Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Variables
2.2.2. Health Status
2.2.3. Helplessness
2.2.4. Self-Efficacy
2.2.5. Condition Impact
2.2.6. Depression
2.3. Procedures
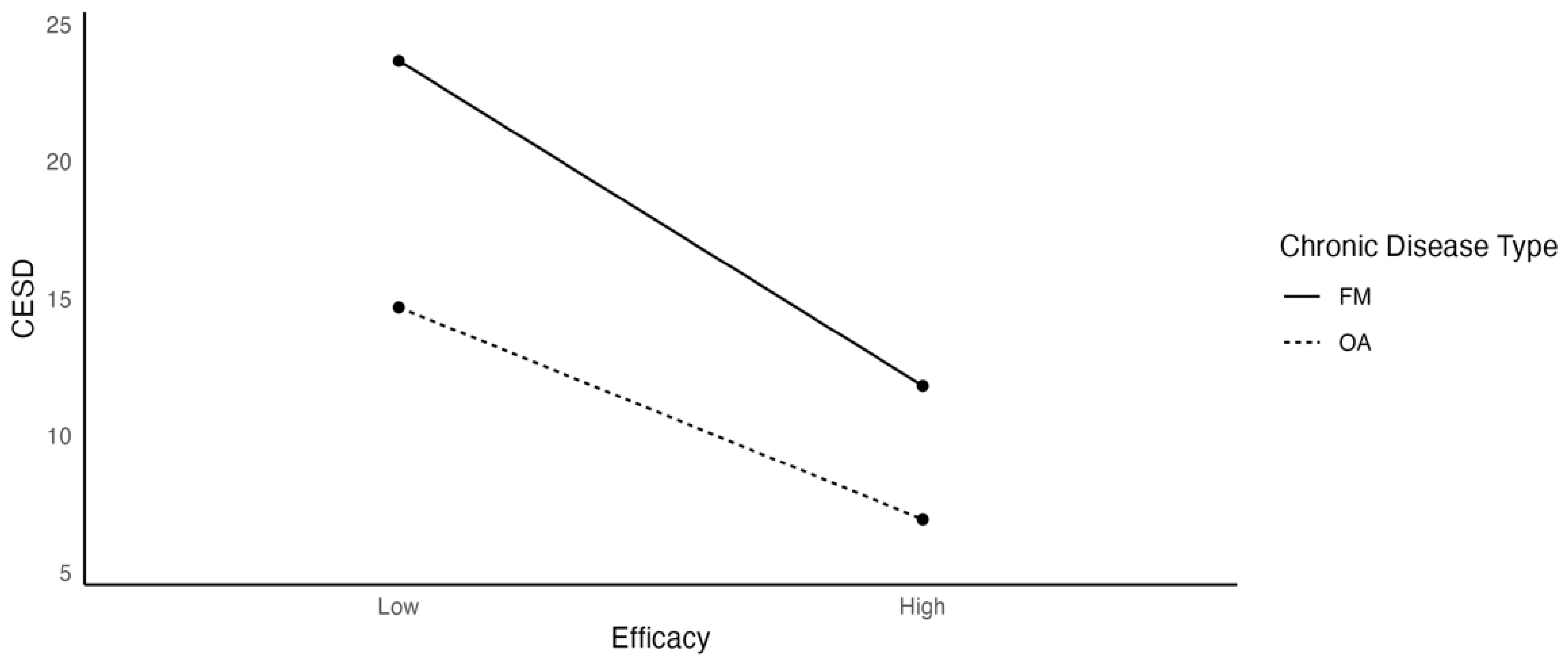
3. Results
3.1. Descriptive Data
3.2. Test of Hypothesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Center for Chronic Disease Prevention and Health Promotion. Available online: https://www.cdc.gov/chronicdisease/about/index.htm (accessed on 2 September 2021).
