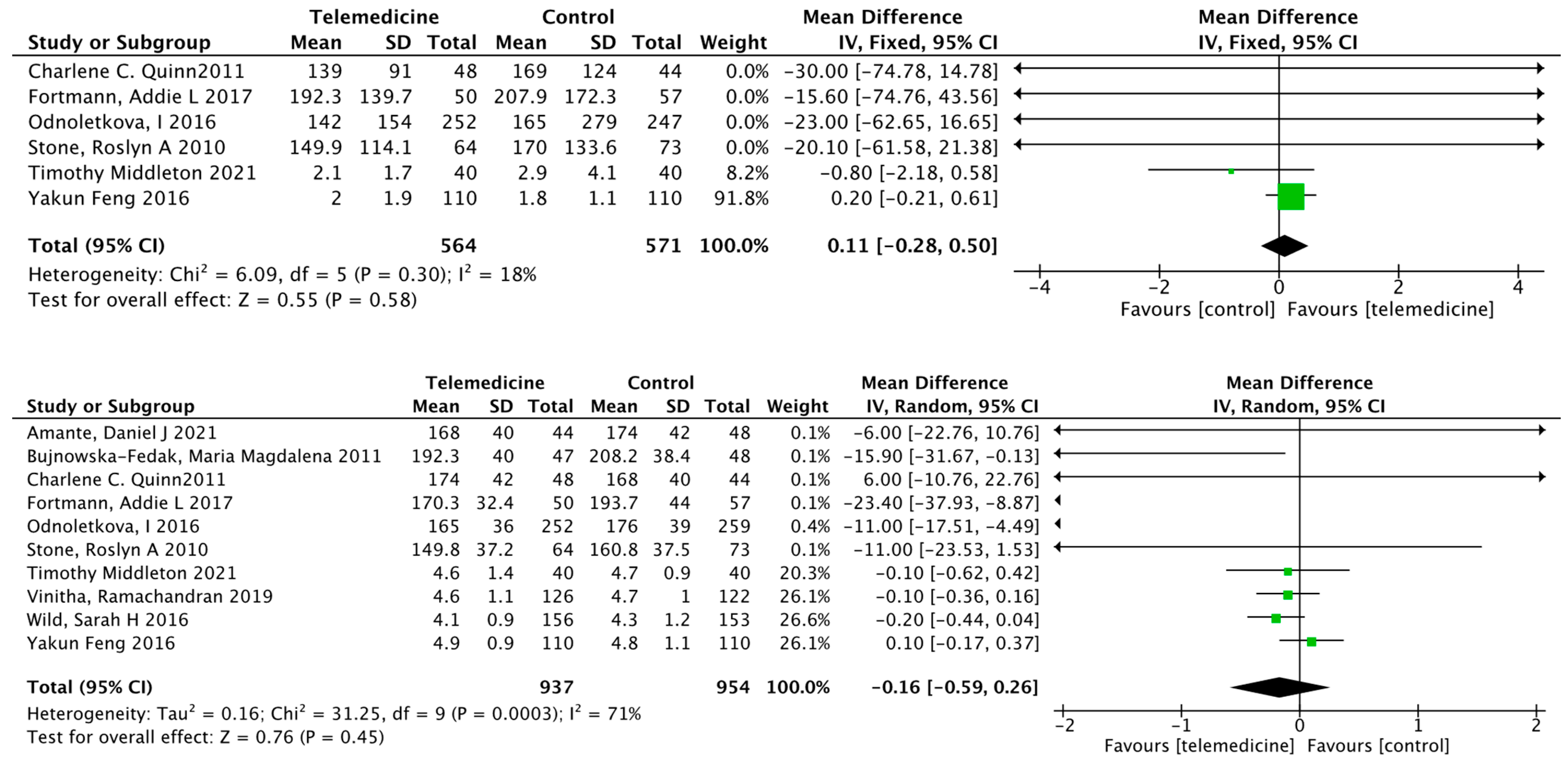
Effect on Body Weight
Effect on Systolic Blood Pressure
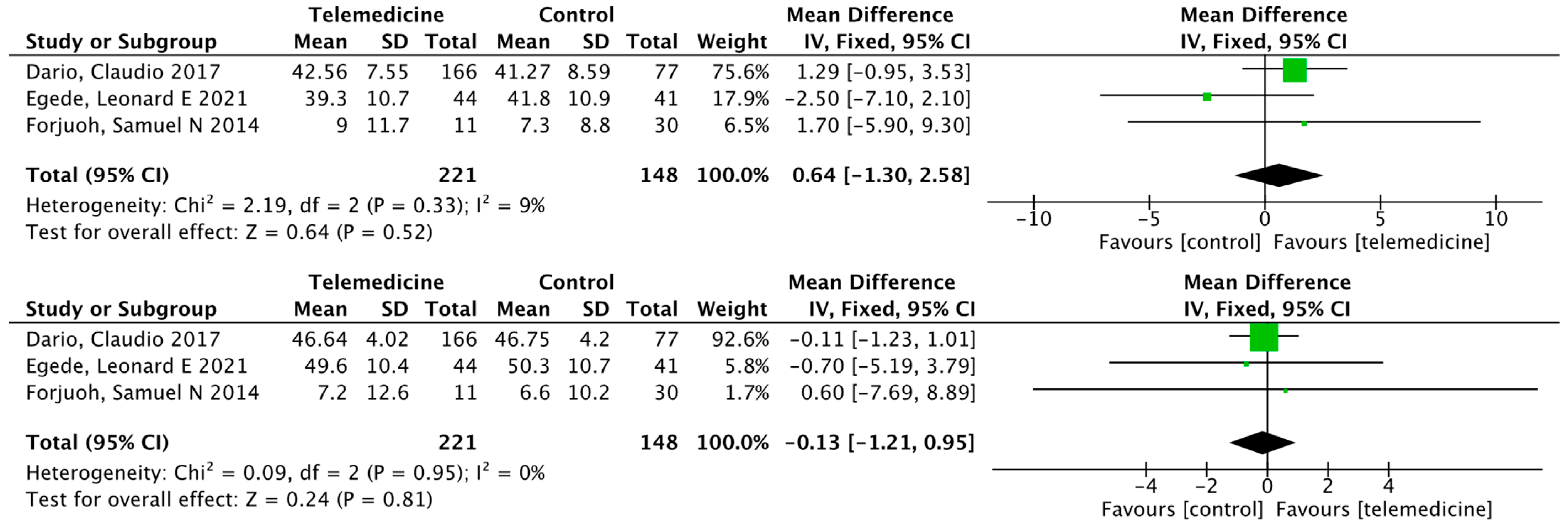
Effects on High-Density Lipoprotein, Low-Density Lipoprotein, Triglycerides, and Total Cholesterol
Effects on Self-Efficacy, Diabetes Knowledge, Physical Health, and Mental Health
Effects on Exercise, Foot Care, and Satisfaction
4. Discussion
4.1. Blood Sugar Control
4.2. Self-Management
4.3. Telemedicine
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9 edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hincapié, M.A.; Gallego, J.C.; Gempeler, A.; Piñeros, J.A.; Nasner, D.; Escobar, M.F. Implementation and Usefulness of Telemedicine During the COVID-19 Pandemic: A Scoping Review. J. Prim. Care Community Health 2020, 11, 2150132720980612. [Google Scholar] [CrossRef]
- Sood, S.; Mbarika, V.; Jugoo, S.; Dookhy, R.; Doarn, C.R.; Prakash, N.; Merrell, R.C. What is telemedicine? A collection of 104 peer-reviewed perspectives and theoretical underpinnings. Telemed. J. e-Health 2007, 13, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gupta, R.; Misra, A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: Guidelines for Physicians. Diabetes Metab. Syndr. 2020, 14, 273–276. [Google Scholar] [CrossRef]
- Shan, R.; Sarkar, S.; Martin, S.S. Digital health technology and mobile devices for the management of diabetes mellitus: State of the art. Diabetologia 2019, 62, 877–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef]
- Seidu, S.; Cos, X.; Brunton, S.; Harris, S.B.; Jansson, S.P.O.; Mata-Cases, M.; Neijens, A.M.J.; Topsever, P.; Khunti, K. A disease state approach to the pharmacological management of Type 2 diabetes in primary care: A position statement by Primary Care Diabetes Europe. Prim. Care Diabetes 2021, 15, 31–51. [Google Scholar] [CrossRef]
- Caro-Bautista, J.; Kaknani-Uttumchandani, S.; Garcia-Mayor, S.; Villa-Estrada, F.; Morilla-Herrera, J.C.; Leon-Campos, A.; Gomez-Gonzalez, A.J.; Morales-Asencio, J.M. Impact of self-care programmes in type 2 diabetes mellitus population in primary health care: Systematic review and meta-analysis. J. Clin. Nurs. 2020, 29, 1457–1476. [Google Scholar] [CrossRef] [PubMed]
