The Presence of Triclosan in Human Hair Samples in Poland—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sampling
2.3. TCS Extraction
2.4. Analysis
2.5. Method Validation
2.6. Statistical Analysis
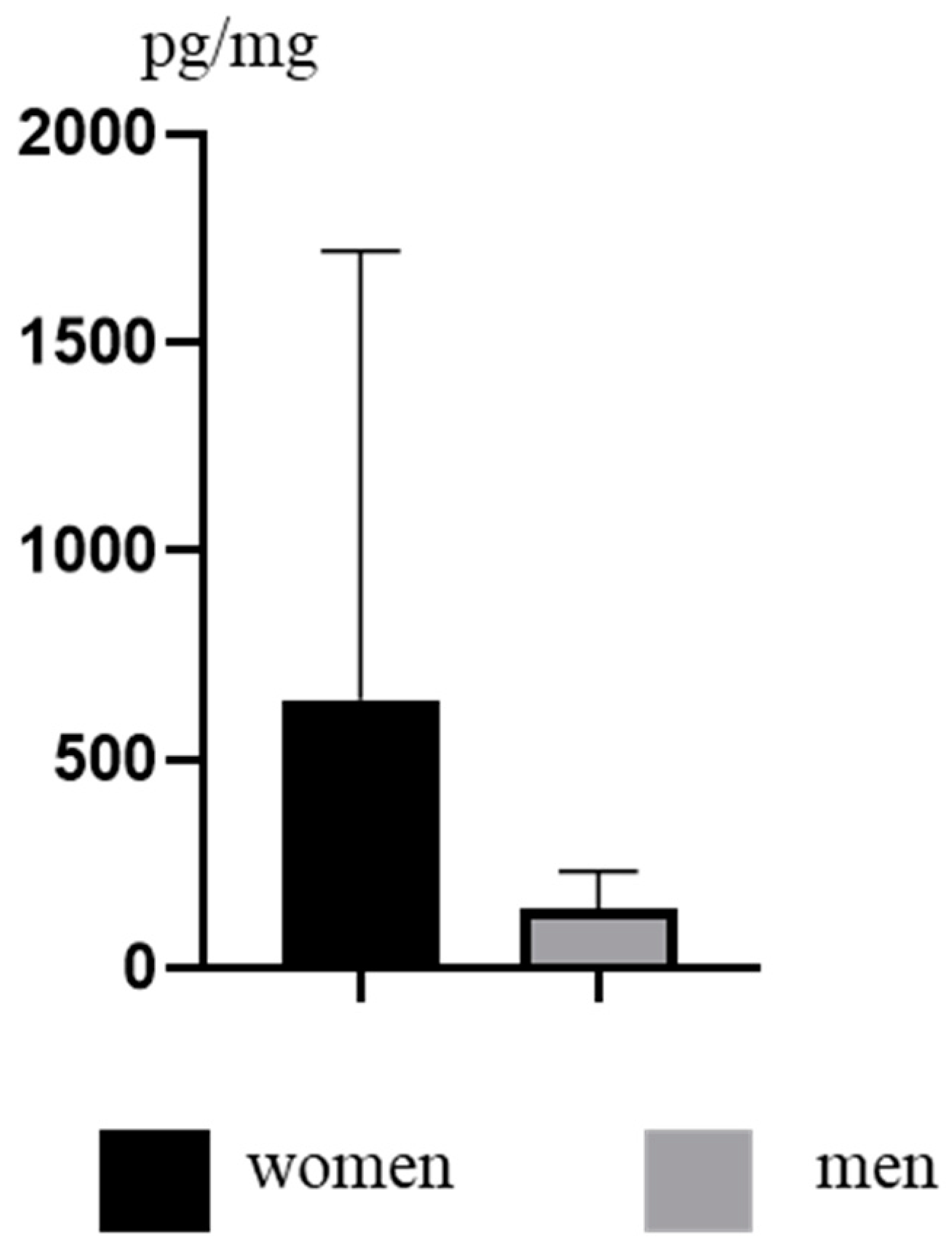
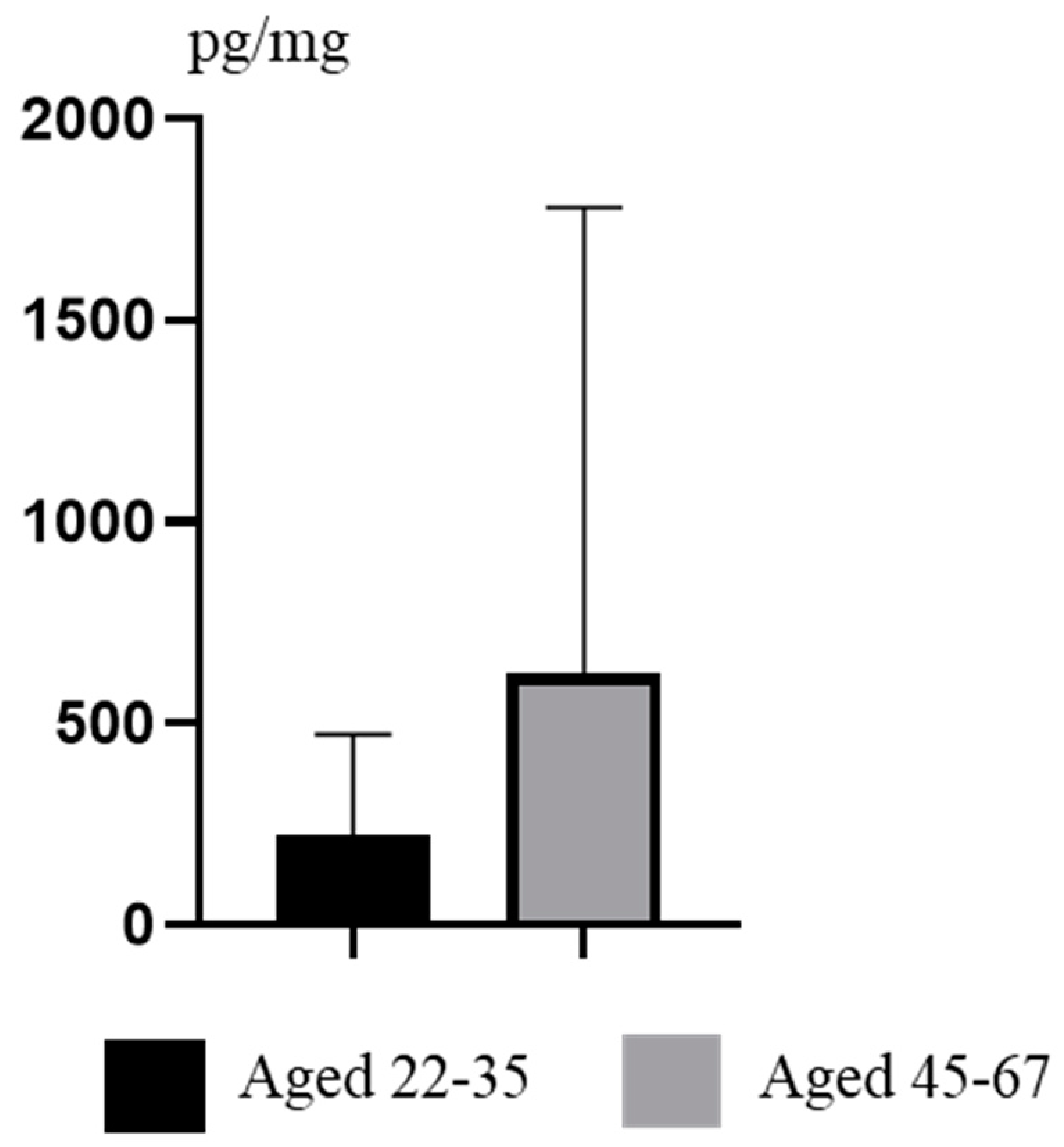
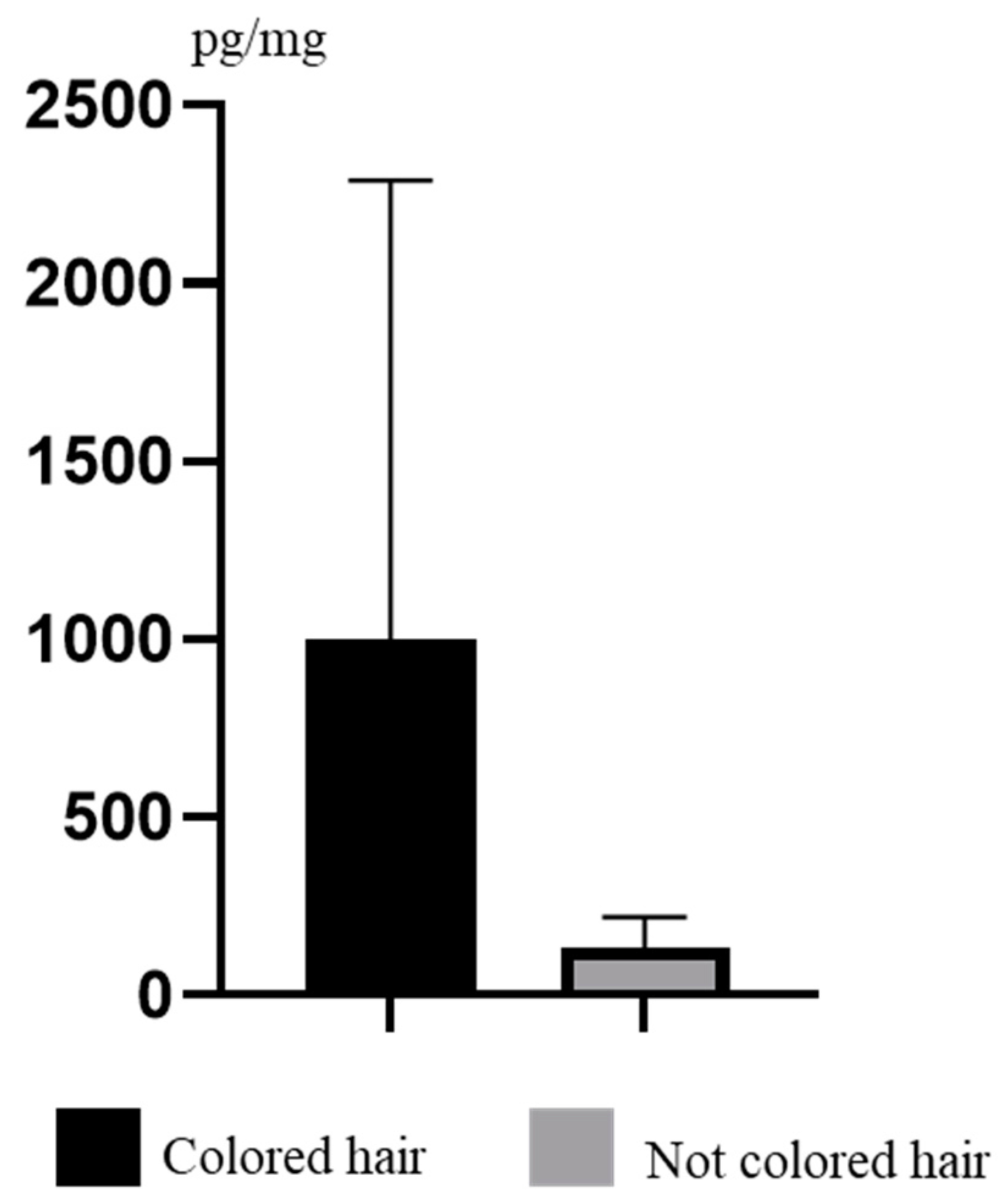
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Zhang, Y.; Chen, W.-J.; Wong, T.-Y. Triclosan: Antimicrobial mechanisms, antibiotics interactions, clinical applications, and human health. J. Environ. Sci. Healh Part C 2020, 38, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive insight into triclosan—from widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. 2021, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.K.; Das Sarkar, S.; Manna, S.K. Triclosan—An antibacterial compound in water, sediment and fish of River Gomti, India. Int. J. Environ. Health Res. 2018, 28, 461–470. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, J.; Li, S.; Yu, M. Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Environ. Pollut. 2020, 266 Pt 3, 115340. [Google Scholar] [CrossRef]
- Safwat, N.; Abdel-Ghany, M.F.; Ayad, M.F. Sensitive Derivative Synchronous and Micellar Enhanced Ecofriendly Spectrofluorimetric Methods for the Determination of Atenolol, Diclofenac, and Triclosan in Drinking Tap Water. J. AOAC Int. 2021, 104, 103–112. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Zhu, Q.; Liao, C. Occurrence of parabens, triclosan and triclocarban in paired human urine and indoor dust from two typical cities in China and its implications for human exposure. Sci. Total Environ. 2021, 786, 147485. [Google Scholar] [CrossRef]
- Emnet, P.; Gaw, S.; Northcott, G.; Storey, B.; Graham, L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2015, 136, 331–342. [Google Scholar] [CrossRef]
- Lin, Y.J. Buccal absorption of triclosan following topical mouthrinse application. Am. J. Dent. 2000, 13, 215–217. [Google Scholar]
- Chedgzoy, P.; Winckle, G.; Heard, C.M. Triclosan: Release from transdermal adhesive formulations and in vitro permeation across human epidermal membranes. Int. J. Pharm. 2002, 235, 229–236. [Google Scholar] [CrossRef]