- Hung, W.W.; Ross, J.S.; Boockvar, K.S.; Siu, A.L. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 2011, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Waters, H.; Graf, M. The Costs of Chronic Disease in the US; The Milken Institute: Santa Monica, CA, USA, 2018. [Google Scholar]
- Johnson, L.M.; Zautra, A.J.; Davis, M.C. The role of illness uncertainty on coping with fibromyalgia symptoms. Health Psychol. 2006, 25, 696–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, E.M.; Dreyer, L.; Jacobsen, S.; Jespersen, A.; Bliddal, H.; Danneskiold-Samsøe, B. Fibromyalgia, diagnosis and prevalence. Are gender differences explainable? Ugeskr. Laeger 2009, 171, 3588–3592. [Google Scholar] [PubMed]
- Fibromyalgia. Available online: https://www.cdc.gov/arthritis/basics/fibromyalgia.htm (accessed on 2 January 2022).
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The Prevalence and Characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Osteoarthritis (OA). Available online: https://www.cdc.gov/arthritis/basics/osteoarthritis.htm (accessed on 16 October 2021).
- Juhakoski, R.; Tenhonen, S.; Anttonen, T.; Kauppinen, T.; Arokoski, J.P. Factors affecting self-reported pain and physical function in patients with hip osteoarthritis. Arch. Phys. Med. Rehabil. 2008, 89, 1066–1073. [Google Scholar] [CrossRef]
- Kawano, M.M.; Araújo, I.L.A.; Castro, M.C.; Matos, M.A. Assessment of quality of life in patients with knee osteoarthritis. Acta Ortopédica Bras. 2015, 23, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, A.; Bourgault, P.; Choinière, M. Fibromyalgia-Related Costs and Loss of Productivity: A Substantial Societal Burden. BMC Musculoskelet. Disord. 2016, 17, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedenbaugh, A.V.; Bonafede, M.; Marchlewicz, E.H.; Lee, V.; Tambiah, J. Real-World Health Care Resource Utilization and Costs Among US Patients with Knee Osteoarthritis Compared with Controls. Clin. Outcomes Res. 2021, 13, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; El-Deredy, W.; Jones, A.K.P. When the brain expects pain: Common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur. J. Neurosci. 2014, 39, 663–672. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, M.; Losilla, J.M.; Monfort, J.; Portell, M.; Gutiérrez, T.; Poca, V.; Garcia-Fructuoso, F.; Llorente, J.; Garcia-Fontanals, A.; Deus, J. Central sensitization in knee osteoarthritis and fibromyalgia: Beyond depression and anxiety. PLoS ONE 2019, 14, e0225836. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Iannuccelli, C.; Bazzichi, L.; Atzeni, F.; Consensi, A.; Salaffi, F.; Pietropaolo, M.; Alessandri, C.; Basili, S.; Olivieri, M.; et al. Misdiagnosis in fibromyalgia: A multicenter study. Clin. Exp. Rheumatol. 2011, 29, S104–S108. [Google Scholar] [PubMed]
- Kratz, A.L.; Davis, M.C.; Zautra, A.J. Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Ann. Behav. Med. 2007, 33, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.C.; Zautra, A.J.; Reich, J.W. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis davis et al. stress vulnerability. Ann. Behav. Med. 2001, 23, 215–226. [Google Scholar] [CrossRef]
- Kleinman, N.; Harnett, J.; Melkonian, A.; Lynch, W.; Kaplan-Machlis, B.; Silverman, S.L. Burden of fibromyalgia and comparisons with osteoarthritis in the workforce. J. Occup. Environ. Med. 2009, 51, 1384–1393. [Google Scholar] [CrossRef]
- Lin, E.H. Depression and osteoarthritis. Am. J. Med. 2008, 121, S16–S19. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Lin, E.H.B.; Bruffaerts, R.; Posada-Villa, J.; Angermeyer, M.C.; Levinson, D.; de Girolamo, G.; Uda, H.; Mneimneh, Z.; et al. Mental disorders among persons with arthritis: Results from the World Mental Health surveys. Psychol. Med. 2008, 38, 1639–1650. [Google Scholar] [CrossRef]