- The King’s Fund. The NHS Long-Term Plan Explained [EB/OL]. 23 January 2019. Available online: https://www.kingsfund.org.uk/publications/nhs-long-term-plan-explained?utm_source=TheKing%27sFundnewsletters%28mainaccount%29&utm_medium=email&utm_campaign=10222788_MKPUB_NHSlong-termplanlongread24012019&utm_content=btnbel (accessed on 1 February 2022).
- Strive to Write a Healthy Chapter in Building a Socialist Modern Country in an All-Round Way [EB/OL]. 28 June 2021. Available online: http://www.nhc.gov.cn/wjw/mtbd/202106/3950e847c71f4d0c88bac4cc5b21ab8a.shtml (accessed on 1 February 2022).
- So, C.F.; Chung, J.W. Telehealth for diabetes self-management in primary healthcare: A systematic review and meta-analysis. J. Telemed. Telecare 2018, 24, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Olyaeemanesh, A.; Mobinizadeh, M.; Nasli-Esfahani, E.; Riazi, H. Assessment of remote patient monitoring (RPM) systems for patients with type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2020, 19, 115–127. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Wang, Z.; Wang, X.; Wang, Y.; Yu, X.; Liu, L. Self-management among type 2 diabetes patients via the WeChat application: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.H.; Chan, C.K.Y.; Chua, S.S.; Chaiyakunapruk, N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: A systematic review and network meta-analysis. Sci. Rep. 2017, 7, 12680. [Google Scholar] [CrossRef] [PubMed]
- Udsen, F.W.; Hangaard, S.; Bender, C.; Andersen, J.; Kronborg, T.; Vestergaard, P.; Hejlesen, O.; Laursen, S. The Effectiveness of Telemedicine Solutions in Type 1 Diabetes Management: A Systematic Review and Meta-analysis. J. Diabetes Sci. Technol. 2022, 19322968221076874. [Google Scholar] [CrossRef] [PubMed]
- Hangaard, S.; Laursen, S.H.; Andersen, J.D.; Kronborg, T.; Vestergaard, P.; Hejlesen, O.; Udsen, F.W. The Effectiveness of Telemedicine Solutions for the Management of Type 2 Diabetes: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Diabetes Sci. Technol. 2021, 19322968211064633. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- American Telemedicine Association (ATA). Telehealth: Defining 21st Century Care. Telehealth Basics—ATA. 2019. 19 January 2021. Available online: https://www.americantelemed.org/resource/why-telemedicine/ (accessed on 2 February 2022).
