Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home
Abstract
:1. Introduction
Study Objectives
2. Materials and Methods
2.1. Design
2.2. Data Collection
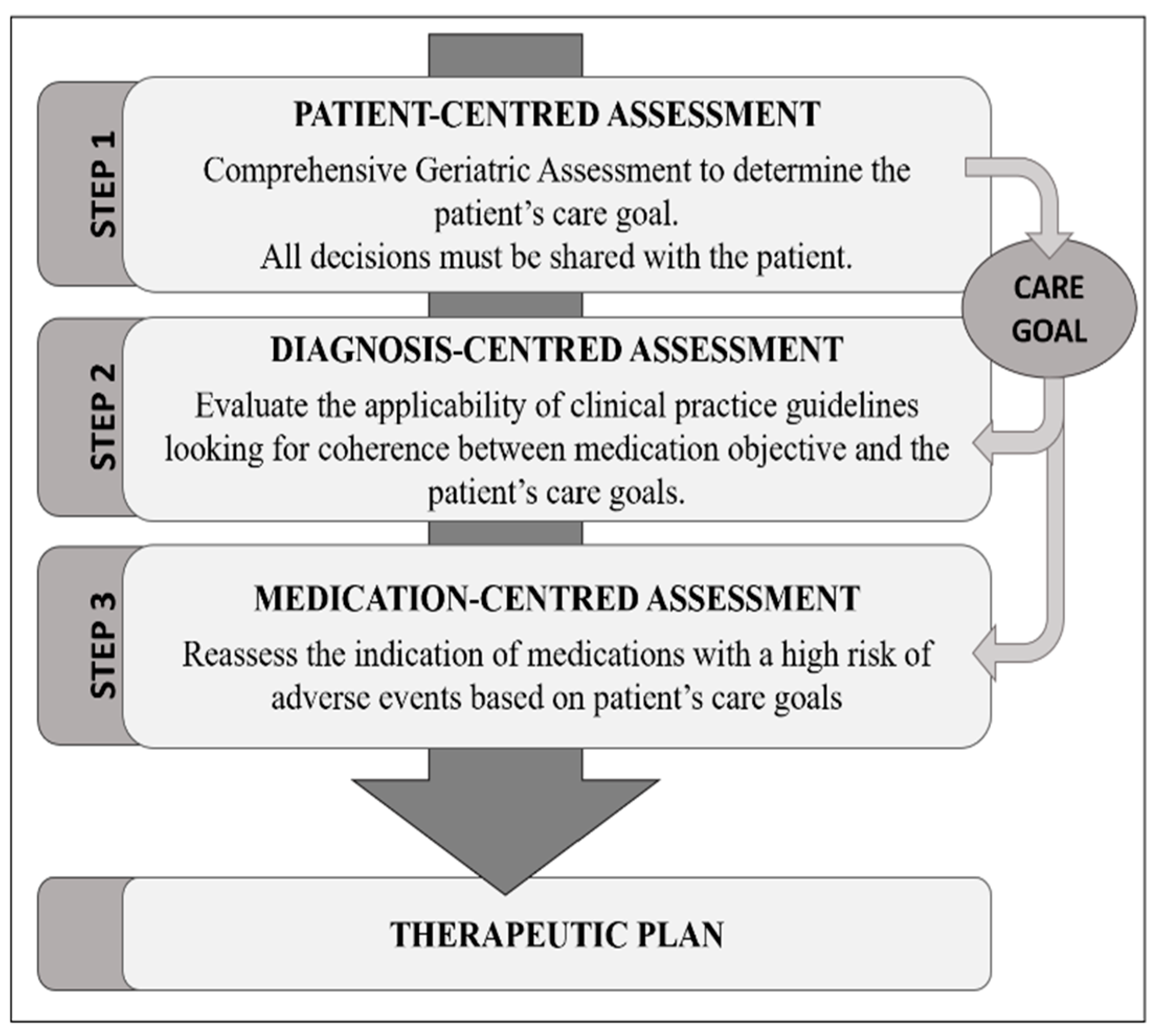
2.3. Medication Review
Criteria Used to Determine IP
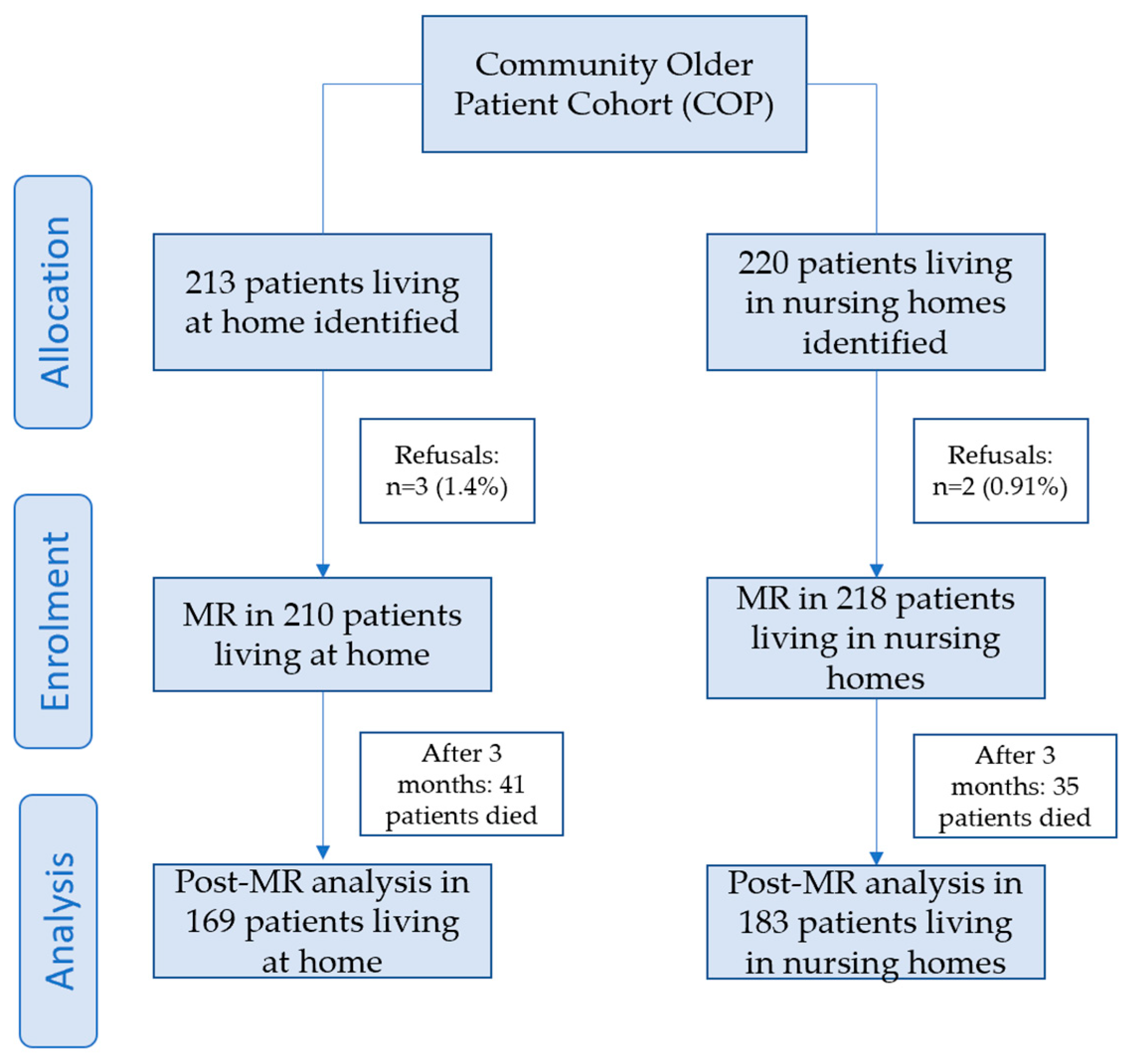
2.4. Sample Size
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kua, C.-H.; Mak, V.S.; Lee, S.W.H. Health Outcomes of Deprescribing Interventions Among Older Residents in Nursing Homes: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 362–372.e11. [Google Scholar] [CrossRef] [PubMed]
- NICE Medicines and Prescribing Centre (UK). Medicines optimisation: The safe and effective use of medicines to enable the best possible outcomes. In NICE Guideline; National Institute for Health and Care Excellence (NICE): London, UK, 2015. [Google Scholar]
- Hilmer, S.; Gnjidic, D. Prescribing for frail older people. Aust. Prescr. 2017, 40, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, M.; Sirven, N.; Grondin, H.; Pichetti, S.; Sermet, C. Frailty, polypharmacy, and potentially inappropriate medications in old people: Findings in a representative sample of the French population. Eur. J. Clin. Pharmacol. 2017, 73, 1165–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilmer, S.N.; Kirkpatrick, C.M.J. New Horizons in the impact of frailty on pharmacokinetics: Latest developments. Age Ageing 2021, 50, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing Inappropriate Polypharmacy. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.; Gómez, F.; Curcio, C.-L.; Pineda, E.; de Souza, J.F. Prevalence and impact of potentially inappropriate medication on community-dwelling older adults. Biomédica 2021, 41, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Reallon, E.; Chavent, B.; Gervais, F.; Dauphinot, V.; Vernaudon, J.; Krolak-Salmon, P.; Mouchoux, C.; Novais, T. Medication exposure and frailty in older community-dwelling patients: A cross-sectional study. Int. J. Clin. Pharm. 2020, 42, 508–514. [Google Scholar] [CrossRef]
