Underestimation in Reporting Excess COVID-19 Death Data in Poland during the First Three Pandemic Waves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
3. Results
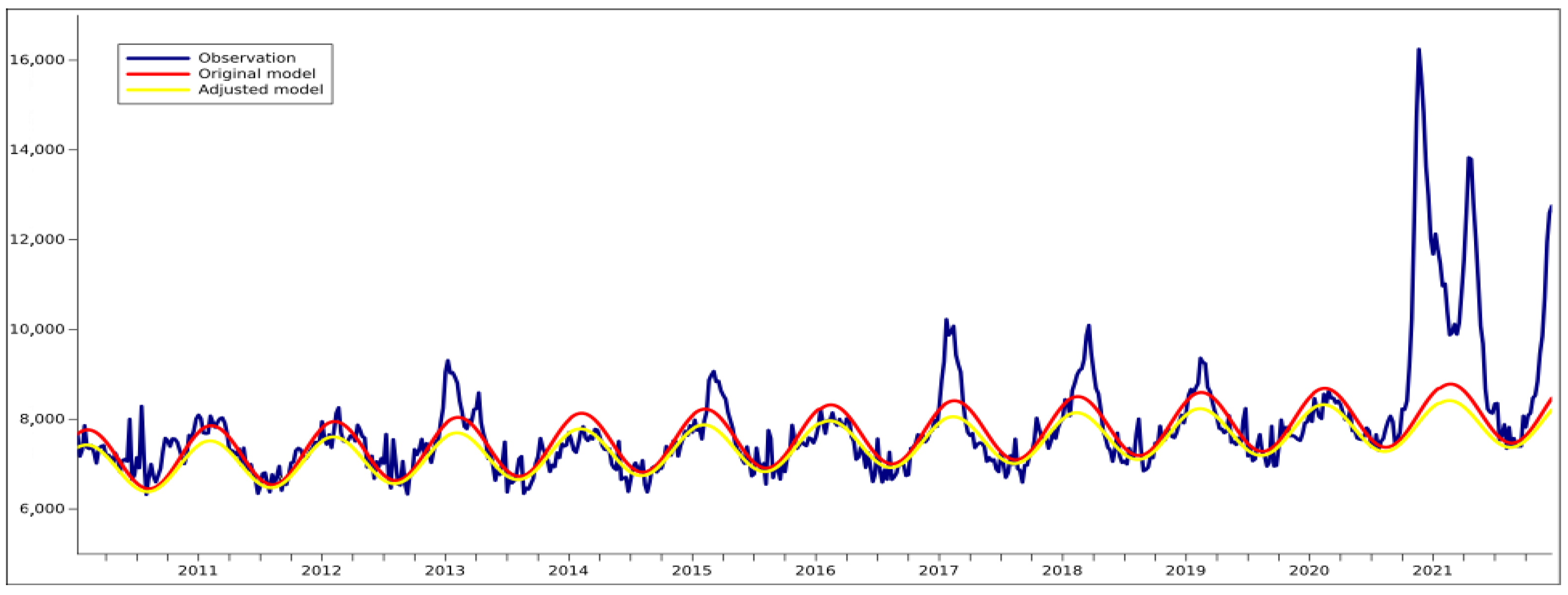
3.1. Validating the Formula for the Calculation of Excess Deaths
3.2. Relation between Excess Deaths and COVID-19 Deaths
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | the coronavirus disease 2019 |
| SD | standard deviation |
| PCA | principal component analysis |
References
- Vestergaard, L.S.; Nielsen, J.; Richter, L.; Schmid, D.; Bustos, N.; Braeye, T.; Denissov, G.; Veideman, T.; Luomala, O.; Möttönen, T.; et al. Excess All-Cause Mortality during the COVID-19 Pandemic in Europe—Preliminary Pooled Estimates from the EuroMOMO Network, March to April 2020. Eurosurveillance 2020, 25, 2001214. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, A.; Rey, G.; Wagner, V.; Laaidi, K.; Empereur-Bissonnet, P.; Le Tertre, A.; Frayssinet, P.; Bessemoulin, P.; Laurent, F.; De Crouy-Chanel, P.; et al. Has the Impact of Heat Waves on Mortality Changed in France since the European Heat Wave of Summer 2003? A Study of the 2006 Heat Wave. Int. J. Epidemiol. 2008, 37, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lytras, T.; Pantavou, K.; Mouratidou, E.; Tsiodras, S. Mortality Attributable to Seasonal Influenza in Greece, 2013 to 2017: Variation by Type/Subtype and Age, and a Possible Harvesting Effect. Eurosurveillance 2019, 24, 1800118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molbak, K.; Espenhain, L.; Nielsen, J.; Tersago, K.; Bossuyt, N.; Denissov, G.; Baburin, A.; Virtanen, M.; Fouillet, A.; Sideroglou, T.; et al. Excess Mortality among the Elderly in European Countries, December 2014 to February 2015. Eurosurveillance 2015, 20, 21065. [Google Scholar] [CrossRef] [Green Version]
- Pirard, P.; Vandentorren, S.; Pascal, M.; Laaidi, K.; Le Tertre, A.; Cassadou, S.; Ledrans, M. Summary of the Mortality Impact Assessment of the 2003 Heat Wave in France. Eurosurveillance 2005, 10, 153–156. [Google Scholar] [CrossRef]