- Critical Appraisal Skills Program (CASP). CASP Checklists from Oxford. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 20 September 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Agboola, S.; Jethwani, K.; Lopez, L.; Searl, M.; O’Keefe, S.; Kvedar, J. Text to Move: A Randomized Controlled Trial of a Text-Messaging Program to Improve Physical Activity Behaviors in Patients With Type 2 Diabetes Mellitus. J. Med. Internet Res. 2016, 18, e307. [Google Scholar] [CrossRef]
- Dario, C.; Toffanin, R.; Calcaterra, F.; Saccavini, C.; Stafylas, P.; Mancin, S.; Vio, E. Telemonitoring of Type 2 Diabetes Mellitus in Italy. Telemed. J. e-Health 2017, 23, 143–152. [Google Scholar] [CrossRef]
- Rodríguez-Idígoras, M.I.; Sepúlveda-Muñoz, J.; Sánchez-Garrido-Escudero, R.; Martínez-González, J.L.; Escolar-Castelló, J.L.; Paniagua-Gómez, I.M.; Bernal-López, R.; Fuentes-Simón, M.V.; Garófano-Serrano, D. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol. Ther. 2009, 11, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Egede, L.E.; Dawson, A.Z.; Walker, R.J.; Garraci, E.; Knapp, R.G. Randomized controlled trial of technology-assisted case management in low-income adults with type 2 diabetes: Effect on quality of life and blood pressure. J. Telemed. Telecare 2021, 1357633X211028491. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.L.; Nunlee-Bland, G.; Callender, C. A patient-centric, provider-assisted diabetes telehealth self-management intervention for urban minorities. Perspect. Health Inf. Manag. 2011, 8, 1b. [Google Scholar]
- Greenwood, D.A.; Blozis, S.A.; Young, H.M.; Nesbitt, T.S.; Quinn, C.C. Overcoming Clinical Inertia: A Randomized Clinical Trial of a Telehealth Remote Monitoring Intervention Using Paired Glucose Testing in Adults With Type 2 Diabetes. J. Med. Internet Res. 2015, 17, e178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odnoletkova, I.; Goderis, G.; Nobels, F.; Fieuws, S.; Aertgeerts, B.; Annemans, L.; Ramaekers, D. Optimizing diabetes control in people with Type 2 diabetes through nurse-led telecoaching. Diabet. Med. 2016, 33, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Overhage, J.M.; Chan, A.S.; Brown, N.L.; Aghighi, B.; Entwistle, M.P.; Hui, S.L.; Hyde, S.M.; Klieman, L.H.; Mitchell, C.J.; et al. Online disease management of diabetes: Engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J. Am. Med. Inform. Assoc. 2013, 20, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Young, H.M.; Miyamoto, S.; Dharmar, M.; Tang-Feldman, Y. Nurse Coaching and Mobile Health Compared With Usual Care to Improve Diabetes Self-Efficacy for Persons With Type 2 Diabetes: Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e16665. [Google Scholar] [CrossRef] [Green Version]
- Holmen, H.; Torbjørnsen, A.; Wahl, A.K.; Jenum, A.K.; Småstuen, M.C.; Arsand, E.; Ribu, L. A Mobile Health Intervention for Self-Management and Lifestyle Change for Persons With Type 2 Diabetes, Part 2: One-Year Results From the Norwegian Randomized Controlled Trial RENEWING HEALTH. JMIR mHealth uHealth 2014, 2, e57. [Google Scholar] [CrossRef]
- Quinn, C.C.; Shardell, M.D.; Terrin, M.L.; Barr, E.A.; Park, D.; Shaikh, F.; Guralnik, J.M.; Gruber-Baldini, A.L. Mobile Diabetes Intervention for Glycemic Control in 45-to 64-Year-Old Persons With Type 2 Diabetes. J. Appl. Gerontol. 2016, 35, 227–243. [Google Scholar] [CrossRef]
- Agarwal, P.; Mukerji, G.; Desveaux, L.; Ivers, N.M.; Bhattacharyya, O.; Hensel, J.M.; Shaw, J.; Bouck, Z.; Jamieson, T.; Onabajo, N.; et al. Mobile App for Improved Self-Management of Type 2 Diabetes: Multicenter Pragmatic Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e10321. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.R.; Christison-Lagay, J.; Villagra, V.; Liu, H.; Dziura, J. Managing the space between visits: A randomized trial of disease management for diabetes in a community health center. J. Gen. Intern. Med. 2010, 25, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Eakin, E.G.; Winkler, E.A.; Dunstan, D.W.; Healy, G.N.; Owen, N.; Marshall, A.M.; Graves, N.; Reeves, M.M. Living well with diabetes: 24-month outcomes from a randomized trial of telephone-delivered weight loss and physical activity intervention to improve glycemic control. Diabetes Care 2014, 37, 2177–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Xing, M.; Javaherian, K.; Peters, R.; Ross, W.; Bernal-Mizrachi, C. Improving HbA(1c) with Glucose Self-Monitoring in Diabetic Patients with EpxDiabetes, a Phone Call and Text Message-Based Telemedicine Platform: A Randomized Controlled Trial. Telemed. J. e-Health 2020, 26, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Bujnowska-Fedak, M.M.; Puchała, E.; Steciwko, A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed. J. e-Health 2011, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Benson, G.A.; Sidebottom, A.; Hayes, J.; Miedema, M.D.; Boucher, J.; Vacquier, M.; Sillah, A.; Gamam, S.; VanWormer, J.J. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) Telemedicine Randomized Controlled Trial on Diabetes Optimal Care Outcomes in Patients with Type 2 Diabetes. J. Acad. Nutr. Diet. 2019, 119, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Amante, D.J.; Harlan, D.M.; Lemon, S.C.; McManus, D.D.; Olaitan, O.O.; Pagoto, S.L.; Gerber, B.S.; Thompson, M.J. Evaluation of a Diabetes Remote Monitoring Program Facilitated by Connected Glucose Meters for Patients With Poorly Controlled Type 2 Diabetes: Randomized Crossover Trial. JMIR Diabetes 2021, 6, e25574. [Google Scholar] [CrossRef] [PubMed]