- Sevilla-Sanchez, D.; Molist-Brunet, N.; Amblàs-Novellas, J.; Roura-Poch, P.; Espaulella-Panicot, J.; Codina-Jané, C. Adverse drug events in patients with advanced chronic conditions who have a prognosis of limited life expectancy at hospital admission. Eur. J. Clin. Pharmacol. 2017, 73, 79–89. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.Á.; Sempere-Verdú, E.; Vicens, C.; González-Rubio, F.; Miguel-García, F.; Palop-Larrea, V.; Orueta-Sánchez, R.; Esteban-Jiménez, Ó.; Sempere-Manuel, M.; Arroyo-Aniés, M.P.; et al. Evolution of polypharmacy in a spanish population (2005–2015): A database study. Pharmacoepidemiol. Drug Saf. 2020, 29, 433–443. [Google Scholar] [CrossRef]
- Woodford, H.J.; Fisher, J. New horizons in deprescribing for older people. Age Ageing 2019, 48, 768–775. [Google Scholar] [CrossRef]
- Garber, J.; Brownlee, S. Medication Overload: America’s Other Drug Problem; The Lown Institute: Needham, MA, USA, 2019; pp. 1–50. [Google Scholar]
- Poudel, A.; Peel, N.; Mitchell, C.; Nissen, L.; Hubbard, R. A systematic review of prescribing criteria to evaluate appropriateness of medications in frail older people. Rev. Clin. Gerontol. 2014, 24, 304–318. [Google Scholar] [CrossRef]
- Liau, S.J.; Lalic, S.; Sluggett, J.K.; Cesari, M.; Onder, G.; Vetrano, D.L.; Morin, L.; Hartikainen, S.; Hamina, A.; Johnell, K.; et al. Medication Management in Frail Older People: Consensus Principles for Clinical Practice, Research, and Education. J. Am. Med. Dir. Assoc. 2020, 22, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Griese-Mammen, N.; Hersberger, K.E.; Messerli, M.; Leikola, S.; Horvat, N.; Van Mil, J.W.F.; Kos, M. PCNE definition of medication review: Reaching agreement. Int. J. Clin. Pharm. 2018, 40, 1199–1208. [Google Scholar] [CrossRef]
- Brunet, N.M.; Sevilla-Sánchez, D.; Novellas, J.A.; Jané, C.C.; Gómez-Batiste, X.; McIntosh, J.; Panicot, J.E. Optimizing drug therapy in patients with advanced dementia: A patient-centered approach. Eur. Geriatr. Med. 2014, 5, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Brunet, N.M.; Panicot, J.E.; Sevilla-Sánchez, D.; Novellas, J.A.; Jané, C.C.; Roset, J.A.; Gómez-Batiste, X. A patient-centered prescription model assessing the appropriateness of chronic drug therapy in older patients at the end of life. Eur. Geriatr. Med. 2015, 6, 565–569. [Google