Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review
Abstract
1. Introduction
| Type | Available Sources | Advantages | Disadvantages |
|---|---|---|---|
| Autogenous [19,20,21] | Extra-oral sites: Crest of the iliac bone, tibia, parietal bone, ribs, sternum. Intra-oral sites: Mandibular symphysis, ramus, maxillary tuberosity, zygomatic buttress, extraction socket, coronoid process, autogenous tooth. | Potential for osteogenesis, osteoinduction, osteoconduction, and osteopromotion. No allergenic and immune-mediated reaction and no possibility of graft rejection. Low cost. | Donor site surgery and morbidity. Increased surgical time which may require general anesthesia. Very large quantities cannot be harvested without significant donor site deficit. |
| Allogenic [21] | FDBA DFDBA DBM | Acts as a scaffold and allows osteoconduction. No donor site surgery/morbidity. Can be combined with other materials such as BMP, GFs, PRF to enhance its healing potential. | Processing required to remove allergenic component Rejection by the host is possible. |
| Xenogenic [19,21] (different species) | Porcine source Bovine source Corals Algae | Acts as a scaffold and allows osteoconduction. No donor site surgery. Can be combined with other materials such as BMP, GFs, PRF to enhance its healing potential. Low cost. Significant quantities can be acquired. | Processing required to remove allergenic components but still can transmit disease. Possibility of rejection. |
| Alloplastic [20] (synthetically produced) | TCP β-TCP Bioactive Glass Bio-ceramics Hydroxyapatite | Acts as a scaffold and allows osteoconduction. No donor site surgery. Can be combined with other materials such as BMP, GFs, PRF to enhance its healing potential. No allergenic potential. | Can be costly. Can act as foreign body. |
| Engineered personalized bone grafts [22,23,24] | Bioactive acellular scaffolds: Biodegradable synthetic materials with osteoinductive factors such as BMPs, PDGF, IGF. Cell seeded scaffolds: Autologous BMSCs in a customized scaffold mixed with PRP. Customized autologous bone grafts: Pluripotent stem cells induced to form bone. | Autologous stem cells with decreased chances of rejection. Can be molded into the desired anatomical shape using 3D modeling. Inclusion of bioactive molecules provides a better healing potential. | Still in infancy and further research needed to bring into clinical use. Requires facilities to harvest and culture stem cells. May have ethical issues. |
2. Composition and Biochemical Properties of AUTO-BG
3. General Characteristics of AUTO-BG
3.1. Biocompatibility of AUTO-BG
3.2. Bioactivity
3.3. Physical Properties
3.4. Clinical Outcome
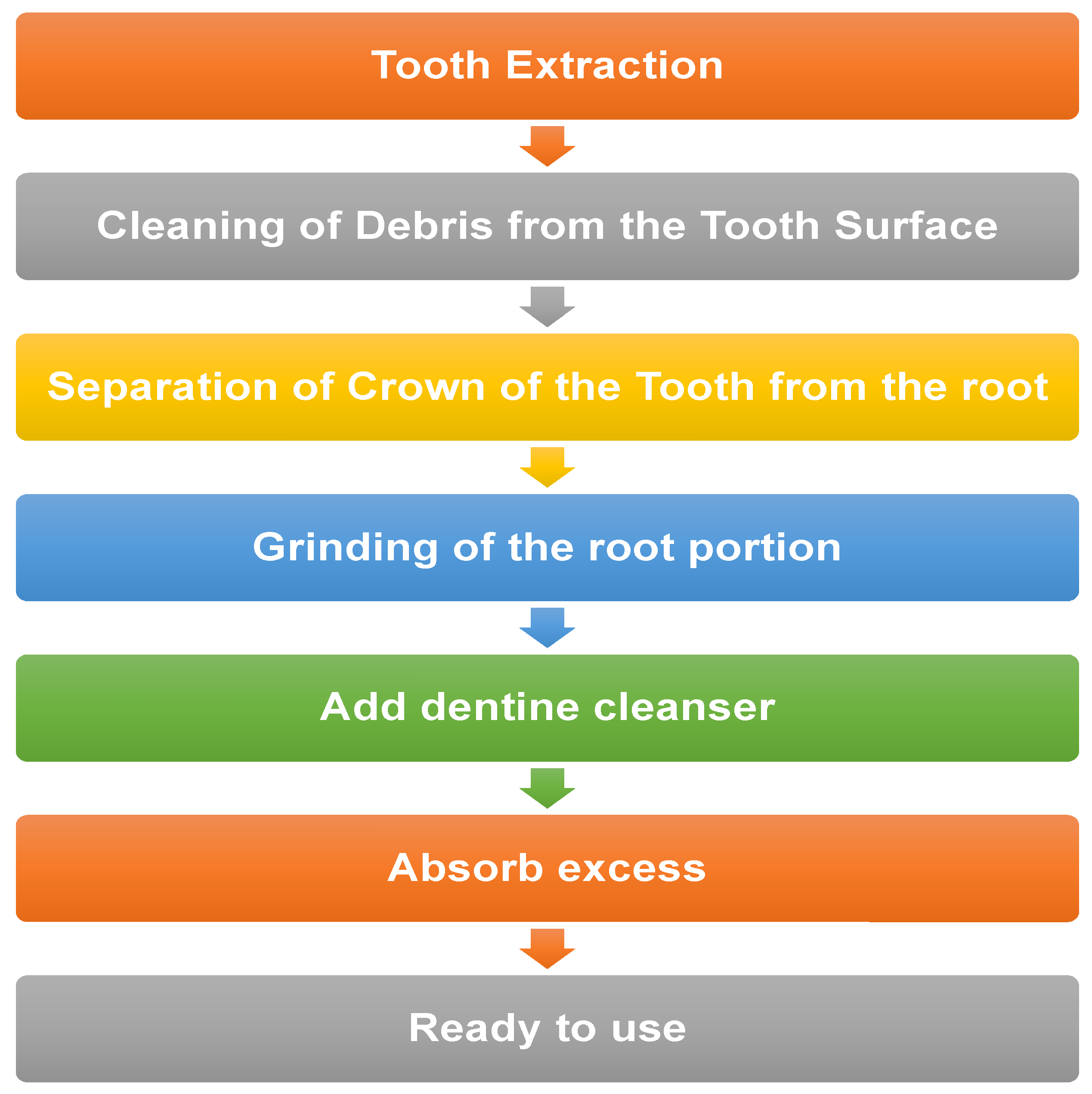
4. Method of Preparation of AUTO-BG
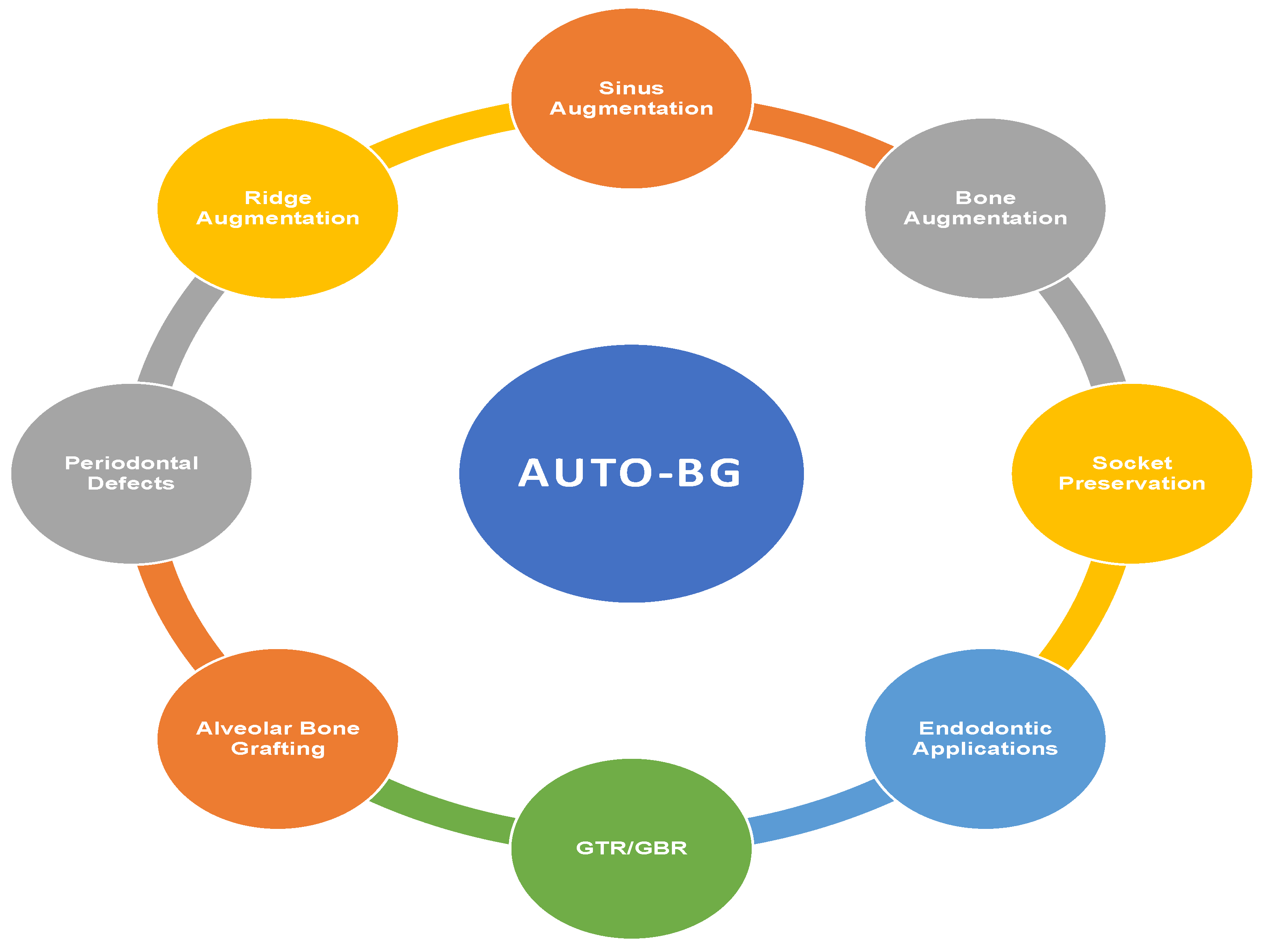
5. Clinical Applications of AUTO-BG
5.1. Bone Augmentation
5.2. Sinus Augmentation
5.3. Periodontal Defects
5.4. Guided Bone Regeneration
5.5. Alveolar Bone Grafting
5.6. Ridge Augmentation