- Shih, M.; Hootman, J.M.; Strine, T.W.; Chapman, D.P.; Brady, T.J. Serious psychological distress in U.S. adults with arthritis. J. Gen. Intern. Med. 2006, 21, 1160–1166. [Google Scholar] [CrossRef]
- Thieme, K.; Turk, D.C.; Flor, H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom. Med. 2004, 66, 837–844. [Google Scholar] [CrossRef]
- Andrade, A.; Steffens, R.d.A.K.; Vilarino, G.T.; Sieczkowska, S.M.; Coimbra, D.R. Does Volume of physical exercise have an effect on depression in patients with fibromyalgia? J. Affect. Disord. 2017, 208, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Harakas, P. Depressive Symptoms, Perceived Stigma, and Perceived Social Support in Fibromyalgia and Osteoarthritis Patients; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2008. [Google Scholar]
- Sale, J.E.M.; Gignac, M.; Hawker, G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J. Rheumatol. 2008, 35, 335–342. [Google Scholar] [PubMed]
- Marr, N.C.; Van Liew, C.; Carovich, T.F.; Cecchini, G.A.; McKinley, L.E.; Cronan, T.A. The effects of racial/ethnic minority status on sleep, mood disturbance, and depression in people with fibromyalgia. Psychol. Res. Behav. Manag. 2020, 13, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, M.S.; Cronan, T.A. Depression, self-efficacy, health status, and health care costs: A comparison of men with fibromyalgia or osteoarthritis. J. Musculoskelet. Pain 2013, 21, 126–134. [Google Scholar] [CrossRef]
- Güven, A.Z.; Kul Panza, E.; Gündüz, O.H. Depression and psychosocial factors in Turkish women with fibromyalgia syndrome. Eur. Med. 2005, 41, 309–313. [Google Scholar]
- Sherman, A.M. Social relations and depressive symptoms in older adults with knee osteoarthritis. Soc. Sci. Med. 2003, 56, 247–257. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of depression and its treatment in the general population. J. Psychiatr. Res. 2007, 41, 207–213. [Google Scholar] [CrossRef]
- Alok, R.; Das, S.K.; Agarwal, G.G.; Salwahan, L.; Srivastava, R. Relationship of severity of depression, anxiety and stress with severity of fibromyalgia. Clin. Exp. Rheumatol. 2011, 29, S70–S72. [Google Scholar] [PubMed]
- Rosemann, T.; Backenstrass, M.; Joest, K.; Rosemann, A.; Szecsenyi, J.; Laux, G. Predictors of depression in a sample of 1021 primary care patients with osteoarthritis. Arthritis Care Res. 2007, 57, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, V.; Ortega, F.; Carbonell-Baeza, A.; Cuevas-Toro, A.; Delgado-Fernández, M.; Ruiz, J. Anxiety, depression and fibromyalgia pain and severity. Behav. Psychol. Conduct. 2013, 21, 381. [Google Scholar]
- McIlvane, J.M.; Schiaffino, K.M.; Paget, S.A. Age differences in the pain-depression link for women with osteoarthritis. Functional impairment and personal control as mediators. Women’s Health Issues 2007, 17, 44–51. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Schmidt, S.M.; Cronan, T.A. Quality of well-being in patients with fibromyalgia. J. Rheumatol. 2000, 27, 785–789. [Google Scholar] [PubMed]
- Van Liew, C.; Brown, K.C.; Cronan, T.A.; Bigatti, S.M.; Kothari, D.J. Predictors of pain and functioning over time in fibromyalgia syndrome: An autoregressive path analysis. Arthritis Care Res. 2013, 65, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Van Liew, C.; Brown, K.C.; Cronan, T.A.; Bigatti, S.M. The effects of self-efficacy on depression and pain in fibromyalgia syndrome: Does initial depression matter? J. Musculoskelet. Pain 2013, 21, 113–125. [Google Scholar] [CrossRef]