Scholar] [CrossRef]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; González-Bueno, J.; Solà-Bonada, N.; Cruz-Grullón, M.; Espaulella-Panicot, J. Optimizing drug therapy in frail patients with type 2 diabetes mellitus. Aging Clin. Exp. Res. 2019, 32, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; González-Bueno, J.; Garcia-Sánchez, V.; Segura-Martín, L.A.; Codina-Jané, C.; Espaulella-Panicot, J. Therapeutic optimization through goal-oriented prescription in nursing homes. Int. J. Clin. Pharm. 2021, 43, 990–997. [Google Scholar] [CrossRef] [PubMed]
- González-Bueno, J.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Molist-Brunet, N.; Codina-Jané, C.; Espaulella-Panicot, J. Improving medication adherence and effective prescribing through a patient-centered prescription model in patients with multimorbidity. Eur. J. Clin. Pharmacol. 2021, 78, 127–137. [Google Scholar] [CrossRef]
- Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Espaulella-Ferrer, M.; Amblàs-Novellas, J.; Espaulella-Panicot, J. Factors Associated with the Detection of Inappropriate Prescriptions in Older People: A Prospective Cohort. Int. J. Environ. Res. Public Health 2021, 18, 11310. [Google Scholar] [CrossRef]
- El Haddad, K.; de Souto Barreto, P.; de Mazieres, C.L.; Rolland, Y. Effect of a geriatric intervention aiming to improve polypharmacy in nursing homes. Eur. Geriatr. Med. 2020, 11, 863–868. [Google Scholar] [CrossRef]
- Boyd, K.; Murray, S.A. Recognising and managing key transitions in end of life care. BMJ 2010, 341, c4863. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, H.; Smit, E.B.; Wattel, E.M.; van der Wouden, J.; Hertogh, C.M.; Terluin, B.; Terwee, C.B. Measurement Properties of the Barthel Index in Geriatric Rehabilitation. J. Am. Med. Dir. Assoc. 2019, 20, 420–425.e1. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, C.; Johnson, L.; Purdy, S.; Valderas, J.M.; Montgomery, A. Epidemiology and impact of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2011, 61, e12–e21. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Brusselaers, N. The Charlson Comorbidity Index in Registry-based Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.; de Leon, M.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Amblàs-Novellas, J.; Martori, J.C.; Espaulella, J.; Oller, R.; Molist-Brunet, N.; Inzitari, M.; Romero-Ortuno, R. Frail-VIG index: A concise frailty evaluation tool for rapid geriatric assessment. BMC Geriatr. 2018, 18, 29. [Google Scholar] [CrossRef] [Green Version]