5.7. Socket Preservation and Reconstruction
5.8. Restorative and Miscellaneous Applications
6. Complications Associated with AUTO-BG
6.1. Wound Dehiscence
6.2. Infection
6.3. Hematoma Formation
6.4. Exposure of Fixation Screws during the Healing Period
6.5. Resorption of Graft in the Form of Crestal or Marginal Bone Loss
6.6. Inability to Achieve Primary Stability while Placing Implants
6.7. Failure to Achieve Osseointegration with Implant Placement
6.8. Fracture of the Block Graft while Drilling at the Time of Fixation
7. Conclusions and Further Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonasson, G.; Rythén, M. Alveolar bone loss in osteoporosis: A loaded and cellular affair? Clin. Cosmet. Investig. Dent. 2016, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, M.K. Bone loss in the oral cavity. J. Bone Miner. Res. 1993, 8, S467–S473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Ahmed, W.; Asim, M.A.; Ehsan, A.; Abbas, Q. Non-vascularized autogenous bone grafts for reconstruction of maxillofacial osseous defects. J. Coll. Phys. Surg. Pak. 2018, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Alberktsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomateri. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Pandit, N.; Pandit, I.K. Autogenous bone grafts in periodontal practice: A literature review. J. Int. Clin. Dent. Res. Organ. 2016, 8, 27–33. [Google Scholar] [CrossRef]
- Kloss, F.R.; Offermanns, V.; Kloss-Brandstätter, A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects—A 12-month retrospective radiographic evaluation. Clin. Oral Implants Res. 2018, 29, 1163–1175. [Google Scholar] [CrossRef]
- Boyan, B.D.; Ranly, D.M.; McMillan, J.; Sunwoo, M.; Roche, K.; Schwartz, Z. Osteoinductive ability of human allograft formulations. J. Periodontol. 2006, 77, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Dehghani, S.; Shafiei, Z.; Nezhad, S.T. Xenogenic demineralized bone matrix and fresh autogenous cortical bone effects on experimental bone healing: Radiological, histopathological and biomechanical evaluation. J. Clin. Orthop. Trauma 2008, 9, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Grisdale, J. The clinical applications of synthetic bone alloplast. J. Can. Dent. Assoc. 1999, 65, 559–562. [Google Scholar]
- Mah, J.; Hung, J.; Wang, J.; Salih, E. The efficacy of various alloplastic bone grafts on the healing of rat calvarial defects. Eur. J. Orthod. 2004, 26, 475–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 3, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J. Synthetic and bone tissue engineering graft substitutes: What is the future? Injury 2021, 52S2, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Ferrario, S.; Poli, P.P.; Manfredini, M. Autogenous Chin Block Grafts in the Aesthetic Zone: A 20-Year Follow-Up Case Report. Case Rep. Dent. 2020, 2020, 6525797. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Um, I.