- Moyano, S.; Scolnik, M.; Vergara, F.; García, M.V.; Sabelli, M.R.; Rosa, J.E.; Catoggio, L.J.; Soriano, E.R. Evaluation of learned helplessness, perceived self-efficacy, and functional capacity in patients with fibromyalgia and rheumatoid arthritis. JCR J. Clin. Rheumatol. 2019, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.A.; Nicassio, P.M.; Greenberg, M.A.; Medina, E.P. Helplessness and loss as mediators between pain and depressive symptoms in fibromyalgia. Pain 2007, 129, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Benyon, K.; Hill, S.; Zadurian, N.; Mallen, C. Coping strategies and self-efficacy as predictors of outcome in osteoarthritis: A systematic review. Musculoskelet. Care 2010, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.M.; Vance, D.E.; Elliott, T.R.; Cuckler, J.M.; Berry, J.W. Hope, self-efficacy, and functional recovery after knee and hip replacement surgery. Rehabil. Psychol. 2008, 53, 521–529. [Google Scholar] [CrossRef]
- Axford, J.; Butt, A.; Heron, C.; Hammond, J.; Morgan, J.; Alavi, A.; Bolton, J.; Bland, M. Prevalence of anxiety and depression in osteoarthritis: Use of the hospital anxiety and depression scale as a screening tool. Clin. Rheumatol. 2010, 29, 1277–1283. [Google Scholar] [CrossRef]
- Marks, R. Comorbid depression and anxiety impact hip osteoarthritis disability. Disabil. Health J. 2009, 2, 27–35. [Google Scholar] [CrossRef]
- Power, J.D.; Kudesia, P.; Nadeem, A.; Perruccio, A.V.; Sundararajan, K.; Mahomed, N.N.; Rampersaud, Y.R.; Gandhi, R. Patterns of depressive symptoms before and after surgery for osteoarthritis: A descriptive study. ACR Open Rheumatol. 2019, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Cavalieri, F.; Nolli, M.; Ferraccioli, G. Analysis of disability in knee osteoarthritis. Relationship with age and psychological variables but not with radiographic score. J. Rheumatol. 1991, 18, 1581–1586. [Google Scholar] [PubMed]
- Cronan, T.A.; Bigatti, S.M. Chronic illness: Psychological and physical characteristics of women with osteoarthritis and fibromyalgia. Psychol. Sci. 2003, 45, 63–74. [Google Scholar]
- Fuller-Thomson, E.; Nimigon-Young, J.; Brennenstuhl, S. Individuals with fibromyalgia and depression: Findings from a nationally representative Canadian survey. Rheumatol. Int. 2012, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Possley, D.; Budiman-Mak, E.; O’Connell, S.; Jelinek, C.; Collins, E.G. Relationship between depression and functional measures in overweight and obese persons with osteoarthritis of the knee. J. Rehabil. Res. Dev. 2009, 46, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Salvi, V.; Maina, G.; Rossetto, I.; Aguglia, E. Fibromyalgia syndrome and depressive symptoms: Comorbidity and clinical correlates. J. Affect. Disord. 2011, 128, 262–266. [Google Scholar] [CrossRef]
- Robinson, R.L.; Birnbaum, H.G.; Morley, M.A.; Sisitsky, T.; Greenberg, P.E.; Wolfe, F. Depression and fibromyalgia: Treatment and cost when diagnosed separately or concurrently. J. Rheumatol. 2004, 31, 1621–1629. [Google Scholar] [PubMed]
- Sharma, A.; Kudesia, P.; Shi, Q.; Gandhi, R. Anxiety and depression in patients with osteoarthritis: Impact and management challenges. Open Access Rheumatol. Res. Rev. 2016, 8, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, H.; Karaca, G.; Demir Polat, H.A.; Akkurt, H.E. Comparison between depression levels of women with knee osteoarthritis, rheumatoid arthritis, and fibromyalgia syndrome: A controlled study. Türkiye Fiz. Tip Ve Rehabil. Derg. 2015, 61, 197–202. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Bush, J.W.; Berry, C.C. Health status: Types of validity and the Index of Well-Being. Health Serv. Res. 1976, 11, 478–507. [Google Scholar]