- Bolcato, M.; Aurilio, M.T.; Aprile, A.; Di Mizio, G.; Della Pietra, B.; Feola, A. Take-Home Messages from the COVID-19 Pandemic: Strengths and Pitfalls of the Italian National Health Service from a Medico-Legal Point of View. Healthcare 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Schneider Dos Santos, R.; De’ Donato, F.; De Sario, M.; Michelozzi, P.; Davoli, M.; Masselot, P.; Sera, F.; Gasparrini, A. Excess Mortality during the COVID-19 Outbreak in Italy: A Two-Stage Interrupted Time-Series Analysis. Int. J. Epidemiol. 2021, 49, 1909–1917. [Google Scholar] [CrossRef]
- Bustos Sierra, N.; Bossuyt, N.; Braeye, T.; Leroy, M.; Moyersoen, I.; Peeters, I.; Scohy, A.; Van der Heyden, J.; Van Oyen, H.; Renard, F. All-Cause Mortality Supports the COVID-19 Mortality in Belgium and Comparison with Major Fatal Events of the Last Century. Arch Public Health 2020, 78, 117. [Google Scholar] [CrossRef]
- Nogueira, P.J.; Nobre, M.D.A.; Nicola, P.J.; Furtado, C.; Vaz Carneiro, A. Excess Mortality Estimation During the COVID-19 Pandemic: Preliminary Data from Portugal. Acta Med. Port. 2020, 33, 376–383. [Google Scholar] [CrossRef]
- Böttcher, L.; D’Orsogna, M.R.; Chou, T. Using Excess Deaths and Testing Statistics to Determine COVID-19 Mortalities. Eur. J. Epidemiol. 2021, 36, 545–558. [Google Scholar] [CrossRef]
- Islam, N.; Shkolnikov, V.M.; Acosta, R.J.; Klimkin, I.; Kawachi, I.; Irizarry, R.A.; Alicandro, G.; Khunti, K.; Yates, T.; Jdanov, D.A.; et al. Excess Deaths Associated with COVID-19 Pandemic in 2020: Age and Sex Disaggregated Time Series Analysis in 29 High Income Countries. BMJ 2021, 373, n1137. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Petti, S.; Noah, N. COVID-19, Non-COVID-19 and Excess Mortality Rates Not Comparable across Countries. Epidemiol. Infect. 2021, 149, e176. [Google Scholar] [CrossRef] [PubMed]
- Sanmarchi, F.; Golinelli, D.; Lenzi, J.; Esposito, F.; Capodici, A.; Reno, C.; Gibertoni, D. Exploring the Gap Between Excess Mortality and COVID-19 Deaths in 67 Countries. JAMA Netw. Open. 2021, 4, e2117359. [Google Scholar] [CrossRef] [PubMed]
- Timonin, S.; Klimkin, I.; Shkolnikov, V.M.; Andreev, E.; McKee, M.; Leon, D.A. Excess Mortality in Russia and Its Regions Compared to High Income Countries: An Analysis of Monthly Series of 2020. SSM Popul. Health 2022, 17, 101006. [Google Scholar] [CrossRef]
- The True Death Toll of COVID-19: Estimating Global Excess Mortality. Available online: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality (accessed on 13 March 2022).