-W.; Murata, M. Tooth bank system for bone regeneration-safety report. J. Hard Tissue Biol. 2014, 23, 371–376. [Google Scholar] [CrossRef][Green Version]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to autogenous bone graft: Efficacy and indications. J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Vunjak-Novakovic, G. Concise review: Personalized human bone grafts for reconstructing head and face. Stem Cells Transl. Med. 2012, 1, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Budak, I.; Mirkovic, S.; Sokac, M.; Santosi, Z.; Puskar, T.; Vukelic, D. An approach to modelling of personalized bone grafts based on advanced technologies. Int. J. Simul. Model. 2016, 15, 637–648. [Google Scholar] [CrossRef]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D printing of personalized artificial bone scaffolds. 3D Print Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Jang, H.-S.; Kim, S.-G.; Lim, S.-C.; Oh, J.-S.; Jeong, M.-A.; Kim, J.-S. Osteogenic ability according to the decalcified modality of auto-tooth bone grafts in peri-implant defects in dogs. Implant Dent. 2014, 23, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Kabir, M.A.; Um, I.-W.; Minamida, Y.; Kim, K.-W.; Kim, Y.-K.; Sun, Y.; Qin, C. Autograft of dentin materials for bone regeneration. Adv. Biomater. Sci. Biomed. Appl. 2013, 15, 391–403. [Google Scholar]
- Zhang, S.; Li, X.; Qi, Y.; Ma, X.; Qiao, S.; Cai, H.; Zhao, B.C.; Jiang, H.B.; Lee, E.-S. Comparison of Autogenous Tooth Materials and Other Bone Grafts. Tissue Eng. Regen. Med. 2021, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Maruta, M.; Shakya, M.; Yamada, K.; Akazawa, T. Bio-Absorption of Human Dentin-Derived Biomaterial in Sheep Critical-Size Iliac Defects. Materials 2021, 14, 223. [Google Scholar] [CrossRef]
- Gual-Vaqués, P.; Polis-Yanes, C.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Marí-Roig, A.; López-López, J. Autogenous teeth used for bone grafting: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e112–e119. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, S.-G.; Oh, J.-S.; Jin, S.-C.; Son, J.-S.; Kim, S.-Y.; Lim, S.-Y. Analysis of the inorganic component of autogenous tooth bone graft material. J. Nanosci. Nanotechnol. 2011, 11, 7442–7445. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Lee, J.K.; Kim, K.-W.; Um, I.-W.; Murata, M. Healing mechanism and clinical application of autogenous tooth bone graft material. In Advances in Biomaterials Science and Biomedical Applications [Internet]. Rijeka, Croatia; IntechOpen Limited: London, UK, 2013; pp. 405–435. [Google Scholar]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Bae, J.-H.; Um, I.-W.; Oh, J.-S.; Jeong, K.-I. Guided bone regeneration using autogenous tooth bone graft in implant therapy: Case series. Implant Dent. 2014, 23, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Pang, K.M.; Yun, P.Y.; Leem, D.H.; Um, I.W. Long-term follow-up of autogenous tooth bone graft blocks with dental implants. Clin. Case Rep. 2017, 5, 108–118. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of autogenous teeth for the reconstruction of alveolar ridge deficiencies: A systematic review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Kim, Y.-K. Bone graft material using teeth. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 134–138. [Google Scholar] [CrossRef]