- Sandborgh-Englund, G.; Adolfsson-Erici, M.; Odham, G.; Ekstrand, J. Pharmacokinetics of Triclosan Following Oral Ingestion in Humans. J. Toxicol. Environ. Health Part A 2006, 69, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.P.; Xue, J.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Wielgomas, B.; Radwan, M.; Karwacka, A.; Klimowska, A.; Dziewirska, E.; Korczak, K.; Zajdel, R.; Radwan, P.; Hanke, W. Triclosan exposure and ovarian reserve. Reprod. Toxicol. 2019, 89, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, Y.; Tang, C.; Fang, H.; Yang, D.; Wu, J.; Wang, H.; Chen, Y.; Jiang, Q. Association of triclosan and triclocarban in urine with obesity risk in Chinese school children. Environ. Int. 2021, 157, 106846. [Google Scholar] [CrossRef] [PubMed]
- Allmyr, M.; Harden, F.; Toms, L.-M.L.; Mueller, J.F.; McLachlan, M.S.; Adolfsson-Erici, M.; Sandborgh-Englund, G. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci. Total Environ. 2008, 393, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Karzi, V.; Tzatzarakis, M.N.; Vakonaki, E.; Alegakis, T.; Katsikantami, I.; Sifakis, S.; Rizos, A.; Tsatsakis, A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018, 38, 1144–1152. [Google Scholar] [CrossRef]
- Karzi, V.; Tzatzarakis, M.; Katsikantami, I.; Stavroulaki, A.; Alegakis, A.; Vakonaki, E.; Xezonaki, P.; Sifakis, S.; Rizos, A.; Tsatsakis, A. Investigating exposure to endocrine disruptors via hair analysis of pregnant women. Environ. Res. 2019, 178, 108692. [Google Scholar] [CrossRef]
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.; Moon, S.-M.; Yang, E.J. Associations of lifestyle factors with phthalate metabolites, bisphenol A, parabens, and triclosan concentrations in breast milk of Korean mothers. Chemosphere 2020, 249, 126149. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Fair, P.A.; Lee, H.-B.; Adams, J.; Darling, C.; Pacepavicius, G.; Alaee, M.; Bossart, G.D.; Henry, N.; Muir, D. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ. Pollut. 2009, 157, 2248–2254. [Google Scholar] [CrossRef]
- Karthikraj, R.; Lee, S.; Kannan, K. Biomonitoring of exposure to bisphenols, benzophenones, triclosan, and triclocarban in pet dogs and cats. Environ. Res. 2020, 180, 108821. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-L.; Vanlandingham, M.; da Costa, G.G.; Beland, F.A. Absorption and metabolism of triclosan after application to the skin of B6C3F1 mice. Environ. Toxicol. 2016, 31, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Kim, T.H.; Bi, K.S.; Chen, X.H.; Kim, H.S. Triclosan exhibits a tendency to accumulate in the epididymis and shows sperm toxicity in male sprague-dawley rats. Environ. Toxicol. 2015, 30, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.M.R.; Todd, M.; Dowd, J.B.; Aiello, A.E. The Impact of Bisphenol A and Triclosan on Immune Parameters in the U.S. Population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119, 390–396. [Google Scholar] [CrossRef]
