Effect of a Digitally-Enabled, Preventive Health Program on Blood Pressure in an Adult, Dutch General Population Cohort: An Observational Pilot Study
Abstract
1. Introduction
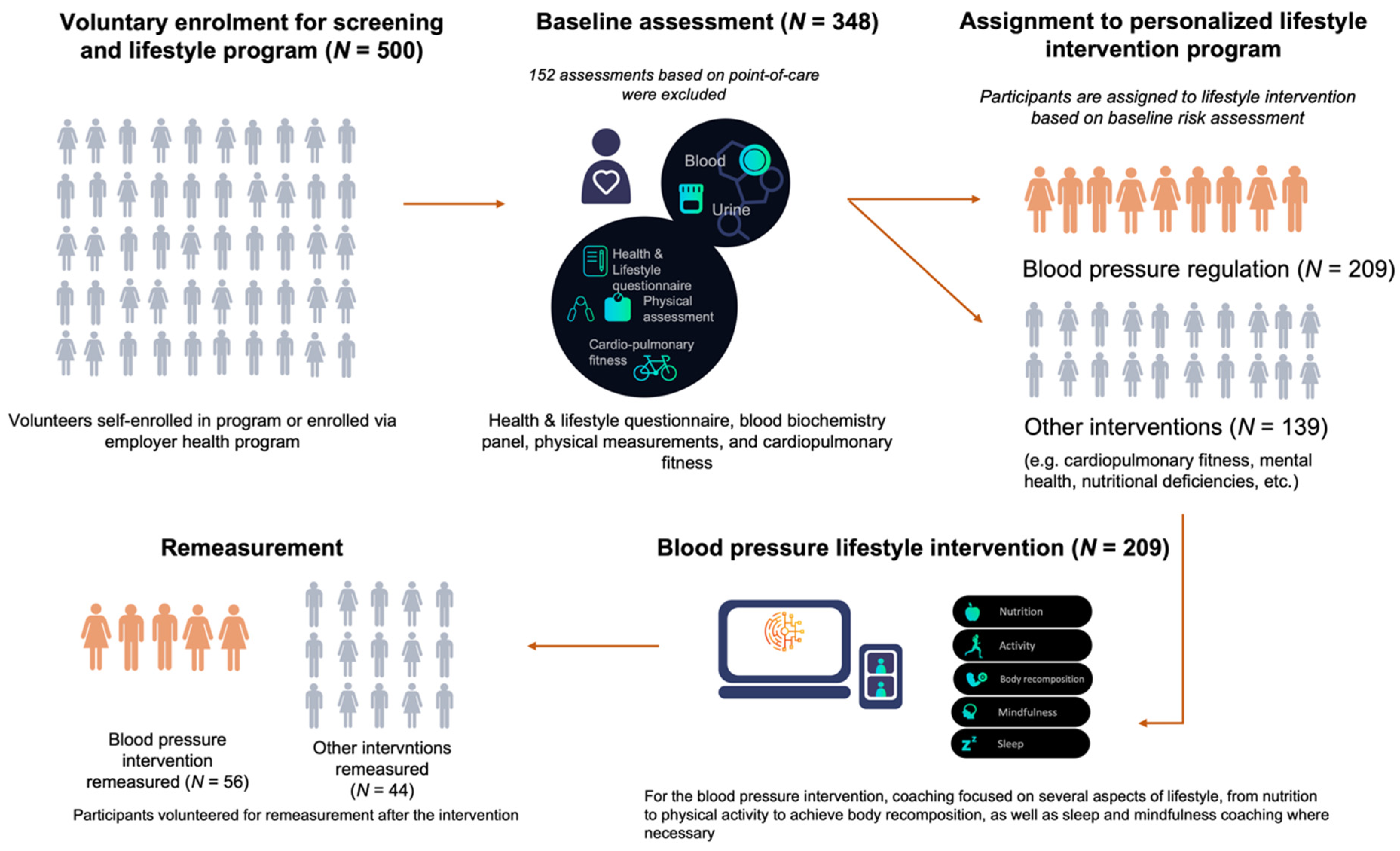
2. Materials and Methods
2.1. Data Collection and Polygenic Risk Scoring
2.2. Risk Stratification and Lifestyle Intervention
2.3. Statistical Analysis
3. Results
3.1. Prevalence of High Blood Pressure and Genetic Risk
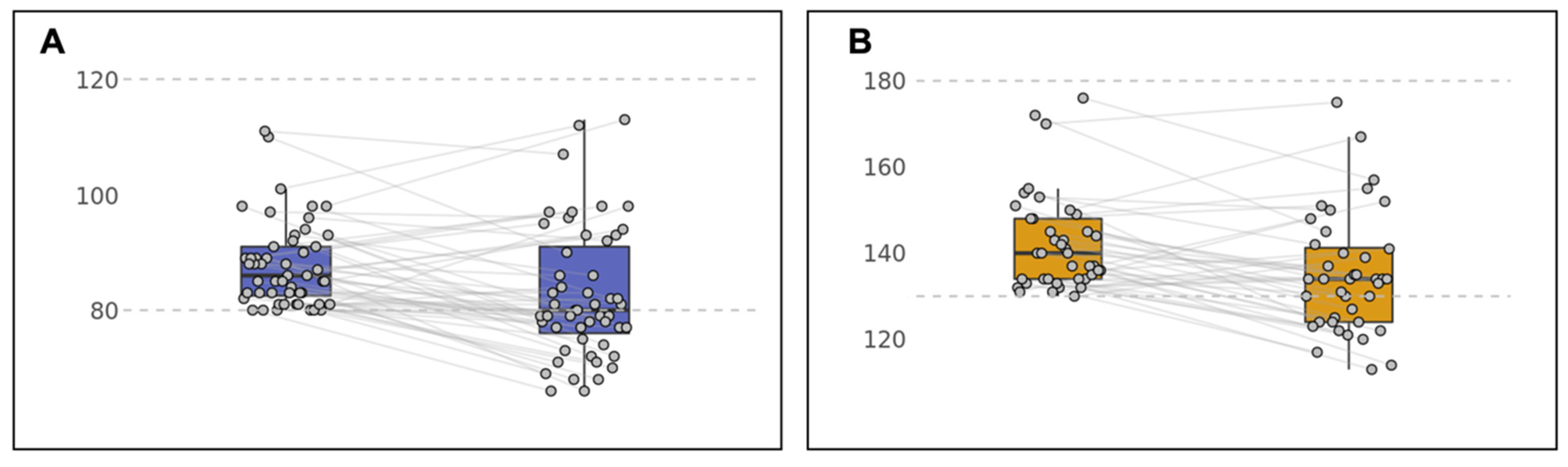
3.2. Effect of the Lifestyle Intervention on Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. ESC Scientific Document Group. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef]
- Xie, X.; Atkins, E.; Lv, J.; Bennett, A.; Neal, B.; Ninomiya, T.; Woodward, M.; MacMahon, S.; Turnbull, F.; Hillis, G.S.; et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 2016, 387, 435–443. [Google Scholar] [CrossRef]
- Duan, Y.; Xie, Z.; Dong, F.; Wu, Z.; Lin, Z.; Sun, N.; Xu, J. Effectiveness of home blood pressure telemonitoring: A systematic review and meta-analysis of randomised controlled studies. J. Hum. Hypertens. 2017, 31, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Carrera-Bastos, P.; Gálvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021, 18, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Pazoki, R.; Dehghan, A.; Evangelou, E.; Warren, H.; Gao, H.; Caulfield, M.; Elliott, P.; Tzoulaki, I. Genetic Predisposition to High Blood Pressure and Lifestyle Factors: Associations with Midlife Blood Pressure Levels and Cardiovascular Events. Circulation 2018, 137, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Kok, F.J.; Grobbee, D.E. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. Eur. J. Public Health 2004, 14, 235–239. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. Hypertension and lifestyle modification: How useful are the guidelines? Br. J. Gen. Pract. 2010, 60, 879–880. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Grobbee, D.E.; Kok, F.J. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. J. Hum. Hypertens. 2005, 19 (Suppl. S3), S1–S4. [Google Scholar] [CrossRef]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.P.; Hering, D.; et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Whelton, P.K.; He, J.; Appel, L.J.; Cutler, J.A.; Havas, S.; Kotchen, T.A.; Roccella, E.J.; Stout, R.; Vallbona, C.; Winston, M.C.; et al. Primary prevention of hypertension: Clinical and public health advisory from the National High Blood Pressure Education Program. JAMA 2002, 288, 1882–1888. [Google Scholar] [CrossRef]