- Gnjidic, D.; Hilmer, S.; Blyth, F.M.; Naganathan, V.; Waite, L.; Seibel, M.; McLachlan, A.; Cumming, R.; Handelsman, D.J.; Le Couteur, D. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012, 65, 989–995. [Google Scholar] [CrossRef]
- Saez de la Fuente, J.; Such Diaz, A.; Cañamares-Orbis, I.; Ramila, E.; Izquierdo-Garcia, E.; Esteban, C.; Escobar-Rodríguez, I. Cross-cultural Adaptation and Validation of the Medication Regimen Complexity Index Adapted to Spanish. Ann. Pharmacother. 2016, 50, 918–925. [Google Scholar] [CrossRef]
- Pantuzza, L.L.; Ceccato, M.D.G.B.; Silveira, M.R.; Pinto, I.V.; Reis, A.M.M. Validation and standardization of the Brazilian version of the Medication Regimen Complexity Index for older adults in primary care. Geriatr. Gerontol. Int. 2018, 18, 853–859. [Google Scholar] [CrossRef]
- Hilmer, S.N. Calculating and using the drug burden index score in research and practice. Expert Rev. Clin. Pharmacol. 2018, 11, 1053–1055. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Batiste, X.; Martínez-Muñoz, M.; Blay, C.; Amblàs, J.; Vila, L.; Costa, X.; Espaulella, J.; Espinosa, J.; Constante, C.; Mitchell, G.K. Prevalence and characteristics of patients with advanced chronic conditions in need of palliative care in the general population: A cross-sectional study. Palliat. Med. 2014, 28, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, C.M. Individualizing Prevention for Older Adults. J. Am. Geriatr. Soc. 2018, 66, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Gallagher, P.; O’Mahony, D. Deprescribing in older people approaching end-of-life: Development and validation of STOPPFrail version 2. Age Ageing 2021, 50, 465–471. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 12-Older Adults: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S168–S179. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Huelgas, R.; Peralta, F.G.; Mañas, L.R.; Formiga, F.; Domingo, M.P.; Bravo, J.M.; Miranda, C.; Ena, J. Treatment of type 2 diabetes mellitus in elderly patients. Rev. Clín. Española (Engl. Ed.) 2018, 218, 74–88. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2014, 44, 213–218. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence (NICE). Hypertension in Adults: Diagnosis and Management; NICE: London, UK, 2016. [Google Scholar]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Johannesen, C.D.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef]
- Van der Steen, J.T.; Radbruch, L.; Hertogh, C.M.; de Boer, M.E.; Hughes, J.C.; Larkin, P.; Francke, A.L.; Jünger, S.; Gove, D.; Firth, P.; et al. White paper defining optimal palliative care in older people with dementia: A Delphi study and recommendations from the European Association for Palliative Care. Palliat. Med. 2014, 28, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Bjerre, L.M.; Farrell, B.; Hogel, M.; Graham, L.; Lemay, G.; McCarthy, L.; Raman-Wilms, L.; Rojas-Fernandez, C.; Sinha, S.; Thompson, W.; et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: Evidence-based clinical practice guideline. Can. Fam. Physician 2018, 64, 17–27. [Google Scholar]
- Fick, D.M.; Semla, T.P.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.E.; Pezzullo, L.; Epplin, J.J.; Flanagan, N.; et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar]
- Scottish Intercollegiate Guidelines Network, (SIGN). Management of Chronic Pain. A National Clinical Guideline; Healthcare Improvement Scotland: Edinburgh, UK, 2019. [Google Scholar]
- Récoché, I.; Lebaudy, C.; Cool, C.; Sourdet, S.; Piau, A.; Lapeyre-Mestre, M.; Vellas, B.; Cestac, P. Potentially inappropriate prescribing in a population of frail elderly people. Int. J. Clin. Pharm. 2016, 39, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Nair, S. New horizons in care home medicine. Age Ageing 2014, 43, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Model D’atenció Sanitària a les Residències de Catalunya. Una Proposta des de l’Atenció Primària de Salut; Societat de Medicina Familiar i Comunitària-Associació d’Infermeria Familiar i Comunitària de Catalunya: Barcelona, Spain, 2020.