- Marshall, N.B.; Lukomska, E.; Long, C.M.; Kashon, M.L.; Sharpnack, D.D.; Nayak, A.P.; Anderson, S.E. Triclosan Induces Thymic Stromal Lymphopoietin in Skin Promoting Th2 Allergic Responses. Toxicol. Sci. 2015, 147, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilburn, W.J.; Jamal, S.; Ismail, F.; Brooks, D.; Whalen, M. Evaluation of triclosan exposures on secretion of pro-inflammatory cytokines from human immune cells. Environ. Toxicol. Pharmacol. 2021, 83, 103599. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Kałużny, P.; Klimowska, A.; Radwan, P.; Hanke, W. Environmental levels of triclosan and male fertility. Environ. Sci. Pollut. Res. 2017, 25, 5484–5490. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhou, W.; Huo, X.; Zhao, S.; Gan, Y.; Wang, B.; Cheng, W.; Ouyang, F.; Wang, W.; Tian, Y.; et al. Triclosan and Female Reproductive Health: A preconceptional cohort study. Epidemiology 2019, 30 (Suppl. 1), S24–S31. [Google Scholar] [CrossRef]
- Saley, A.; Hess, M.; Miller, K.; Howard, D.; King-Heiden, T.C. Cardiac Toxicity of Triclosan in Developing Zebrafish. Zebrafish 2016, 13, 399–404. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Romano, K.A.; Gu, M.; Sanidad, K.Z.; Kim, D.; Yang, J.; Schmidt, B.; Panigrahy, D.; Pei, R.; et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018, 10, 4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yueh, M.-F.; He, F.; Chen, C.; Vu, C.; Tripathi, A.; Knight, R.; Karin, M.; Chen, S.; Tukey, R.H. Triclosan leads to dysregulation of the metabolic regulator FGF21 exacerbating high fat diet-induced nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 31259–31266. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, T.P.; Artacho-Cordón, F.; Swaab, D.F.; Struik, D.; Makris, K.C.; Wolffenbuttel, B.H.R.; Frederiksen, H.; Van Vliet-Ostaptchouk, J.V. Distribution of Non-Persistent Endocrine Disruptors in Two Different Regions of the Human Brain. Int. J. Environ. Res. Public Health 2017, 14, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruszkiewicz, J.A.; Li, S.; Rodriguez, M.B.; Aschner, M. Is Triclosan a neurotoxic agent? J. Toxicol. Environ. Health Part B 2017, 20, 104–117. [Google Scholar] [CrossRef]