- Berry, L.L.; Flynn, A.G.; Seiders, K.; Haws, K.L.; Quach, S.Q. Physician counseling of overweight patients about preventive health behaviors. Am. J. Prev. Med. 2014, 46, 297–302. [Google Scholar] [CrossRef]
- Thomas, K.; Krevers, B.; Bendtsen, P. Implementing healthy lifestyle promotion in primary care: A quasi-experimental cross-sectional study evaluating a team initiative. BMC Health Serv. Res. 2015, 15, 31. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Leppin, A.; Gyrd-Hansen, D.E.; Jarbøl, D.E.; Søndergaard, J.; Larsen, P.V. Barriers to lifestyle changes for prevention of cardiovascular disease-a survey among 40–60 years old Danes. BMC Cardiovasc. Disord. 2017, 17, 245. [Google Scholar] [CrossRef]
- Alessa, T.; Hawley, M.S.; Hock, E.S.; de Witte, L. Smartphone Apps to Support Self-Management of Hypertension: Review and Content Analysis. JMIR mHealth uHealth 2019, 7, e13645. [Google Scholar] [CrossRef]
- Predmore, Z.S.; Roth, E.; Breslau, J.; Fischer, S.H.; Uscher-Pines, L. Assessment of Patient Preferences for Telehealth in Post–COVID-19 Pandemic Health Care. JAMA Netw. Open 2021, 4, e2136405. [Google Scholar] [CrossRef]
- Vosburg, R.W.; Robinson, K.A. Telemedicine in Primary Care During the COVID-19 Pandemic: Provider and Patient Satisfaction Examined. Telemed. J. E-Health Off. J. Am. Telemed. Assoc. 2022, 28, 167–175. [Google Scholar] [CrossRef]
- Lu, X.; Yang, H.; Xia, C.; Lu, X.; Lin, J.; Liu, F.; Gu, D. Interactive Mobile Health Intervention and Blood Pressure Management in Adults: A Meta-Analysis of Randomized Controlled Trials. Hypertension 2019, 74, 697–704. [Google Scholar] [CrossRef]
- Akinosun, A.S.; Polson, R.; Diaz-Skeete, Y.; De Kock, J.H.; Carragher, L.; Leslie, S.; Grindle, M.; Gorely, T. Digital Technology Interventions for Risk Factor Modification in Patients with Cardiovascular Disease: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2021, 9, e21061. [Google Scholar] [CrossRef]