- Middleton, T.; Constantino, M.; McGill, M.; D’Souza, M.; Twigg, S.M.; Wu, T.; Thiagalingam, A.; Chow, C.; Wong, J. An Enhanced SMS Text Message-Based Support and Reminder Program for Young Adults With Type 2 Diabetes (TEXT2U): Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e27263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Zhao, X.; Pan, X.; Lu, M.; Lu, J.; Hu, Y. Effects of continuous care for patients with type 2 diabetes using mobile health application: A randomised controlled trial. Int. J. Health Plan. Manag. 2019, 34, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Peimani, M.; Rambod, C.; Omidvar, M.; Larijani, B.; Ghodssi-Ghassemabadi, R.; Tootee, A.; Esfahani, E.N. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: A feasibility study. Prim. Care Diabetes 2016, 10, 251–258. [Google Scholar] [CrossRef]
- Vinitha, R.; Nanditha, A.; Snehalatha, C.; Satheesh, K.; Susairaj, P.; Raghavan, A.; Ramachandran, A. Effectiveness of mobile phone text messaging in improving glycaemic control among persons with newly detected type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 158, 107919. [Google Scholar] [CrossRef]
- Kang, J.; Chen, Y.; Zhao, Y.; Zhang, C. Effect of remote management on comprehensive management of diabetes mellitus during the COVID-19 epidemic. Prim. Care Diabetes 2021, 15, 417–423. [Google Scholar] [CrossRef]
- Damayanti, M.R.; Antari, G.A.A.; Nopriani, N.L.P. Effect of a Ten-Week Short Message Service-Based Intervention on Self-Management of Type-2 Diabetes Patients in Bali, Indonesia. Nurse Media J. Nurs. 2021, 11, 177–186. [Google Scholar] [CrossRef]
- Iljaž, R.; Brodnik, A.; Zrimec, T.; Cukjati, I. E-healthcare for Diabetes Mellitus Type 2 Patients—A Randomised Controlled Trial in Slovenia. Zdr. Varst. 2017, 56, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortmann, A.L.; Gallo, L.C.; Garcia, M.I.; Taleb, M.; Euyoque, J.A.; Clark, T.; Skidmore, J.; Ruiz, M.; Dharkar-Surber, S.; Schultz, J.; et al. Dulce Digital: An mHealth SMS-Based Intervention Improves Glycemic Control in Hispanics With Type 2 Diabetes. Diabetes Care 2017, 40, 1349–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, A.; Bobrow, K.; Leon, N.; Williams, N.; Phiri, E.; Namadingo, H.; Cooper, S.; Prince, J.; Crampin, A.; Besada, D.; et al. Digital messaging to support control for type 2 diabetes (StAR2D): A multicentre randomised controlled trial. BMC Public Health 2021, 21, 1907. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Hanley, J.; Lewis, S.C.; McKnight, J.A.; McCloughan, L.B.; Padfield, P.L.; Parker, R.A.; Paterson, M.; Pinnock, H.; Sheikh, A.; et al. Correction: Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med. 2016, 13, e1002163. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.C.; Shardell, M.D.; Terrin, M.L.; Barr, E.A.; Ballew, S.H.; Gruber-Baldini, A.L. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011, 34, 1934–1942. [Google Scholar] [CrossRef] [Green Version]
- Forjuoh, S.N.; Bolin, J.N.; Huber, J.C.; Vuong, A.M.; Adepoju, O.E.; Helduser, J.W.; Begaye, D.S.; Robertson, A.; Moudouni, D.M.; Bonner, T.J.; et al. Behavioral and technological interventions targeting glycemic control in a racially/ethnically diverse population: A randomized controlled trial. BMC Public Health 2014, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Liu, Y.; Gang, X.; Sun, L.; Wang, G.; Sun, C. The application of telemedicine in the integrated management of type 2 diabetes mellitus. Chin. J. Diabetes 2016, 24, 439–442. [Google Scholar]
- Stone, R.A.; Rao, R.H.; Sevick, M.A.; Cheng, C.; Hough, L.J.; Macpherson, D.S.; Franko, C.M.; Anglin, R.A.; Obrosky, D.S.; Derubertis, F.R. Active care management supported by home telemonitoring in veterans with type 2 diabetes: The DiaTel randomized controlled trial. Diabetes Care 2010, 33, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Eberle, C.; Stichling, S. Clinical Improvements by Telemedicine Interventions Managing Type 1 and Type 2 Diabetes: Systematic Meta-review. J. Med. Internet Res. 2021, 23, 12. [Google Scholar] [CrossRef]
- Haider, R.; Sudini, L.; Chow, C.K.; Cheung, N.W. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 150, 27–37. [Google Scholar] [CrossRef]
- Timpel, P.; Oswald, S.; Schwarz, P.E.H.; Harst, L. Mapping the Evidence on the Effectiveness of Telemedicine Interventions in Diabetes, Dyslipidemia, and Hypertension: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Med. Internet Res. 2020, 22, e16791. [Google Scholar] [CrossRef] [PubMed]
- Michaud, T.L.; Ern, J.; Scoggins, D.; Su, D. Assessing the Impact of Telemonitoring-Facilitated Lifestyle Modifications on Diabetes Outcomes: A Systematic Review and Meta-Analysis. Telemed. J. e-Health 2021, 27, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Min, J.; Khuri, J.; Xue, H.; Xie, B.; Kaminsky, L.A.; Cheskin, L.J. Effectiveness of Mobile Health Interventions on Diabetes and Obesity Treatment and Management: Systematic Review of Systematic Reviews. JMIR mHealth uHealth 2020, 8, e15400. [Google Scholar] [CrossRef]