- Kobak, D. Excess Mortality Reveals COVID’S True Toll in Russia. Significance 2021, 18, 16–19. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Latest Insights—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/deaths (accessed on 13 March 2022).
- Deaths in the UK|Coronavirus in the UK. Available online: https://coronavirus.data.gov.uk/details/deaths (accessed on 13 March 2022).
- Alicandro, G.; Remuzzi, G.; Centanni, S.; Gerli, A.; La Vecchia, C. Excess Total Mortality in 2021 in Italy Was about One Third of That Observed in 2020. Med. Lav. 2021, 112, 414–421. [Google Scholar] [CrossRef]
- Barnard, S.; Chiavenna, C.; Fox, S.; Charlett, A.; Waller, Z.; Andrews, N.; Goldblatt, P.; Burton, P.; De Angelis, D. Methods for Modelling Excess Mortality across England during the COVID-19 Pandemic. Stat. Methods Med. Res. 2021; Online ahead of print. 9622802211046384. [Google Scholar] [CrossRef]
- Konstantinoudis, G.; Cameletti, M.; Gómez-Rubio, V.; Gómez, I.L.; Pirani, M.; Baio, G.; Larrauri, A.; Riou, J.; Egger, M.; Vineis, P.; et al. Regional Excess Mortality during the 2020 COVID-19 Pandemic in Five European Countries. Nat. Commun. 2022, 13, 482. [Google Scholar] [CrossRef]
- Portugal, L. Mortality and Excess Mortality: Improving FluMOMO. J. Environ. Public Health 2021, 2021, 5582589. [Google Scholar] [CrossRef]
- Verbeeck, J.; Faes, C.; Neyens, T.; Hens, N.; Verbeke, G.; Deboosere, P.; Molenberghs, G. A Linear Mixed Model to Estimate COVID-19-induced Excess Mortality. Biometrics, 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Stan Sanitarny Kraju w 2020 Roku—Główny Inspektorat Sanitarny—Portal Gov.pl. Available online: https://www.gov.pl/web/gis/stan-sanitarny-kraju-w-2020-roku (accessed on 30 January 2022).
- Wytyczne-Do-Karty-Zgonu-01.04.2020-1.Pdf. Available online: https://www.pzh.gov.pl/wp-content/uploads/2020/04/wytyczne-do-karty-zgonu-01.04.2020-1.pdf (accessed on 13 March 2022).
- EUROMOMO Partners. Available online: https://euromomo.eu/dev-404-page/ (accessed on 30 January 2022).
- BASiW—Statystyki Zgonów w 2020 Roku. Available online: https://basiw.mz.gov.pl/index.html#/visualization?id=3653 (accessed on 13 March 2022).
- Internetowa Stacja Meteorologiczna Warszawa—Historia Pomiarów. Available online: https://www.meteo.waw.pl/hist.pl (accessed on 13 March 2022).