- Gordon, A.L.; Franklin, M.; Bradshaw, L.; Logan, P.; Elliott, R.; Gladman, J.R. Health status of UK care home residents: A cohort study. Age Ageing 2014, 43, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falch, C.; Alves, G. Pharmacists’ Role in Older Adults’ Medication Regimen Complexity: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 8824. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Alfahmi, A.; Farooq, A.; Akhtar, M.; Ajaz, S.; Alotaibi, S.; Faiz, M.; Ali, S. Effectiveness of Interventions to Improve the Anticholinergic Prescribing Practice in Older Adults: A Systematic Review. J. Clin. Med. 2022, 11, 714. [Google Scholar] [CrossRef]
- Delgado-Silveira, E.; Bermejo-Vicedo, T. The role of pharmacists in geriatric teams: The time is now. Eur. Geriatr. Med. 2021, 12, 1119–1121. [Google Scholar] [CrossRef]
- Vestergaard Ravn-Nielsen, L.; Duckert, M.-L.; Hallas, J. Effect of an In-Hospital Multifaceted Clinical Pharmacist Intervention on the Risk of Readmission A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Fournier, A.; Anrys, P.; Beuscart, J.-B.; Dalleur, O.; Henrard, S.; Foulon, V.; Spinewine, A. Use and Deprescribing of Potentially Inappropriate Medications in Frail Nursing Home Residents. Drugs Aging 2020, 37, 917–924. [Google Scholar] [CrossRef]
- Ibrahim, K.; Cox, N.J.; Stevenson, J.M.; Lim, S.; Fraser, S.D.S.; Roberts, H.C. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021, 21, 258. [Google Scholar] [CrossRef]
- Dautzenberg, L.; Bretagne, L.; Koek, H.L.; Tsokani, S.; Zevgiti, S.; Rodondi, N.; Scholten, R.J.P.M.; Rutjes, A.W.; Di Nisio, M.; Raijmann, R.C.M.A.; et al. Medication review interventions to reduce hospital readmissions in older people. J. Am. Geriatr. Soc. 2021, 69, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Lenders, D.; Jansen, J.; Stoffers, H.; Winkens, B.; Aretz, K.; Twellaar, M.; Schols, J.; van der Kuy, P.-H.; Knottnerus, J.; Akker, M.V.D. The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. J. Clin. Med. 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, H.; Stafford, A.; Etherton-Beer, C.; Flicker, L. Optimisation of medications used in residential aged care facilities: A systematic review and meta-analysis of randomised controlled trials. BMC Geriatr. 2020, 20, 236. [Google Scholar] [CrossRef] [PubMed]
| Target | Patients | ||
|---|---|---|---|
| Healthy Older Adults * | Frail Older Adults † | Older Adults in a Probable EOL Situation ‡ | |
| Qualitative Glycaemic | Similar to those for diabetic young adults | Assess the decrease of therapeutic intensity | Quality of life preservation: avoid hypoglycaemic and symptomatic hyperglycaemic episodes |
| Quantitative HbA1c | ≤7–7.5% | ≤8.0% | Avoid reliance on A1C ** |
| Therapeutic Goal †† | Prolong survival | Maintain functionality | Symptomatic treatment |
| Baseline Data | Home n = 210 (49.1%) | Nursing Home n = 218 (50.9%) | p | |