- Hao, Z.; Wu, Q.; Li, Z.; Li, Y.; Li, Q.; Lai, X.; Liu, H.; Zhang, M.; Yang, T.; Chen, J.; et al. Maternal exposure to triclosan constitutes a yet unrecognized risk factor for autism spectrum disorders. Cell Res. 2019, 29, 866–869. [Google Scholar] [CrossRef]
- Hao, Y.; Meng, L.; Zhang, Y.; Chen, A.; Zhao, Y.; Lian, K.; Guo, X.; Wang, X.; Du, Y.; Wang, X.; et al. Effects of chronic triclosan exposure on social behaviors in adult mice. J. Hazard. Mater. 2022, 424, 127562. [Google Scholar] [CrossRef]
- Jackson-Browne, M.S.; Henderson, N.; Patti, M.; Spanier, A.; Braun, J.M. The Impact of Early-Life Exposure to Antimicrobials on Asthma and Eczema Risk in Children. Curr. Environ. Health Rep. 2019, 6, 214–224. [Google Scholar] [CrossRef]
- Naffaa, V.; Laprévote, O.; Schang, A.-L. Effects of endocrine disrupting chemicals on myelin development and diseases. NeuroToxicology 2021, 83, 51–68. [Google Scholar] [CrossRef]
- Xie, X.; Lu, C.; Wu, M.; Liang, J.; Ying, Y.; Liu, K.; Huang, X.; Zheng, S.; Du, X.; Liu, D.; et al. Association between triclocarban and triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ. Int. 2020, 136, 105445. [Google Scholar] [CrossRef]
- Dinwiddie, M.T.; Terry, P.D.; Chen, J. Recent Evidence Regarding Triclosan and Cancer Risk. Int. J. Environ. Res. Public Health 2014, 11, 2209–2217. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Exposure assessment to parabens, bisphenol A and perfluoroalkyl compounds in children, women and men by hair analysis. Sci. Total Environ. 2019, 695, 133864. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Tzatzarakis, M.N.; Karzi, V.; Stavroulaki, A.; Xezonaki, P.; Vakonaki, E.; Alegakis, A.K.; Sifakis, S.; Rizos, A.K.; Tsatsakis, A.M. Biomonitoring of bisphenols A and S and phthalate metabolites in hair from pregnant women in Crete. Sci. Total Environ. 2020, 712, 135651. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Martín, J.; Rychlik, A.; Aparicio, I.; Santos, J.L.; Alonso, E.; Gonkowski, S. Biomonitoring parabens in dogs using fur sample analysis—Preliminary studies. Sci. Total Environ. 2022, 807, 150757. [Google Scholar] [CrossRef]
- Alves, A.; Jacobs, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. New approach for assessing human perfluoroalkyl exposure via hair. Talanta 2015, 144, 574–583. [Google Scholar] [CrossRef]
- Martín, J.; Möder, M.; Gaudl, A.; Alonso, E.; Reemtsma, T. Multi-class method for biomonitoring of hair samples using gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 8725–8734. [Google Scholar] [CrossRef] [PubMed]