- Wollschläger, D.; Schmidtmann, I.; Fückel, S.; Blettner, M.; Gianicolo, E. Explaining the age-adjusted excess mortality with COVID-19-attributed deaths from January 2020 to July 2021. Bundesgesundheitsblatt Gesundh. Gesundh. 2021, 65, 378–387. [Google Scholar] [CrossRef]
- Yasin, Y.J.; Grivna, M.; Abu-Zidan, F.M. Global Impact of COVID-19 Pandemic on Road Traffic Collisions. World J. Emerg. Surg. 2021, 16, 51. [Google Scholar] [CrossRef]
- Bogos, K.; Kiss, Z.; Kerpel Fronius, A.; Temesi, G.; Elek, J.; Madurka, I.; Cselkó, Z.; Csányi, P.; Abonyi-Tóth, Z.; Rokszin, G.; et al. Different Trends in Excess Mortality in a Central European Country Compared to Main European Regions in the Year of the COVID-19 Pandemic (2020): A Hungarian Analysis. Pathol. Oncol. Res. 2021, 27, 1609774. [Google Scholar] [CrossRef] [PubMed]
- Golinelli, D.; Lenzi, J.; Adja, K.Y.C.; Reno, C.; Sanmarchi, F.; Fantini, M.P.; Gibertoni, D. Small-Scale Spatial Analysis Shows the Specular Distribution of Excess Mortality between the First and Second Wave of the COVID-19 Pandemic in Italy. Public Health 2021, 194, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, M.P.; Walkowiak, D. Predictors of COVID-19 Vaccination Campaign Success: Lessons Learnt from the Pandemic So Far. A Case Study from Poland. Vaccines 2021, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kraska: Nikt w Polsce Nie Zmarł z Powodu Podania Szczepionki Przeciw COVID-19. Available online: https://serwisy.gazetaprawna.pl/zdrowie/artykuly/8310526,kraska-nikt-w-polsce-nie-zmarl-z-powodu-szczepionki.html (accessed on 13 March 2022).