- Vergès, B. Combination lipid therapy in type 2 diabetes mellitus. Expert Opin. Pharm. 2011, 12, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S73–S84. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wen, X.; Wang, F.; Yang, D.; Liu, S.; Li, P.; Xu, J. Effect of telemedicine intervention on hypoglycaemia in diabetes patients: A systematic review and meta-analysis of randomised controlled trials. J. Telemed. Telecare 2019, 25, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Yerrakalva, D.; Yerrakalva, D.; Hajna, S.; Griffin, S. Effects of Mobile Health App Interventions on Sedentary Time, Physical Activity, and Fitness in Older Adults: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2019, 21, e14343. [Google Scholar] [CrossRef] [PubMed]
- Faruque, L.I.; Wiebe, N.; Ehteshami-Afshar, A.; Liu, Y.; Dianati-Maleki, N.; Hemmelgarn, B.R.; Manns, B.J.; Tonelli, M. Effect of telemedicine on glycated hemoglobin in diabetes: A systematic review and meta-analysis of randomized trials. CMAJ 2017, 189, E341–E364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, D.; Zhou, J.; Kelley, M.S.; Michaud, T.L.; Siahpush, M.; Kim, J.; Wilson, F.; Stimpson, J.P.; Pagán, J.A. Does telemedicine improve treatment outcomes for diabetes? A meta-analysis of results from 55 randomized controlled trials. Diabetes Res. Clin. Pract. 2016, 116, 136–148. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [Green Version]
- De Groot, J.; Wu, D.; Flynn, D.; Robertson, D.; Grant, G.; Sun, J. Efficacy of telemedicine on glycaemic control in patients with type 2 diabetes: A meta-analysis. World J. Diabetes 2021, 12, 170–197. [Google Scholar] [CrossRef]
- Robson, N.; Hosseinzadeh, H. Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 12171. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Greenfield, G.; Pappas, Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: A systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv. Res. 2018, 18, 495. [Google Scholar] [CrossRef]
- Mann, D.M.; Chen, J.; Chunara, R.; Testa, P.A.; Nov, O. COVID-19 transforms health care through telemedicine: Evidence from the field. J. Am. Med. Inform. Assoc. 2020, 27, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, B. Telemedicine: Barriers and opportunities in the 21st century. J. Intern. Med. 2000, 247, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H. Systematic review of studies of the cost-effectiveness of telemedicine and telecare. Changes in the economic evidence over twenty years. J. Telemed. Telecare 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Kruk, M.E.; Freedman, L.P. Assessing health system performance in developing countries: A review of the literature. Health Policy 2008, 85, 263–276. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Author (Year) Country | Interveners | Intervention Basis | Intervention Method | Routine Group | Intervention Time; Frequency | Primary Outcome | Secondary Outcome | Intervention Method | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Education | Feedback | Counseling | Goals | Prompt | Motivation | Monitoring | ||||||||
| Stephen Agboola (2016) USA [22] | Health literacy concepts, the transtheoretical model of behavior change | SMS, pedometers (monitoring and uploading data) | Reminder telephone calls for those participants who did not upload their activity data after 5 consecutive days, and usual care. | 6 months; 2 text messages daily | Mean step counts (collected by the wireless pedometers) | HbA1c, weight, physical activity stage of change questionnaire, usability and satisfaction | Y | Y | Y | Y | Y | |||
| Daniel J. Amante (2021) USA [39] | AADE National Standards for Diabetes Self-Management Education curriculum | SMBG, the Livongo Care Team of CDEs would contact participants by their preferred communication method (either phone call or text message) within 3 min of receiving an abnormal SMBG notification from the Smart Cloud. | Usual care | 6 months; within 3 min after receiving the abnormal SMBG notification | Changes in HbA1c during each time period | Diabetes Treatment Satisfaction Questionnaire (DTSQ) | Y | Y | ||||||
| Charlene C. Quinn (2011) USA [50] | Doctor | Mobile diabetes management software application and a web portal | Usual care | 12 months; real time based on feedback | HbA1c | Patient Health Questionnaire-9 (PHQ), Diabetes Distress Scale, Self-Completion Patient Outcome Instrument, diabetes complications (blood pressure, lipid levels) | Y | Y | Y | |||||
| Daren R. Anderson (2010) Spain [34] | EHR nurses | Telephonic disease management | Usual care | 12 months; patients were called weekly, bi-weekly, or monthly depending on their risk stratification. | HbA1c | BMI, SBP, DBP, and LDL | Y | Y | ||||||
| Rade Iljaž (2017) Slovenia [46] | Health-care providers | e-Diabetes application: upload data and send automatic alerts via simple email and text messages | Usual care | 12 months; data were recorded every two weeks. These reminders were sent if a user had not entered body weight, blood pressure, physical activity, and diet data within 2 weeks of the deadline or not completed the COOP-WONCA questionnaire within 8 weeks. | Change from baseline of HbA1c at 1 year | HbA1c at 6 months, BMI, COOP-WONCA Questionnaire, blood lipids, SBP, DBP | Y | Y | Y | |||||
| Ramachandran Vinitha (2019) India [43] | Health education SMS | Usual care | 24 months; 2–3 educatory text messages per week | HbA1c | FPG, 2hPG, lipid parameters, weight, waist circumference, blood pressure, physical activity, quality of life and dietary aspects, acceptability of text messages | Y | ||||||||
| Samuel N. Forjuoh (2014) USA [51] | a. CDSMP(chronic disease self-management program); b. PDA(diabetes self-care software); c. a combination of both interventions (CDSMP + PDA) | Usual care | 12 months; enter every day | HbA1c | BMI and Blood pressure, along with several self-management behavioral measures (e.g., foot care) | Y | ||||||||