|---|---|---|---|---|
| Age (years), mean (SD *) | 85.74 (6.74) | 85.31 (8.48) | 0.558 | |
| Gender | Men | 83 (39.5%) | 60 (27.5%) | 0.09 |
| Women | 127 (60.5%) | 158 (72.5%) | ||
| Medication management | 57 (27.1%) | 1 (0.5%) | <0.001 | |
| Barthel Index (BI), mean (SD) | 65.21 (28.08) | 35.21 (28.79) | <0.001 | |
| Functional status, BI † degrees | Independence: BI ≥ 95 | 46 (21.9%) | 5 (2.3%) | <0.001 |
| Mild dependence: BI 90–65 | 75 (35.7%) | 45 (20.6%) | ||
| Mod. dependence: BI 60–25 | 70 (33.3%) | 59 (27.1%) | ||
| Severe dependence: BI ≤ 20 | 19 (9.0%) | 109 (50.0%) | ||
| Cognitive status | No dementia | 75 (35.7%) | 37 (17.0%) | <0.001 |
| Mild dementia (GDS 4) | 29 (13.9%) | 33 (15.1%) | ||
| Moderate dementia (from GDS 5 to GDS 6B) | 66 (31.4%) | 46 (21.1%) | ||
| Advanced dementia (from GDS 6C) | 40 (19.0%) | 102 (46.8%) | ||
| Emotional status | Euthymic | 102 (48.6%) | 82 (37.6%) | 0.02 |
| Depressive syndrome | 93 (44.3%) | 105 (48.2%) | 0.421 | |
| Anxiety syndrome | 19 (9.0%) | 16 (7.3%) | 0.519 | |
| Other psychiatric disorders | 5 (2.4%) | 29 (13.3%) | <0.001 | |
| Frailty Index (FI): VIG-Frail index, mean (SD) | 0.34 (0.13) | 0.43 (0.11) | <0.001 | |
| VIG-Frailty index degrees | No frailty (FI < 0.20) | 30 (14.3%) | 2 (0.9%) | <0.001 |
| Mild frailty (0.20–0.35) | 69 (32.9%) | 44 (20.2%) | ||
| Moderate frailty (0.36–0.50) | 86 (41.1%) | 115 (52.8%) | ||
| Severe frailty (FI > 0.50) | 25 (11.9%) | 115 (52.8%) | ||
| End-of-life patients | No | 168 (80.0%) | 105 (48.2%) | <0.001 |
| Yes | 42 (20.0%) | 113 (51.8%) | ||
| Number of geriatric syndromes, mean (SD) | 2.78 (1.50) | 3.07 (1.53) | 0.047 | |
| Type of geriatric syndrome | Falls | 76 (36.2%) | 68 (31.2%) | 0.274 |
| Dysphagia | 36 (17.1%) | 48 (22.0%) | 0.204 | |
| Pain | 47 (22.4%) | 52 (23.9%) | 0.718 | |
| Pressure ulcers | 10 (4.8%) | 10 (4.6%) | 0.932 | |
| Constipation | 67 (31.9%) | 68 (31.2%) | 0.874 | |
| Insomnia | 106 (50.5%) | 123 (56.4%) | 0.218 | |
| Malnutrition | 16 (7.6%) | 25 (11.5%) | 0.176 | |
| Incontinence | 79 (37.6%) | 153 (70.2%) | <0.001 | |
| Previous delirium | 32 (23.4%) | 23 (44.2%) | 0.007 | |
| Morbidities | Number of morbidities, mean (SD) | 5.51 (2.21) | 4.32 (1.94) | <0.001 |
| Age-adjusted Charlson Index, mean (SD) | 3.37 (2.38) | 3.15 (2.17) | 0.33 | |
| Main therapeutic aim | Survival | 31 (14.8%) | 10 (4.6%) | <0.001 |
| Functional | 128 (61.0%) | 95 (43.6%) | ||
| Symptomatic | 51 (24.3%) | 113 (51.8%) | ||
| Mortality | 41 (19.5%) | 35 (16.1%) | 0.348 | |
| Baseline Pharmacological Data | Home n = 210 (49.1%) | Nursing Home n = 218 (50.9%) | p | |
|---|---|---|---|---|
| No. of med ** | Mean (SD *) | 8.84 (3.93) | 7.45 (3.70) | <0.001 |
| Polypharmacy | No polypharmacy: 0–4 med ** | 29 (13.8%) | 51 (23.4%) | 0.04 |
| 5–9 med ** | 97 (46.2%) | 108 (49.5%) | ||
| ≥10 med ** | 84 (40.0%) | 59 (27.1%) | ||