- Tzatzarakis, M.N.; Vakonaki, E.; Kavvalakis, M.P.; Barmpas, M.; Kokkinakis, E.N.; Xenos, K.; Tsatsakis, A.M. Biomonitoring of bisphenol A in hair of Greek population. Chemosphere 2015, 118, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Pirard, C.; Sagot, C.; Deville, M.; Dubois, N.; Charlier, C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ. Int. 2012, 48, 78–83. [Google Scholar] [CrossRef]
- Rocha, B.A.; de Oliveira, A.R.M.; Barbosa, F. A fast and simple air-assisted liquid-liquid microextraction procedure for the simultaneous determination of bisphenols, parabens, benzophenones, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. Talanta 2018, 183, 94–101. [Google Scholar] [CrossRef]
- Provencher, G.; Bérubé, R.; Dumas, P.; Bienvenu, J.-F.; Gaudreau, É.; Bélanger, P.; Ayotte, P. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1348, 97–104. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Y.; He, W.; Huang, S.; Li, Q.; Guo, C.; Ma, S.; Li, G.; Yu, Y. Co-exposure and health risks of parabens, bisphenols, triclosan, phthalate metabolites and hydroxyl polycyclic aromatic hydrocarbons based on simultaneous detection in urine samples from guangzhou, south China. Environ. Pollut. 2020, 272, 115990. [Google Scholar] [CrossRef]
- Tkalec, Ž.; Kosjek, T.; Tratnik, J.S.; Stajnko, A.; Runkel, A.A.; Sykiotou, M.; Mazej, D.; Horvat, M. Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: Urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 2021, 146, 106172. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Frederiksen, H.; Andersson, A.-M.; Juul, A.; Thankamony, A.; Ong, K.K.; Dunger, P.D.; Hughes, I.A.; Acerini, C.L. Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Front. Endocrinol. 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Leung, K.-F.; Tong, S.-F.; Lam, C.-W. Organochlorine isotopic pattern-enhanced detection and quantification of triclosan and its metabolites in human serum by ultra-high-performance liquid chromatography/quadrupole time-of-flight/mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 26, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef]
- Allmyr, M.; Adolfsson-Erici, M.; McLachlan, M.; Englund, G.S. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372, 87–93. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Urinary Concentrations of Triclosan in the U.S. Population: 2003–2004. Environ. Health Perspect. 2008, 116, 303–307. [Google Scholar] [CrossRef]