- Koronawirus w Polsce. Wiceszef MZ Podał Liczbę Nowych Zakażeń. Available online: https://polskieradio24.pl/art129_2864886 (accessed on 30 January 2022).
- NOP—Niepożądany Odczyn Poszczepienny. Czym Jest i Jak Często Występuje?—Szczepienie Przeciwko COVID-19—Portal Gov.pl. Available online: https://www.gov.pl/web/szczepimysie/niepozadane-odczyny-poszczepienne (accessed on 13 March 2022).
- Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events|CDC. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 13 March 2022).
- Walkowiak, M.P.; Walkowiak, J.B.; Walkowiak, D. COVID-19 Passport as a Factor Determining the Success of National Vaccination Campaigns: Does It Work? The Case of Lithuania vs. Poland. Vaccines 2021, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
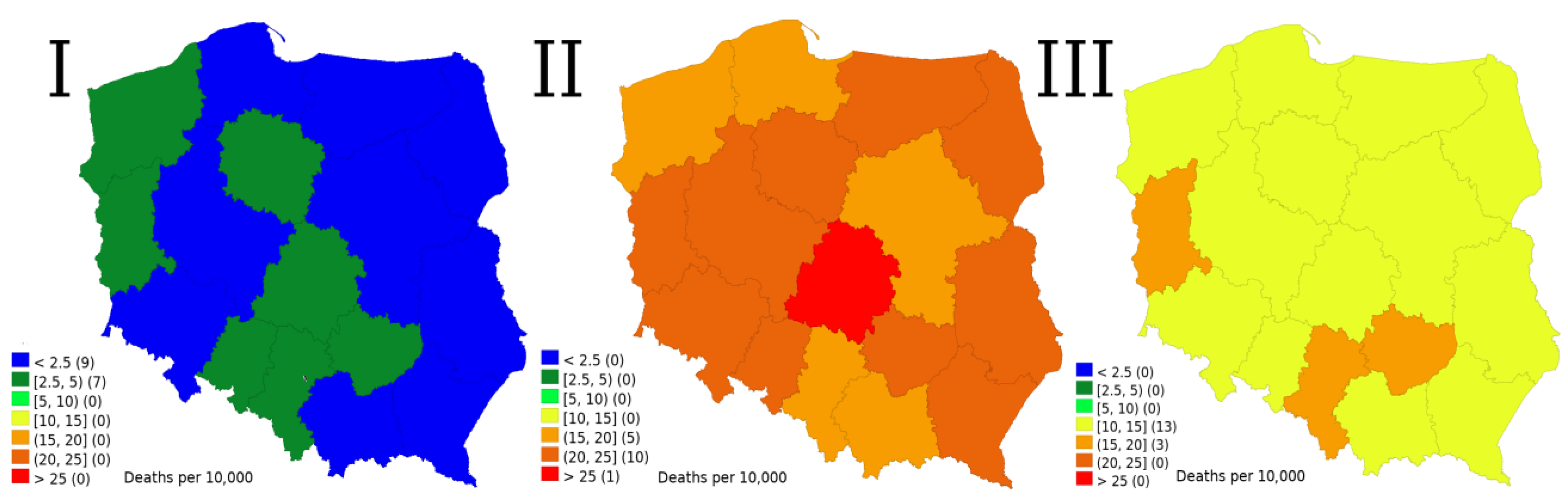
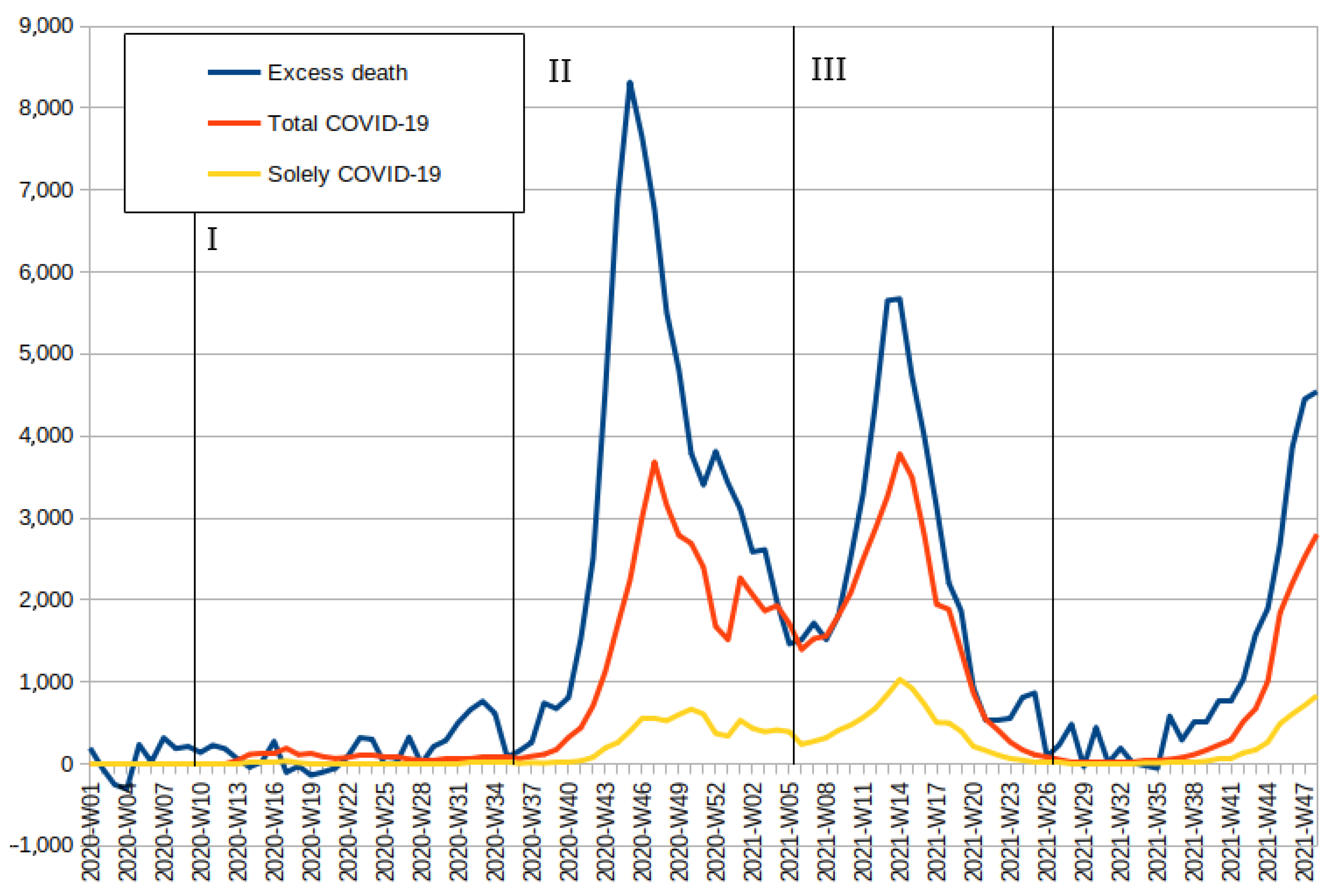
| Demographics | Excess Mortality | PCA Squared | Inferred COVID-19 Deaths | Officially Reported COVID-19 Deaths | Detection Rate |
|---|---|---|---|---|---|
| M0_39 | 1400 | 58% | 830 | 450 | 54% |
| M40_49 | 2800 | 85% | 2400 | 1000 | 43% |
| M50_59 | 6300 | 94% | 5900 | 2700 | 45% |
| M60_69 | 16,400 | 97% | 15,800 | 10,000 | 63% |
| M70_79 | 20,900 | 98% | 20,400 | 13,500 | 66% |
| M80_89 | 17,400 | 96% | 16,800 | 10,600 | 63% |
| M90plus | 4500 | 86% | 3900 | 2700 | 70% |