- Bessho, K.; Tagawa, T.; Murata, M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J. Oral Maxillofac. Surg. 1990, 48, 162–169. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Chen, Z.; Zhang, L. Immunohistochemical Localization of LIM Mineralization Protein 1 in Pulp–Dentin Complex of Human Teeth with Normal and Pathologic Conditions. J. Endod. 2008, 34, 143–147. [Google Scholar] [CrossRef]
- Ravindran, S.; George, A. Dentin matrix proteins in bone tissue engineering. Eng. Miner. Load Bear. Tissues 2015, 881, 129–142. [Google Scholar] [CrossRef]
- Hao, J.; Zou, B.; Narayanan, K.; George, A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone 2004, 34, 921–932. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, Y.-K.; Yi, Y.-J.; Choi, J.-H. Clinical evaluation of ridge augmentation using autogenous tooth bone graft material: Case series study. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Ahn, J.-S.; Lee, J.-I.; Ahn, K.-J.; Yun, P.-Y.; Kim, Y.-K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Um, I.-W.; Kim, Y.-K.; Kim, K.-W. Clinical application of auto-tooth bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 2–8. [Google Scholar] [CrossRef]
- Ghahroudi, A.A.R.; Homayouni, A.; Rokn, A.R.; Kia, F.; Kharazifard, M.J.; Khorsand, A. Frequency of dental implants placed in the esthetic zone in dental clinic of Tehran University: A descriptive study. J. Dent. 2015, 12, 906–912. [Google Scholar]
- Bathla, S.C.; Fry, R.R.; Majumdar, K. Maxillary sinus augmentation. J. Indian Soc. Periodontol. 2018, 22, 468–473. [Google Scholar] [CrossRef]
- Zaniol, T.; Zaniol, A. A rational approach to sinus augmentation: The low window sinus lift. Case Rep. Dent. 2017, 2017, 7610607. [Google Scholar] [CrossRef]
- Jeong, T.M.; Lee, J.K. The efficacy of the graft materials after sinus elevation: Retrospective comparative study using panoramic radiography. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 146–153. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Yun, J.-Y.; Yun, P.-Y.; Um, I.-W. Comparison of autogenous tooth bone graft and synthetic bone graft materials used for bone resorption around implants after crestal approach sinus lifting: A retrospective study. J. Periodontal Implant Sci. 2014, 44, 216–221. [Google Scholar] [CrossRef]
- Shavit, E.; Shavit, I.; Pinchasov, D.; Shavit, D.; Pinchasov, G.; Juodzbalys, G. The Use of tooth derived bone graft materials in sinus augmentation procedures: A systematic review. J. Oral Maxillofac. Res. 2019, 10, e1. [Google Scholar] [CrossRef]