- Apel, P.; Angerer, J.; Wilhelm, M.; Kolossa-Gehring, M. New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2017, 220, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Blume-Peytavi, U.; Massoudy, L.; Patzelt, A.; Lademann, J.; Dietz, E.; Rasulev, U.; Bartels, N.G. Follicular and percutaneous penetration pathways of topically applied minoxidil foam. Eur. J. Pharm. Biopharm. 2010, 76, 450–453. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin. Drug Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Radwan, P.; Wielgomas, B.; Radwan, M.; Krasiński, R.; Klimowska, A.; Zajdel, R.; Kaleta, D.; Jurewicz, J. Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environ. Sci. Pollut. Res. 2021, 28, 12993–12999. [Google Scholar] [CrossRef]
- Urbaniak, M.; Tygielska, A.; Krauze, K.; Mankiewicz-Boczek, J. Effects of Stormwater and Snowmelt Runoff on ELISA-EQ Concentrations of PCDD/PCDF and Triclosan in an Urban River. PLoS ONE 2016, 11, e0151756. [Google Scholar] [CrossRef] [Green Version]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Chen, Y.; Wang, D.; Xu, W.; Gao, Y. Profiles of parabens, benzophenone-type ultraviolet filters, triclosan, and triclocarban in paired urine and indoor dust samples from Chinese university students: Implications for human exposure. Sci. Total Environ. 2021, 798, 149275. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Aksglaede, L.; Sorensen, K.; Nielsen, O.; Main, K.M.; Skakkebaek, N.E.; Juul, A.; Andersson, A.-M. Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC–MS/MS. Int. J. Hyg. Environ. Health 2013, 216, 710–720. [Google Scholar] [CrossRef]
- Geens, T.; Dirtu, A.C.; Dirinck, E.; Malarvannan, G.; Van Gaal, L.; Jorens, P.G.; Covaci, A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ. Int. 2015, 76, 98–105. [Google Scholar] [CrossRef]
- Covaci, A.; Tutudaki, M.; Tsatsakis, A.M.; Schepens, P. Hair analysis: Another approach for the assessment of human exposure to selected persistent organochlorine pollutants. Chemosphere 2002, 46, 413–418. [Google Scholar] [CrossRef]
- Appenzeller, B.M.; Tsatsakis, A. Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: State of the art, critical review and future needs. Toxicol. Lett. 2012, 210, 119–140. [Google Scholar] [CrossRef]
- Gerace, E.; Veronesi, A.; Martra, G.; Salomone, A.; Vincenti, M. Study of cocaine incorporation in hair damaged by cosmetic treatments. Forensic Chem. 2017, 3, 69–73. [Google Scholar] [CrossRef]