- Khoong, E.C.; Olazo, K.; Rivadeneira, N.A.; Thatipelli, S.; Barr-Walker, J.; Fontil, V.; Lyles, C.R.; Sarkar, U. Mobile health strategies for blood pressure self-management in urban populations with digital barriers: Systematic review and meta-analyses. NPJ Digit. Med. 2021, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Castela Forte, J.; Folkertsma, P.; Gannamani, R.; Kumaraswamy, S.; Mount, S.; de Koning, T.J.; van Dam, S.; Wolffenbuttel, B.H.R. Development and Validation of Decision Rules Models to Stratify Coronary Artery Disease, Diabetes, and Hypertension Risk in Preventive Care: Cohort Study of Returning UK Biobank Participants. J. Pers. Med. 2021, 11, 1322. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [CrossRef]
- Vilhjálmsson, B.J.; Yang, J.; Finucane, H.K.; Gusev, A.; Lindström, S.; Ripke, S.; Genovese, G.; Loh, P.-R.; Bhatia, G.; Do, R.; et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Hum. Genet. 2015, 97, 576–592. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Ehret, G.B.; Nandakumar, P.; Ranatunga, D.; Schaefer, C.; Kwok, P.Y.; Iribarren, C.; Chakravarti, A.; Risch, N. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat. Genet. 2017, 49, 54–64. [Google Scholar] [CrossRef]
- Campbell, N.R.; Burgess, E.; Taylor, G.; Wilson, E.; Cléroux, J.; Fodor, J.G.; Leiter, L.; Spence, J.D. Lifestyle changes to prevent and control hypertension: Do they work? A summary of the Canadian consensus conference. CMAJ 1999, 160, 1341–1343. [Google Scholar]
- Schelleman, H.; Klungel, O.; Kromhout, D.; de Boer, A.; Stricker, B.; Verschuren, W.M.M. Prevalence and determinants of undertreatment of hypertension in the Netherlands. J. Hum. Hypertens. 2004, 18, 317–324. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu). Bloeddruk en Hypertensie, Naar Leeftijd en Geslacht. Available online: https://www.rivm.nl/bloeddruk-en-hypertensie-naar-leeftijd-en-geslacht (accessed on 19 February 2022).
- Niu, M.; Zhang, L.; Wang, Y.; Tu, R.; Liu, X.; Wang, C.; Bie, R. Lifestyle Score and Genetic Factors With Hypertension and Blood Pressure Among Adults in Rural China. Front. Public Health 2021, 9, 687174. [Google Scholar] [CrossRef]
- Appel, L.J.; Champagne, C.M.; Harsha, D.W.; Cooper, L.S.; Obarzanek, E.; Elmer, P.J.; Stevens, V.J.; Vollmer, W.M.; Lin, P.H.; Svetkey, L.P.; et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA 2003, 289, 2083–2093. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Stevens, S.; Stevens, R.; Martin, U.; Mant, J.; Hobbs, F.R.; McManus, R.J. Benefits and Harms of Antihypertensive Treatment in Low-Risk Patients with Mild Hypertension. JAMA Intern. Med. 2018, 178, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, H.J.; Belo, J.N.; Blom, J.W.; Velberg, I.D.; Assendelft, W.J. Revised guidelines for cardiovascular risk management-time to stop medication? A practice-based intervention study. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2011, 61, e347–e352. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.A.; Janick, J.J. Lifestyle modifications in the prevention and treatment of hypertension. Endocr. Pract. 2001, 7, 392–399. [Google Scholar] [CrossRef]
- Gazit, T.; Gutman, M.; Beatty, A.L. Assessment of Hypertension Control Among Adults Participating in a Mobile Technology Blood Pressure Self-management Program. JAMA Netw. Open 2021, 4, e2127008. [Google Scholar] [CrossRef]
- Kario, K.; Nomura, A.; Harada, N.; Okura, A.; Nakagawa, K.; Tanigawa, T.; Hida, E. Efficacy of a digital therapeutics system in the management of essential hypertension: The HERB-DH1 pivotal trial. Eur. Heart J. 2021, 42, 4111–4122. [Google Scholar] [CrossRef]
- Fleming, T.; Bavin, L.; Lucassen, M.; Stasiak, K.; Hopkins, S.; Merry, S. Beyond the trial: Systematic review of real-world uptake and engagement with digital self-help interventions for depression, low mood, or anxiety. J. Med. Internet Res. 2018, 20, e199. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of Attrition and Dropout in App-Based Interventions for Chronic Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e20283. [Google Scholar] [CrossRef]
- Verberne, L.D.; Hendriks, M.R.; Rutten, G.M.; Spronk, I.; Savelberg, H.H.; Veenhof, C.; Nielen, M.M. Evaluation of a combined lifestyle intervention for overweight and obese patients in primary health care: A quasi-experimental design. Fam. Pract. 2016, 33, 671–677. [Google Scholar] [CrossRef][Green Version]
| Entire Population (n = 348) | Blood Pressure Intervention Group w/Follow-Up a (n = 56) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 44.6 (11.1) | 46.4 (10) | <0.0001 |
| Sex, female | 195 (56%) | 22 (39.3%) | 0.02 |
| Blood pressure | |||
| Systolic (mmHg) | 131 (16.4) | 137 (12.9) | <0.0001 |
| Diastolic (mmHg) | 81 (11.2) | 87.4 (9.3) | <0.0001 |
| Previously diagnosed with hypertension | 13 (3.7%) | 0 (0%) | 0.268 |
| Taking antihypertensive medication | 6 (1.7%) | 0 (0%) | 0.268 |
| Genetic risk | |||
| High | 20 (5.7%) | 5 (8.9%) | 0.706 |
| Elevated | 58 (16.7%) | 9 (16.1%) | 0.987 |
| Not elevated | 251 (72.2%) | 29 (51.8%) | 0.239 |
| Not available | 19 (5.4%) | 13 (23.2%) | |
| Anthropometrics | |||