| Deborah A. Greenwood (2015) USA [27] | Diabetes educators | In-home tablet computer: telehealth remote monitoring system | Usual care | 6 months; every day | HbA1c | Diabetes Knowledge Test (DKT), Summary of Diabetes Self-Care Activities (SDSCA), Diabetes Empowerment Scale short form (DES-SF) | Y | Y | Y | |||||
| Jing Kang (2021) China [44] | Doctor, nurse | WeChat app | Usual care | 3 months; twice a week | FBG, PBG, BMI, blood glucose, TIR, and blood pressure | Y | Y | Y | Y | |||||
| Maryam Peimani (2016) Iran [42] | Social cognitive theory | a. Tailored SMS group; b. non-tailored SMS group | Usual care | 3 months; 7 messages per week | HgA1C levels, FBS, lipid profile, BMI, Self-Care Inventory (SCI), Diabetes Management Self-Efficacy Scale (DMSES) | Y | Y | Y | ||||||
| Paul C. Tang (2013) USA [29] | Nurse-led, multidisciplinary health team | Universal models of behavior change, motivational interviewing techniques, Chronic Care Model | The PAMF Online-mediated Personalized Health Care Program, which couples a multidisciplinary diabetes care management team with an EHR-integrated Online Disease Management (ODM) system | Usual care | 12 months; periodically uploading data | HbA1c | Blood pressure, LDL, 10-year Framingham cardiovascular risk, satisfactionand psychosocial well-being | Y | Y | Y | ||||
| Payal Agarwal (2019) Canada [33] | Transtheoretical Model of Behavior Change | BlueStar mobile app: customized, evidence-based messages are delivered in real time based on information uploaded by patients. | Usual care | 3 months; every day | HbA1c | Patient-reported outcomes measures (PROMs), patient-reported experience measures (PREMs), EuroQol-5D (EQ-5D), Problem Areas in Diabetes (PAID), The Summary of Diabtes Self-Care Activities Measure (SDSCA-6), availability | Y | Y | Y | |||||
| Gretchen A. Benson (2019) USA [38] | Registered dietitian nutritionist (RDN) | Health Belief Model, Transtheoretical Model | Phone coaching intervention: medical nutrition therapy (MNT), combined with pharmacotherapy | No intervention | 12 months; intervention calls generally lasted about 30 min, with a specific call frequency tailored to patient preferences. | The composite number of diabetes optimal care goals met | BMI, LDL, Morisky scale | Y | Y | Y | ||||
| Maria Magdalena Bujnowska-Fedak (2011) Poland [37] | Nurse | Telehome monitoring system | Usual care | 6 months; upload data at least once a week and receive a text message if you exceed the alarm line. | Regular glucometry, HbA1c, blood cell count, erythrocyte sedimentation rate, cholesterol balance, body mass index, creatinine concentration, urine analysis, blood serum electrolytes, blood pressure | Quality of life, doctor–patient communication, sense of control over the disease | Y | Y | ||||||
| Claudio Dario (2017) Italy [23] | Telehealth service: Patients were equipped at home with a glucometer and a gateway for data transmission to a Regional eHealth Center (ReHC). | Usual care | 12 months; every day | HRQoL: SF-20 questionnaire | HbA1c, outpatient, emergency, hospitalization rates, bed days of hospital care, Hospital Anxiety and Depression Scale (HADS) | Y | ||||||||
| Addie L. Fortmann (2017) USA [47] | Culturally appropriate DSME curriculum | Dulce Digital participants received up to motivational, educational, and/or call-to-action SMS. | Usual care | 6 months; two to three messages a day were sent at study start, with frequency tapering over 6 months. | HbA1c and lipids (TC, LDL, HDL, and TG), SBP, DBP, body weight and height, self-report items, feasibility, acceptability | Y | Y | Y | ||||||
| Heidi Holmen, MSc (2014) Norway [31] | GPs, health providers | a. Few Touch Application (FTA): diabetes diary app; b. the FTA with health counseling (FTA-HC): increase health counseling | Usual care | 12 months | HbA1c | Health Education Impact Questionnaire (heiQ), Short-Form 36v2 Health Survey (SF-36), Center for Epidemiologic Studies Depression Scale (CES-D) | Y | Y | Y | Y | ||||
| Yanmei Wang (2019) China [41] | Nurse | Mobile application: health monitoring, health guide, gealth advice, follow-up | Usual care | 6 months; patients upload data on a daily basis and the nurses evaluate them 2 to 3 days a week. | Glycemic control compliance rate, self-management ability of patients with diabetes, questionnaire on disease awareness, rehospitalization rate and number of hospital visits | Y | Y | Y | Y | Y | ||||
| Sarah H. Wild (2016) UK [49] | Nurse | Telemonitoring and glycemic control | Usual care | 9 months; one fasting and one nonfasting blood glucose at least twice weekly and BP and weight measured at least weekly | HbA1c | Ambulatory systolic, DBP, weight, anxiety, depression, quality of life, self-efficacy, self-reported physical activity, self-reported exercise tolerance, self-reported alcohol intake, diabetes knowledge | Y | Y | ||||||
| I Odnoletkova (2016) Belgium [28] | Nurse | COACH Program: an update of the best practice guidelines for the management of type 2 diabetes, motivational interviewing techniques and software program use | Usual care | 6 months; an average of 5 times over 6 months, each time 10–45 min | HbA1c | TC, LDL, HDL, TG, blood pressure, BMI, self-perceived health status, diabetes-specific emotional distress, satisfaction, annual healthcare utilization | Y | Y | Y | Y | ||||