- Claessens, J.; Pirard, C.; Charlier, C. Determination of contamination levels for multiple endocrine disruptors in hair from a non-occupationally exposed population living in Liege (Belgium). Sci. Total Environ. 2021, 815, 152734. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Tzatzarakis, M.; Vakonaki, E.; Makowska, K.; Gonkowski, S. Evaluation of human exposure to parabens in north eastern Poland through hair sample analysis. Sci. Rep. 2021, 11, 23673. [Google Scholar] [CrossRef]


| No. | Age | Gender | Hair Color | Hair Coloring |
|---|---|---|---|---|
| 1 | 46 | Male | Black | No |
| 2 | 45 | Male | Black | No |
| 3 | 28 | Female | Brown | Yes |
| 4 | 23 | Male | Black | No |
| 5 | 32 | Male | Black | No |
| 6 | 34 | Male | Black | No |
| 7 | 50 | Female | Blond | Yes |
| 8 | 53 | Female | Black | Yes |
| 9 | 28 | Male | Black | No |
| 10 | 22 | Female | Blond | No |
| 11 | 27 | Female | Black | No |
| 12 | 24 | Female | Brown | No |
| 13 | 23 | Female | Brown | No |
| 14 | 23 | Male | Brown | No |
| 15 | 61 | Male | Black-Gray | No |
| 16 | 35 | Male | Black | No |
| 17 | 55 | Male | Black-Gray | No |
| 18 | 49 | Male | Black | No |
| 19 | 23 | Male | Brown | No |
| 20 | 22 | Female | Black-Brown | Yes |
| 21 | 56 | Female | Brown | Yes |
| 22 | 55 | Female | Brown-Red | Yes |
| 23 | 48 | Female | Brown | Yes |
| 24 | 46 | Female | Black | Yes |
| 25 | 50 | Male | Grey | No |
| 26 | 31 | Female | Brown | Yes |
| 27 | 27 | Female | Brown | No |
| 28 | 67 | Female | White | No |
| 29 | 34 | Male | Black | No |
| 30 | 59 | Male | Black | No |
| n = 3 | |
| Mean % recovery | 117.0 |
| ±SD | 18.6 |
| Mean % accuracy | 115.6 |
| ±SD | 24.8 |
| Precision (% RSD) | 15.6 |
| ±SD | 7.3 |
| LOD (pg/mg) | 0.7 |
| LOQ (pg/mg) | 2.4 |
| r2 (spiked curves) | 0.9937 |
| r2 (standard curves) | 0.9998 |
| Country | Matrix | Method | Range of TCS Concentration | References |
|---|---|---|---|---|
| Belgium | urine | GC-MS/MS | ND–598.95 | [47] |
| Brazil | urine | LC-MS/MS | <0.5–294 | [48] |
| Canada | urine | LC-MS/MS | <0.008–19.8 | [49] |
| China | urine | LC-MS/MS | <0.003–178 | [50] |
| China | urine | LC-MS/MS | 0.08–1600 | [12] |
| Greece | urine | LC-MS/MS | 0.08–386 | [12] |
| India | urine | LC-MS/MS | 0.08–898 | [12] |
| Japan | urine | LC-MS/MS | 0.08–287 | [12] |
| Kuwait | urine | LC-MS/MS | 0.26–288 | [12] |
| Saudi Arabia | urine | LC-MS/MS | 0.08–34.4 | [12] |
| Slovenia | urine | GC-MS/MS | <0.25–25 | [51] |
| South Korea | urine | LC-MS/MS | 0.08–558 | [12] |
| USA | urine | LC-MS/MS | 0.21–819 | [12] |
| Vietnam | urine | LC-MS/MS | 0.08–27 | [12] |