- Needleman, I.; Tucker, R.; Giedrys-Leeper, E.; Worthington, H. Guided tissue regeneration for periodontal intrabony defects–a Cochrane Systematic Review. Periodontol. 2000 2005, 37, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Sys. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Petsos, H.; Ratka-Krüger, P.; Neukranz, E.; Raetzke, P.; Eickholz, P.; Nickles, K. Infrabony defects 20 years after open flap debridement and guided tissue regeneration. J. Clin. Periodontol. 2019, 46, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Heijl, L.; Heden, G.; Svärdström, G.; Ostgren, A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J. Clin. Periodontol. 1997, 24, 705–714. [Google Scholar] [CrossRef]
- Seshima, F.; Aoki, H.; Takeuchi, T.; Suzuki, E.; Irokawa, D.; Makino-Oi, A.; Sugito, H.; Tomita, S.; Saito, A. Periodontal regenerative therapy with enamel matrix derivative in the treatment of intrabony defects: A prospective 2-year study. BMC Res. Notes 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Pisani, F.; De Vito, D.; Lone, M.A.; Almasri, M. Long-term Clinical Performance of Regeneration versus Conservative Surgery in the Treatment of Infra-bony Defects: A systematic review. J. Int. Acad. Periodontol. 2021, 23, 31–56. [Google Scholar]
- Shaikh, M.S.; Lone, M.A.; Matabdin, H.; Lone, M.A.; Soomro, A.H.; Zafar, M.S. Regenerative Potential of Enamel Matrix Protein Derivative and Acellular Dermal Matrix for Gingival Recession: A Systematic Review and Meta-Analysis. Proteomes 2021, 9, 11. [Google Scholar] [CrossRef]
- Lynch, S.E.; Williams, R.; Poison, A.; Howell, T.; Reddy, M.; Zappa, U.; Antoniades, H. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J. Clin. Periodontol. 1989, 16, 545–548. [Google Scholar] [CrossRef]
- Darby, I.B.; Morris, K.H. A systematic review of the use of growth factors in human periodontal regeneration. J. Periodontol. 2013, 84, 465–476. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Xu, X.; Li, C.; Huang, D.; Zhou, X.; Ye, L.; Zheng, L. Evaluation of recombinant human FGF-2 and PDGF-BB in periodontal regeneration: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 65. [Google Scholar] [CrossRef]
- Wei, L.; Teng, F.; Deng, L.; Liu, G.; Luan, M.; Jiang, J.; Liu, Z.; Liu, Y. Periodontal regeneration using bone morphogenetic protein 2 incorporated biomimetic calcium phosphate in conjunction with barrier membrane: A pre-clinical study in dogs. J. Clin. Periodontol. 2019, 46, 1254–1263. [Google Scholar] [CrossRef]
- Roselló-Camps, À.; Monje, A.; Lin, G.-H.; Khoshkam, V.; Chávez-Gatty, M.; Wang, H.-L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. Platelet-rich plasma for periodontal regeneration in the treatment of intrabony defects: A meta-analysis on prospective clinical trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Agwan, M.A.S.; Ansari, S.A.; Zafar, M.S.; Matinlinna, J.P. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: A systematic review of clinical studies. Tissue Eng. Regen. Med. 2017, 14, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L. Regeneration of periodontal tissue: Bone replacement grafts. Dent. Clin. N. Am. 2010, 54, 55–71. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Husain, S.; Lone, M.A.; Lone, M.A.; Akhlaq, H.; Zafar, M.S. Clinical effectiveness of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide grafts for regeneration of periodontal defects: A systematic review and meta-analysis. Regen. Med. 2020, 15, 2379–2395. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A. Comparing Nanohydroxyapatite Graft and Other Bone Grafts in the Repair of Periodontal Infrabony Lesions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 12021. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A.; Javed, F. Nanocrystalline hydroxyapatite in regeneration of periodontal intrabony defects: A systematic review and meta-analysis. Ann. Anat. 2022, 240, 151877. [Google Scholar] [CrossRef]
- Kataria, S.; Chandrashekar, K.T.; Mishra, R.; Tripathi, V. Autogenous bone graft for management of periodontal defects. J. Int. Clin. Dent. Res. Organ. 2016, 8, 70–75. [Google Scholar] [CrossRef]
- Upadhyay, P.; Blaggana, V.; Tripathi, P.; Jindal, M. Treatment of Furcation Involvement Using Autogenous Tooth Graft With 1-Year Follow-Up: A Case Series. Clin. Adv. Periodontics 2019, 9, 4–8. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Polis-Yanes, C.; Cadenas-Sebastián, C.; Gual-Vaqués, P.; Ayuso-Montero, R.; Marí-Roig, A.; López-López, J. Guided bone regeneration of an atrophic maxilla using heterologous cortical lamina. Case Rep. Dent. 2019, 2019, 5216362. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided bone regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, J.; Kim, Y.-K. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J. Dent. Sci. 2013, 8, 281–286. [Google Scholar] [CrossRef]
- Coots, B.K. Alveolar bone grafting: Past, present, and new horizons. Semin. Plast. Surg. 2012, 26, 178–183. [Google Scholar] [CrossRef][Green Version]
- Datarkar, A.; Bhawalkar, A. Utility of tooth as an autogenous graft material in the defects of alveolar cleft–A novel case report. J. Oral Biol. Craniofac. Res. 2020, 10, 470–473. [Google Scholar] [CrossRef]
- Jeong, K.-I.; Lee, J.; Kim, K.-W.; Um, I.-W.; Hara, S.; Mitsugi, M.; Kim, Y.-K. Alveolar Cleft Reconstruction Using Chin Bone and Autogenous Tooth Bone Graft Material: Reports of 5 Cases. J. Korean Dent. Sci. 2013, 6, 13–21. [Google Scholar] [CrossRef]
- Hara, S.; Mitsugi, M.; Kanno, T.; Tatemoto, Y. Bone transport and bone graft using auto-tooth bone for alveolar cleft repair. J. Craniofac. Surg. 2013, 24, e65–e68. [Google Scholar] [CrossRef]
- Mitsugi, M.; Kim, Y.; Hara, S.; Kim, K.; Um, I. Alveolar cleft reconstruction using autogenous tooth bone graft material. Int. J. Oral Maxillofac. Surg. 2013, 42, 1201. [Google Scholar] [CrossRef]