| MRCI † | Mean | 33.12 (16.83) | 28.44 (15.39) | 0.03 |
| DBI ‖ | Mean (SD *) | 1.08 (0.84) | 1.26 (0.83) | 0.031 |
| IP ‡ | Mean (SD *) | 3.31 (2.42) | 2.97 (2.10) | 0.108 |
| IP ‡ | 0 IP | 25 (11.9%) | 18 (8.3%) | 0.209 |
| 1 or more IP ‡ | 185 (88.1%) | 200 (91.7%) | ||
| Home | Nursing Home | p | |||
|---|---|---|---|---|---|
| Medication number, mean (SD *) | Pre-MR | 8.84 (3.94) | 7.45 (3.70) | <0.001 | |
| Post-MR | 7.75 (3.58) | 5.66 (3.58) | <0.001 | ||
| Difference | −1.20 (2.07) | −1.68 (1.84) | 0.020 | ||
| Polypharmacy N (%) | Pre-MR † | No polypharmacy | 29 (13.8%) | 51 (23.4%) | 0.004 |
| 5–9 medications | 97 (46.2%) | 108 (49.5%) | |||
| ≥10 medications | 84 (40.0%) | 59 (27.1%) | |||
| Post-MR | No polypharmacy | 28 (16.6%) | 77 (42.1%) | <0.001 | |
| 5–9 medications | 91 (53.8%) | 80 (43.7%) | |||
| ≥10 medications | 50 (29.6%) | 26 (14.2%) | |||
| MRCI ‡, mean (SD) | Pre-MR | 33.1 (16.8) | 28.4 (15.4) | 0.003 | |
| Post-MR | 28.7 (14.9) | 21.7 (14.4) | <0.001 | ||
| Difference | −4.8 (8.9) | −6.9 (7.4) | 0.016 | ||
| DBI ‖, mean (SD) | Pre-MR | 1.08 (0.84) | 1.26 (0.84) | 0.031 | |
| Post-MR | 1.01 (0.78) | 1.17 (0.86) | 0.079 | ||
| Difference | −0.09 (0.35) | −0.13 (0.35) | 0.359 | ||
| MDE **, median (Q1; Q3) | Pre-MR | 71.24 (29.6; 130.5) | 51.81 (25.3; 99,1) | 0.012 | |
| Post-MR | 60.59 (26.3; 122.2) | 36.46 (17.7; 83,0) | <0.001 | ||
| Difference | −2.17 (−16.3; 2.0) | −6.41 (−16.9; −1.9) | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molist-Brunet, N.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Bajo-Peñas, L.; Cantizano-Baldo, I.; Cabanas-Collell, L.; Espaulella-Panicot, J. Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home. Int. J. Environ. Res. Public Health 2022, 19, 3423. https://doi.org/10.3390/ijerph19063423
Molist-Brunet N, Sevilla-Sánchez D, Puigoriol-Juvanteny E, Bajo-Peñas L, Cantizano-Baldo I, Cabanas-Collell L, Espaulella-Panicot J. Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home. International Journal of Environmental Research and Public Health. 2022; 19(6):3423. https://doi.org/10.3390/ijerph19063423
Chicago/Turabian StyleMolist-Brunet, Núria, Daniel Sevilla-Sánchez, Emma Puigoriol-Juvanteny, Lorena Bajo-Peñas, Immaculada Cantizano-Baldo, Laia Cabanas-Collell, and Joan Espaulella-Panicot. 2022. "Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home" International Journal of Environmental Research and Public Health 19, no. 6: 3423. https://doi.org/10.3390/ijerph19063423
APA StyleMolist-Brunet, N., Sevilla-Sánchez, D., Puigoriol-Juvanteny, E., Bajo-Peñas, L., Cantizano-Baldo, I., Cabanas-Collell, L., & Espaulella-Panicot, J. (2022). Individualized Medication Review in Older People with Multimorbidity: A Comparative Analysis between Patients Living at Home and in a Nursing Home. International Journal of Environmental Research and Public Health, 19(6), 3423. https://doi.org/10.3390/ijerph19063423