| Great Britain | blood serum | LC-MS/MS | <0.22–178.75 | [52] |
| China | blood serum | UHPLC-Q-TOF/MS | 0.15–217 | [53] |
| Spain | blood serum | GC-MS | <1.4–12 ± 1 | [54] |
| Spain | breast milk | GC-MS | <1.3–0.70 ± 0.05 | [54] |
| Sweden | breast milk | GC/ECNI/MS | <0.018–0.32 | [55] |
| China | nails | UHPLC-MS/MS | ND–5049.19 | [18] |
| Germany | hair | GC-MS | 276–1870 | [45] |
| Greece | hair | LC-MS | 3.6–8564.9 | [16] |
| Greece | hair | LC-MS | 8.8–8070.2 | [17] |
| Poland | hair | LC-MS | 37.9–3386.5 | This study |
| Matrix | Method | TCS Concentration Levels | References |
|---|---|---|---|
| Human urine: women | GC–MS/MS | 0.3–1677.68 | [13] |
| Human urine: women | GC–MS/MS | 0.3–265.17 | [60] |
| Human urine: men | GC–MS/MS | <LOD–789.20 | [28] |
| Surface water | ELISA | 0.021–2.457 | [61] |
| Surface water | LC-MS/MS | 0.0446–0.2715 | [52] |
| Wastewater | LC-MS/MS | 0.0498 ± 0.0007–6.7217 ± 0.0068 | [62] |
| Human hair | LC-MS | 37.9–3386.5 | This study |
| Country | Groups of Volunteers | Median Value of TCS Concentration Levels | References |
|---|---|---|---|
| Greece | |||
| Children of both gender | 48.8 | [16] | |
| Adults of both gender | 181.6 | [16] | |
| Greece | |||
| Adult pregnant women | 61.6 | [17] | |
| Poland | |||
| Adults of both gender | 103 | This study | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonkowski, S.; Tzatzarakis, M.; Vakonaki, E.; Makowska, K.; Tsatsakis, A.M.; Wojtkiewicz, J. The Presence of Triclosan in Human Hair Samples in Poland—A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 3796. https://doi.org/10.3390/ijerph19073796
Gonkowski S, Tzatzarakis M, Vakonaki E, Makowska K, Tsatsakis AM, Wojtkiewicz J. The Presence of Triclosan in Human Hair Samples in Poland—A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(7):3796. https://doi.org/10.3390/ijerph19073796
Chicago/Turabian StyleGonkowski, Slawomir, Manolis Tzatzarakis, Elena Vakonaki, Krystyna Makowska, Aristidis M. Tsatsakis, and Joanna Wojtkiewicz. 2022. "The Presence of Triclosan in Human Hair Samples in Poland—A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 7: 3796. https://doi.org/10.3390/ijerph19073796
APA StyleGonkowski, S., Tzatzarakis, M., Vakonaki, E., Makowska, K., Tsatsakis, A. M., & Wojtkiewicz, J. (2022). The Presence of Triclosan in Human Hair Samples in Poland—A Pilot Study. International Journal of Environmental Research and Public Health, 19(7), 3796. https://doi.org/10.3390/ijerph19073796