- Mazzucchi, G.; Lollobrigida, M.; Lamazza, L.; Serafini, G.; Di Nardo, D.; Testarelli, L.; De Biase, A. Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study. Materials 2022, 5, 1431. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Ku, J.-K. Ridge augmentation in implant dentistry. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, F.; Pacifici, A.; Giudice, A.; Polimeni, A.; Pacifici, L. Horizontal Ridge Augmentation and Contextual Implant Placement with a Resorbable Membrane and Particulated Anorganic Bovine Bone-Derived Mineral. Case Rep. Dent. 2019, 2019, 6710340. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: A focused review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [PubMed]
- Faria-Almeida, R.; Astramskaite-Januseviciene, I.; Puisys, A.; Correia, F. Extraction socket preservation with or without membranes, soft tissue influence on post extraction alveolar ridge preservation: A systematic review. J. Oral Maxillofac. Res. 2019, 10, e5. [Google Scholar] [CrossRef]
- Radoczy-Drajko, Z.; Windisch, P.; Svidro, E.; Tajti, P.; Molnar, B.; Gerber, G. Clinical, radiographical and histological evaluation of alveolar ridge preservation with an autogenous tooth derived particulate graft in EDS class 3–4 defects. BMC Oral Health 2021, 21, 63. [Google Scholar] [CrossRef]
- Kizildag, A.; Tasdemir, U.; Arabaci, T.; Özmen, Ö.; Kizildag, C.A.; Iyilikci, B. Evaluation of new bone formation using autogenous tooth bone graft combined with platelet-rich fibrin in calvarial defects. J. Craniofac. Surg. 2019, 30, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Arad, D.; Levin, L. Intraoral autogenous block onlay bone grafting for extensive reconstruction of atrophic maxillary alveolar ridges. J. Periodontol. 2005, 76, 636–641. [Google Scholar] [CrossRef]
- Cenicante, J.; Botelho, J.; Machado, V.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G.; Santos, A. The Use of Autogenous Teeth for Alveolar Ridge Preservation: A Literature Review. Appl. Sci. 2021, 11, 1853. [Google Scholar] [CrossRef]
- Gharpure, A.S.; Bhatavadekar, N.B. Clinical efficacy of tooth-bone graft: A systematic review and risk of bias analysis of randomized control trials and observational studies. Implant Dent. 2018, 27, 119–134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janjua, O.S.; Qureshi, S.M.; Shaikh, M.S.; Alnazzawi, A.; Rodriguez-Lozano, F.J.; Pecci-Lloret, M.P.; Zafar, M.S. Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3690. https://doi.org/10.3390/ijerph19063690
Janjua OS, Qureshi SM, Shaikh MS, Alnazzawi A, Rodriguez-Lozano FJ, Pecci-Lloret MP, Zafar MS. Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(6):3690. https://doi.org/10.3390/ijerph19063690
Chicago/Turabian StyleJanjua, Omer Sefvan, Sana Mehmood Qureshi, Muhammad Saad Shaikh, Ahmad Alnazzawi, Francisco J. Rodriguez-Lozano, Maria Pilar Pecci-Lloret, and Muhammad Sohail Zafar. 2022. "Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 6: 3690. https://doi.org/10.3390/ijerph19063690
APA StyleJanjua, O. S., Qureshi, S. M., Shaikh, M. S., Alnazzawi, A., Rodriguez-Lozano, F. J., Pecci-Lloret, M. P., & Zafar, M. S. (2022). Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review. International Journal of Environmental Research and Public Health, 19(6), 3690. https://doi.org/10.3390/ijerph19063690