| Heather M. Young (2020) USA [30] | Nurse | Motivational interviewing | Wearable tracking device (Basis Peak, then Garmin VivoSmart Heart Rate), telephone | Usual care | 9 months; conference call every 2 weeks for 3 months, real-time data upload | Diabetes self-efficacy (Diabetes Empowerment Scale [DES]–Short Form) | Depression severity (Patient Health Questionnaire-9 (PHQ-9), physical function, emotional distress, anxiety, Perceived Stress Scale (PSS) | Y | Y | Y | Y | |||
| Elizabeth G. Eakin (2014) Australia [35] | Telephone counselor | Motivational interviewing, social cognitive theory | Telephone counseling | Usual care | 18 months; 4 initial weekly calls, fortnightly calls for 5 months, monthly calls for 12 months | Weight, accelerometer-derived MVPA, HbA1c | Dietary energy intake, diet quality, waist circumference, fasting blood lipid levels, blood pressure | Y | Y | Y | Y | |||
| Roslyn A. Stone (2010) USA [53] | Nurse | ACM + HT: home telemonitoring coupled with active medication management by a nurse practitioner | Monthly telephone calls from the study’s diabetes nurse educator | 6 months; patients upload data daily and nurses provide timely phone contact. | HbA1c, blood pressure, weight, a fasting lipid panel | Medication regimen (dose), changes in the regimen (dose and date) | Y | Y | Y | |||||
| Yakun Feng (2016) China [52] | Doctor, nurse | U-Healthcare: Patients upload data and doctors give feedback over the phone or on the web. | Usual care | 6 months; 2 times a week | HbA1c, FPG, 2 hPG, TG, TC, HDL, LDL, BMI, SBP, DBP, BUN, Scr, AST, ALT, r-GT | Y | Y | Y | ||||||
| Leonard E. Egede (2021) USA [25] | Nurse case manager, doctor | Web-based TACM intervention: the FORA 2-in-1 Telehealth System for diabetes to link a case manager to poorly controlled diabetics in real time. | Usual care | 6 months; patients upload data on a daily basis and nurses adjust patients’ medication weekly or biweekly under the supervision of doctors. | BP, QOL: 12-item Short-Form Health Survey (SF-12) | Y | ||||||||
| María I. Rodríguez-Idígoras (2009) Spain [24] | Doctor, nurse | Telemedicine system: possibility of sending the SMBG values of the patients to a web page via mobile SMS messages. The HCP had a password access to this web page to check the blood glucose values of the patients and if necessary send to them SMS messages with recommendations. | No intervention | 12 months; send text messages if necessary | HbA1c | Blood glucose, TC, HDL, LDL, TG, BMI, SBP, DBP, and system adherence | Y | Y | Y | |||||
| A. Farmer (2021) Sub-Saharan Africa [48] | Capability, opportunity, Motivation Behavior Model | SMS | Usual care | 12 months; three to four times a week | HbA1c | Systolic blood pressure, Lipids, EuroQol 5-Dimension 3-Level (EQ-5D-3L), cardiovascular risk and the proportion of participants reaching treatment goals | Y | Y | Y | |||||
| Timothy Middleton (2021) Australia [40] | SMS: personalized support and reminder programs based on text messages | Usual care | 12 months; two a week for the first two months; one per week in the third month; one per month after the fourth month | All scheduled follow-up appointments | Overall clinic attendance, HbA1c, BMI, total cholesterol, triglycerides, diabetes self-management practices | Y | Y | Y | ||||||
| Made Rini Damayanti (2021) India [45] | SMS | Usual care | 10 weeks; three times a day | Diabetes Self-Care Activities Measure | Y | Y | Y | |||||||
| Ran Xu (2020) USA [36] | Self-reported FBG data were collected by EpxDiabetes automated phone calls or text messages. | Usual care | 6 months; three times a week. | HbA1c, FBG | Response rate, engagement rate | Y | Y | |||||||
| Ernest L. Carter (2011) USA [26] | Nurse | Laptop, wireless scale, blood pressure cuff, glucometer: Upload biometric data, make action plans with nurses through video conferencing, and watch related health videos. | Usual care | 9 months; every two weeks. | HbA1c, BMI, blood pressure, qualitative interview | Y | Y | |||||||
| Charlene C. Quinn (2016) USA [32] | Doctor | Software application, glucose meter and glucose testing supplies: Upload blood glucose monitoring data, receive health education information, and check health files. | Usual care | 12 months; real time based on feedback | HbA1c | Y | Y | Y | ||||||
| Author (Year) | Diabetes Management | Diet | Exercise | Diabetes Complications (Eye, Foot) | Diabetes Symptoms (Hypoglycemia) | Medication Compliance | Other | Diabetes Managemen | Diet | Exercise | Diabetes Complications (Eye, Foot) | Diabetes Symptoms (Hypoglycemia) | Medication Compliance | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Group | Control Group | |||||||||||||
| Stephen Agboola (2016) [22] | Y | Y | Y | Y | Y | |||||||||
| Daniel J. Amante (2021) [39] | Y | Y | Y | Y | ||||||||||
| Charlene C. Quinn (2011) [50] | Y | Y | Y | Y | Y | Y | Y | |||||||
| Deborah A. Greenwood (2015) [27] | Y | Y | Y | Y | Y | Y | ||||||||
| Jing Kang (2021) [44] | Y | Y | Y | Y | Y | Psychological counselling | Y | Y | Y | |||||
| Maryam Peimani (2016) [42] | Y | Y | Y | Y | Blood glucose monitoring | Y | ||||||||
| Paul C. Tang (2013) [29] | Y | Y | Y | Y | Y | Y | ||||||||
| Yanmei Wang (2019) [41] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| Sarah H. Wild (2016) [49] | Y | Y | Lifestyle change | Y | ||||||||||
| Heather M. Young (2020) [30] | Y | Y | Y | Self-efficacy | Y | |||||||||
| Daren R. Anderson (2010) [34] | Y | Y | Y | Y | Y | |||||||||