- Kaplan, R.M.; Anderson, J.P.; Wu, A.W.; Mathews, W.C.; Kozin, F.; Orenstein, D. The Quality of Well-Being Scale: Applications in AIDS, cystic Fibrosis, and arthritis. Med. Care 1989, 27, S27–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.P.; Kaplan, R.M.; Berry, C.C.; Bush, J.W.; Rumbaut, R.G. Interday reliability of function assessment for a health status measure: The Quality of Well-Being Scale. Med. Care 1989, 27, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coons, S.J.; Rao, S.; Keininger, D.L.; Hays, R.D. A Comparative Review of Generic Quality-of-Life Instruments. Pharmacoeconomics 2000, 17, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Nicassio, P.; Wallston, K.; Callahan, L.; MA, H.; Pincus, T. The measurement of helplessness in rheumatoid arthritis. The development of the Arthritis Helplessness Index. J. Rheumatol. 1985, 12, 462–467. [Google Scholar]
- Engle, E.W.; Callahan, L.F.; Pincus, T.; Hochberg, M.C. Learned helplessness in systemic lupus erythematosus: Analysis using the Rheumatology Attitudes Index. Arthritis Rheum. 1990, 33, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.; Chastain, R.L.; Ung, E.; Shoor, S.; Holman, H.R. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989, 32, 37–44. [Google Scholar] [CrossRef]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The Fibromyalgia Impact Questionnaire: Development and validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar]
- Calandre, E.P.; Garcia-Carrillo, J.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M. Subgrouping patients with fibromyalgia according to the results of the Fibromyalgia Impact Questionnaire: A Replication Study. Rheumatol. Int. 2011, 31, 1555–1559. [Google Scholar] [CrossRef]
- Henriksen, M.; Lund, H.; Christensen, R.; Jespersen, A.; Dreyer, L.; Bennett, R.M.; Danneskiold-SamsØe, B.; Bliddal, H. Relationships between the Fibromyalgia Impact Questionnaire, tender point count, and muscle strength in female patients with fibromyalgia: A Cohort Study. Arthritis Care Res. 2009, 61, 732–739. [Google Scholar] [CrossRef]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A Validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar]
- Meenan, R.F.; Mason, J.H.; Anderson, J.J.; Guccione, A.A.; Kazis, L.E. AIMS2. The Content and properties of a revised and expanded Arthritis Impact Measurement Scales health status questionnaire. Arthritis Rheum. 1992, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meenan, R.F.; Gertman, P.M.; Mason, J.H. Measuring health status in arthritis. The Arthritis Impact Measurement Scales. Arthritis Rheum. 1980, 23, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Turk, D.C.; Okifuji, A. Detecting depression in chronic pain patients: Adequacy of self-reports. Behav. Res. Ther. 1994, 32, 9–16. [Google Scholar] [CrossRef]
- Miller, W.C.; Anton, H.A.; Townson, A.F. Measurement properties of the CESD Scale among individuals with spinal cord injury. Spinal Cord 2008, 46, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011, 63, S454–S466. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Frerichs, R.R.; Aneshensel, C.S.; Yokopenic, P.A.; Clark, V.A. Physical health and depression: An epidemiologic survey. Prev. Med. 1982, 11, 639–646. [Google Scholar] [CrossRef]
- Goodwin, R.D.; Kroenke, K.; Hoven, C.W.; Spitzer, R.L. Major depression, physical illness, and suicidal ideation in primary care. Psychosom. Med. 2003, 65, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Perry, G.S.; Strine, T.W. The vital link between chronic disease and depressive disorders. Prev. Chronic. Dis. 2005, 2, A14. [Google Scholar]
- Hirsch, J.K.; Treaster, M.K.; Kaniuka, A.R.; Brooks, B.D.; Sirois, F.M.; Kohls, N.; Nöfer, E.; Toussaint, L.L.; Offenbächer, M. Fibromyalgia impact and depressive symptoms: Can perceiving a silver lining make a difference? Scand. J. Psychol. 2020, 61, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.R.; Choy, E.; Mainguy, Y.; Gracely, R.; Ingvar, M.; et al. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis Rheum. 2010, 62, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.M. The Effects of Depression and Attention on the Health Status of Fibromyalgia Patient; San Diego State University: San Diego, CA, USA, 2018. [Google Scholar]