| Weight (kg) | 77.2 (14.4) | 78.3 (13) | 0.017 |
| BMI (kg/m2) | 25.0 (4.7) | 24.4 (3.9) | 0.003 |
| Body fat percentage (%) | 24.9 (9.8) | 23.0 (9.6) | 0.13 |
| Blood Pressure | Blood Pressure Intervention Group w/Follow-Up | p-Value | ||
|---|---|---|---|---|
| Baseline | After Intervention | Change | ||
| Systolic blood pressure (mmHg) | 142.3 (11.3) | 135.1 (13.8) | −7.2 | <0.01 |
| Diastolic blood pressure (mmHg) | 88.4 (8.7) | 83 (11.4) | −5.4 | <0.008 |
| Variable | β | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Sex (female) | −0.35 | 0.71 (0.38–1.33) | 0.28 |
| Age (above 60 years old) | −0.34 | 0.71 (0.09–5.4) | 0.74 |
| Stress management score | 0.41 | 1.5 (0.63–3.57) | 0.36 |
| Physical activity score | 0.31 | 1.36 (0.65–2.83) | 0.41 |
| Nutrition score | −0.4 | 0.67 (0.35–1.3) | 0.24 |
| Weight loss | 0.02 | 1.02 (0.55–1.89) | 0.95 |
| High genetic risk | −0.15 | 0.86 (0.25–2.93) | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castela Forte, J.; Folkertsma, P.; Gannamani, R.; Kumaraswamy, S.; van Dam, S.; Hoogsteen, J. Effect of a Digitally-Enabled, Preventive Health Program on Blood Pressure in an Adult, Dutch General Population Cohort: An Observational Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 4171. https://doi.org/10.3390/ijerph19074171
Castela Forte J, Folkertsma P, Gannamani R, Kumaraswamy S, van Dam S, Hoogsteen J. Effect of a Digitally-Enabled, Preventive Health Program on Blood Pressure in an Adult, Dutch General Population Cohort: An Observational Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(7):4171. https://doi.org/10.3390/ijerph19074171
Chicago/Turabian StyleCastela Forte, José, Pytrik Folkertsma, Rahul Gannamani, Sridhar Kumaraswamy, Sipko van Dam, and Jan Hoogsteen. 2022. "Effect of a Digitally-Enabled, Preventive Health Program on Blood Pressure in an Adult, Dutch General Population Cohort: An Observational Pilot Study" International Journal of Environmental Research and Public Health 19, no. 7: 4171. https://doi.org/10.3390/ijerph19074171
APA StyleCastela Forte, J., Folkertsma, P., Gannamani, R., Kumaraswamy, S., van Dam, S., & Hoogsteen, J. (2022). Effect of a Digitally-Enabled, Preventive Health Program on Blood Pressure in an Adult, Dutch General Population Cohort: An Observational Pilot Study. International Journal of Environmental Research and Public Health, 19(7), 4171. https://doi.org/10.3390/ijerph19074171