| F0_39 | 730 | 48% | 350 | 230 | 66% |
| F40_49 | 1200 | 74% | 920 | 450 | 49% |
| F50_59 | 2700 | 89% | 2400 | 1300 | 54% |
| F60_69 | 8600 | 95% | 8200 | 5000 | 62% |
| F70_79 | 14,900 | 97% | 14,400 | 8800 | 61% |
| F80_89 | 20,200 | 97% | 19,600 | 11,100 | 57% |
| F90plus | 8400 | 92% | 7700 | 4500 | 58% |
| Total | 126,400 | 95% | 119,600 | 72,300 | 60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak, M.P.; Walkowiak, D. Underestimation in Reporting Excess COVID-19 Death Data in Poland during the First Three Pandemic Waves. Int. J. Environ. Res. Public Health 2022, 19, 3692. https://doi.org/10.3390/ijerph19063692
Walkowiak MP, Walkowiak D. Underestimation in Reporting Excess COVID-19 Death Data in Poland during the First Three Pandemic Waves. International Journal of Environmental Research and Public Health. 2022; 19(6):3692. https://doi.org/10.3390/ijerph19063692
Chicago/Turabian StyleWalkowiak, Marcin Piotr, and Dariusz Walkowiak. 2022. "Underestimation in Reporting Excess COVID-19 Death Data in Poland during the First Three Pandemic Waves" International Journal of Environmental Research and Public Health 19, no. 6: 3692. https://doi.org/10.3390/ijerph19063692
APA StyleWalkowiak, M. P., & Walkowiak, D. (2022). Underestimation in Reporting Excess COVID-19 Death Data in Poland during the First Three Pandemic Waves. International Journal of Environmental Research and Public Health, 19(6), 3692. https://doi.org/10.3390/ijerph19063692