- Gracely, R.H.; Ceko, M.; Bushnell, M.C. Fibromyalgia and depression. Pain Res. Treat. 2011, 2012, e486590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offenbaecher, M.; Kohls, N.; Ewert, T.; Sigl, C.; Hieblinger, R.; Toussaint, L.L.; Sirois, F.; Hirsch, J.; Vallejo, M.A.; Kramer, S.; et al. Pain is not the major determinant of quality of life in fibromyalgia: Results from a retrospective “real world” data analysis of fibromyalgia patients. Rheumatol. Int. 2021, 41, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Arnstein, P.; Caudill, M.; Mandle, C.L.; Norris, A.; Beasley, R. Self-efficacy as a mediator of the relationship between pain intensity, disability and depression in chronic pain patients. Pain 1999, 80, 483–491. [Google Scholar] [CrossRef]
- Buckelew, S.P.; Murray, S.E.; Hewett, J.E.; Johnson, J.; Huyser, B. Self-Efficacy, pain, and physical activity among fibromyalgia subjects. Arthritis Rheum. 1995, 8, 43–50. [Google Scholar] [CrossRef]
- Reibel, M.D.; Hutti, M.H. The Role of helplessness in the appraisal of illness uncertainty in women with fibromyalgia. Nurs. Sci. Q. 2020, 33, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.W.; Johnson, L.M.; Zautra, A.J.; Davis, M.C. Uncertainty of illness relationships with mental health and coping processes in fibromyalgia patients. J. Behav. Med. 2006, 29, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Nicassio, P.M.; Schuman, C.; Radojevic, V.; Weisman, M.H. Helplessness as a mediator of health status in fibromyalgia. Cogn. Ther. Res. 1999, 23, 181–196. [Google Scholar] [CrossRef]
- Peterson, C. Learned Helplessness and Health Psychology. Health Psychol. 1982, 1, 153–168. [Google Scholar] [CrossRef]
- Dexter, P.; Brandt, K. Distribution and predictors of depressive symptoms in osteoarthritis. J. Rheumatol. 1994, 21, 279–286. [Google Scholar]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in patients with knee osteoarthritis: Risk factors and associations with joint symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Conversano, C.; Poli, A.; Ciacchini, R.; Hitchcott, P.; Bazzichi, L.; Gemignani, A. A psychoeducational intervention is a treatment for fibromyalgia syndrome. Clin. Exp. Rheumatol. 2019, 37, 98–104. [Google Scholar]
- Allen, K.D.; Helmick, C.G.; Schwartz, T.A.; DeVellis, R.F.; Renner, J.B.; Jordan, J.M. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: The Johnston county osteoarthritis project. Osteoarthr. Cartil. 2009, 17, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chang, H.J.; Tirodkar, M.; Chang, R.W.; Manheim, L.M.; Dunlop, D.D. Racial/ethnic differences in activities of daily living disability in older adults with arthritis: A longitudinal study. Arthritis Care Res. 2007, 57, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Raphael, K.G.; Janal, M.N.; Nayak, S.; Schwartz, J.E.; Gallagher, R.M. Psychiatric Comorbidities in a Community Sample of Women with Fibromyalgia. Pain 2006, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Faison, W.E.; Harrell, P.G.; Semel, D. Disparities across Diverse Populations in the Health and Treatment of Patients with Osteoarthritis. Healthcare 2021, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Sibille, K.T.; Goodin, B.R.; Petrov, M.E.; Bartley, E.J.; Riley, J.L.; King, C.D.; Glover, T.L.; Sotolongo, A.; Herbert, M.S.; et al. Racial and Ethnic Differences in Older Adults With Knee Osteoarthritis. Arthritis Rheumatol. 2014, 66, 1800–1810. [Google Scholar] [CrossRef] [PubMed]