| Rade Iljaž (2017) [46] | Y | Y | Y | Y | ||||||||||
| RamachandranVinitha (2019) [43] | Y | Y | Y | Y | Y | Y | Y | |||||||
| Payal Agarwal (2019) [33] | Y | Y | Y | Y | Y | Y | ||||||||
| Gretchen A. Benson (2019) [38] | Y | Y | Y | |||||||||||
| Maria Magdalena Bujnowska-Fedak (2011) [37] | Y | Y | Y | Y | ||||||||||
| Claudio Dario (2017) [23] | Blood glucose monitoring | |||||||||||||
| Addie L. Fortmann (2017) [47] | Y | Y | Y | Y | ||||||||||
| Yakun Feng (2016) [52] | Y | Y | Y | Y | Y | Lifestyle and weight control | Y | |||||||
| Elizabeth G. Eakin (2014) [35] | Y | Y | Y | Weight loss | Y | |||||||||
| Leonard E. Egede (2021) [25] | Y | Y | Y | |||||||||||
| Roslyn A. Stone (2010) [53] | Y | Y | Y | |||||||||||
| María I. Rodríguez-Idígoras (2009) [24] | Y | Blood glucose monitoring | Y | |||||||||||
| Heidi Holmen, MSc (2014) [31] | Y | Y | Y | Y | ||||||||||
| I Odnoletkova (2016) [28] | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| Samuel N. Forjuoh (2014) [51] | Y | Y | Y | Y | Y | |||||||||
| Timothy Middleton (2021) [40] | Y | Y | Y | Y | Y | |||||||||
| A. Farmer (2021) [48] | Y | Y | Y | |||||||||||
| Made Rini Damayanti (2021) [45] | Y | Y | Y | Y | Y | |||||||||
| Ran Xu (2020) [36] | Y | Blood glucose monitoring | Y | |||||||||||
| Ernest L. Carter (2011) [26] | Y | Y | Y | Y | Y | Y | ||||||||
| Charlene C. Quinn (2016) [32] | Y | Y | Y | Y | Y | Y | Y | |||||||
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stephen Agboola et al. (2016) [22] | Y | Y | Y | Y | Y | L | Y | Y | ? | Y | ? |
| Daniel J. Amante et al. (2021) [39] | Y | Y | Y | Y | Y | L | Y | Y | N | Y | Y |
| Charlene C. Quinn et al. (2011) [50] | Y | Y | Y | ? | Y | S | Y | Y | N | Y | Y |
| Daren R. Anderson et al. (2010) [34] | Y | Y | Y | N | Y | S | Y | Y | N | Y | Y |
| Rade Iljaž et al. (2017) [46] | Y | Y | Y | ? | Y | S | Y | Y | N | Y | Y |
| RamachandranVinitha et al. (2019) [43] | Y | Y | ? | Y | Y | L | Y | Y | N | Y | Y |
| Samuel N. Forjuoh et al. (2014) [51] | Y | ? | Y | N | Y | S | Y | Y | N | Y | ? |
| Deborah A. Greenwood et al. (2015) [27] | Y | Y | Y | ? | Y | S | Y | Y | ? | Y | Y |
| Jing Kang et al. (2021) [44] | Y | N | ? | ? | Y | L | Y | Y | ? | Y | Y |
| Maryam Peimani et al. (2016) [42] | Y | Y | Y | N | N | L | Y | Y | ? | Y | Y |
| Paul C. Tang et al. (2013) [29] | Y | N | Y | ? | Y | S | Y | N | ? | Y | Y |
| Payal Agarwal et al. (2019) [33] | Y | Y | Y | ? | Y | S | Y | Y | Y | Y | ? |
| Gretchen A. Benson RDN et al. (2019) [38] | Y | Y | Y | ? | Y | N | Y | Y | ? | Y | Y |
| Maria Magdalena Bujnowska-Fedak et al. (2011) [37] | Y | N | ? | N | Y | S | Y | N | ? | Y | Y |
| Claudio Dario et al. (2017) [23] | Y | Y | Y | N | Y | S | Y | Y | ? | Y | Y |
| Addie L. Fortmann et al. (2017) [47] | Y | Y | ? | ? | Y | S | Y | N | ? | Y | Y |
| Heidi Holmen, MSc et al. (2014) [31] | Y | Y | Y | ? | Y | S | Y | Y | N | Y | ? |
| Yanmei Wang et al. (2019) [41] | Y | Y | Y | ? | Y | S | Y | N | ? | Y | Y |
| Sarah H. Wild et al. (2016) [49] | Y | Y | Y | Y | Y | S | Y | N | N | Y | Y |
| I Odnoletkova et al. (2016) [28] | Y | Y | Y | ? | Y | S | Y | Y | Y | Y | Y |
| Heather M. Young et al. (2020) [30] | Y | Y | Y | ? | Y | S | Y | N | ? | Y | Y |
| Elizabeth G. Eakin et al. (2014) [35] | Y | Y | ? | N | Y | S | Y | Y | N | Y | ? |
| Roslyn A. Stone et al. (2010) [53] | Y | Y | Y | ? | Y | L | Y | Y | N | Y | Y |
| Yakun Feng et al. (2016) [52] | Y | ? | Y | N | ? | S | Y | N | N | Y | Y |
| Leonard E. Egede et al. (2021) [25] | Y | Y | Y | ? | Y | S | Y | Y | ? | Y | Y |
| María I. Rodríguez-Idígoras et al. (2009) [24] | Y | Y | Y | ? | Y | N | Y | Y | N | Y | Y |
| A. Farmer et al. (2021) [48] | Y | Y | Y | Y | Y | L | Y | Y | Y | Y | Y |
| Timothy Middleton et al. (2021) [40] | Y | Y | Y | ? | Y | L | Y | Y | ? | Y | Y |
| Made Rini Damayanti et al. (2021) [45] | Y | Y | Y | N | Y | S | N | Y | Y | Y | Y |
| Ran Xu et al. (2020) [36] | Y | Y | Y | N | Y | S | Y | Y | ? | Y | Y |
| Ernest L. Carter et al. (2011) [26] | Y | Y | Y | ? | Y | L | Y | Y | N | Y | Y |
| Charlene C. Quinn et al. (2016) [32] | Y | Y | ? | N | Y | S | Y | N | ? | Y | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Wang, J.; Wan, X.; Zhang, Z.; Zhao, S.; Guo, Z.; Wang, C. A Meta-Analysis of the Effectiveness of Telemedicine in Glycemic Management among Patients with Type 2 Diabetes in Primary Care. Int. J. Environ. Res. Public Health 2022, 19, 4173. https://doi.org/10.3390/ijerph19074173
Zhang A, Wang J, Wan X, Zhang Z, Zhao S, Guo Z, Wang C. A Meta-Analysis of the Effectiveness of Telemedicine in Glycemic Management among Patients with Type 2 Diabetes in Primary Care. International Journal of Environmental Research and Public Health. 2022; 19(7):4173. https://doi.org/10.3390/ijerph19074173
Chicago/Turabian StyleZhang, Anqi, Jinsong Wang, Xiaojuan Wan, Ziyi Zhang, Shuhan Zhao, Zihe Guo, and Chufan Wang. 2022. "A Meta-Analysis of the Effectiveness of Telemedicine in Glycemic Management among Patients with Type 2 Diabetes in Primary Care" International Journal of Environmental Research and Public Health 19, no. 7: 4173. https://doi.org/10.3390/ijerph19074173
APA StyleZhang, A., Wang, J., Wan, X., Zhang, Z., Zhao, S., Guo, Z., & Wang, C. (2022). A Meta-Analysis of the Effectiveness of Telemedicine in Glycemic Management among Patients with Type 2 Diabetes in Primary Care. International Journal of Environmental Research and Public Health, 19(7), 4173. https://doi.org/10.3390/ijerph19074173