| Variable | Mean/Percentage | SD/Range | ||
|---|---|---|---|---|
| FM | OA | FM | OA | |
| Gender/Women | 95.5% | 64.2% | - | - |
| Age | 53.918 | 69.213 | 11.447 | 5.626 |
| Ethnicity/White | 85.0% | 92.3% | - | - |
| Education 1 | 3.205 | 3.465 | 0.914 | 1.394 |
| Income 2 | 4.712 | 3.742 | 2.130 | 1.768 |
| Well-Being | 559.648 | 642.840 | 73.469 | 89.832 |
| Helplessness | 3.120 | 2.612 | 0.695 | 0.800 |
| Efficacy | 55.592 | 72.874 | 17.716 | 16.124 |
| Body Mass Index (BMI) | 29.464 | 26.958 | 6.516 | 5.261 |
| Condition Impact 3 | 0.000 | 0.000 | 1.000 | 1.000 |
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 Gender | |||||||||
| 2 Age | −0.250 *** | ||||||||
| 3 Ethnicity | −0.036 | 0.176 *** | |||||||
| 4 Education | −0.157 *** | 0.023 | 0.007 | ||||||
| 5 Income | −0.036 | −0.255 *** | −0.026 | 0.249 *** | |||||
| 6 Well-being | −0.271 *** | 0.276 *** | 0.055 | 0.102 * | 0.025 | ||||
| 7 Helplessness | 0.147 *** | 0.221 *** | −0.079 | −0.181 *** | −0.042 | −0.406 *** | |||
| 8 Efficacy | −0.238 *** | 0.261 *** | 0.081 | 0.214 *** | 0.068 | 0.547 *** | −0.632 *** | ||
| 9 BMI | 0.064 | −0.163 *** | −0.047 | −0.042 | −0.020 | −0.186 *** | 0.134 *** | −0.226 *** | |
| 10 Impact | 0.081 | −0.134 *** | −0.086 | −0.090 | −0.135 ** | −0.430 *** | 0.456 *** | −0.528 *** | 0.091 |
| Well-Being | Condition Impact | Body Mass Index (BMI) | ||||
|---|---|---|---|---|---|---|
| b | p-Value | b | p-Value | b | p-Value | |
| Intercept | 59.342 | <0.001 | 19.740 | <0.001 | 18.355 | <0.001 |
| Variable | −0.071 | <0.001 | 8.122 | <0.001 | 0.049 | 0.447 |
| Chronic Condition | −36.256 | <0.001 | −11.153 | <0.001 | −10.921 | 0.002 |
| Variable × Chronic Condition | 0.048 | <0.001 | −3.157 | <0.001 | −0.009 | 0.943 |
| Helplessness | Efficacy | |||
|---|---|---|---|---|
| b | p-Value | b | p-Value | |
| Intercept | −2.812 | 0.106 | 37.033 | <0.001 |
| Variable | 7.228 | <0.001 | −0.311 | <0.001 |
| Chronic Condition | 3.509 | 0.149 | −13.648 | <0.001 |
| Variable × Chronic Condition | −4.217 | <0.001 | 0.108 | 0.003 |
| Gender | Age | Ethnicity | Education | Income | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | p-Value | b | p-Value | b | p-Value | b | p-Value | b | p-Value | |
| Intercept | 19.333 | <0.001 | 30.994 | <0.001 | 22.767 | <0.001 | 25.839 | <0.001 | 23.550 | <0.001 |
| Variable | 0.426 | 0.832 | −0.209 | <0.001 | −3.561 | 0.002 | −1.846 | <0.001 | −0.809 | <0.001 |
| Chronic Condition | −12.032 | <0.001 | −19.546 | 0.005 | −16.878 | <0.001 | −17.081 | <0.001 | −13.361 | <0.001 |
| Variable × Chronic Condition | 1.547 | 0.502 | 0.167 | 0.106 | 6.457 | 0.006 | 1.787 | 0.003 | 0.374 | 0.329 |
| FM | OA | |||
|---|---|---|---|---|
| b | p-Value | b | p-Value | |
| Education | −1.846 | <0.001 | −0.059 | 0.881 |
| Quality of Well-Being | −0.071 | <0.001 | −0.023 | <0.001 |
| Condition Impact | 8.122 | <0.001 | 4.965 | <0.001 |
| Helplessness | 7.228 | <0.001 | 3.011 | <0.001 |
| Efficacy | −0.311 | <0.001 | −0.203 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Dyne, A.; Moy, J.; Wash, K.; Thompson, L.; Skow, T.; Roesch, S.C.; Cronan, T. Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 3413. https://doi.org/10.3390/ijerph19063413
Van Dyne A, Moy J, Wash K, Thompson L, Skow T, Roesch SC, Cronan T. Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis. International Journal of Environmental Research and Public Health. 2022; 19(6):3413. https://doi.org/10.3390/ijerph19063413
Chicago/Turabian StyleVan Dyne, Angelina, Jason Moy, Kalila Wash, Linda Thompson, Taylor Skow, Scott C. Roesch, and Terry Cronan. 2022. "Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis" International Journal of Environmental Research and Public Health 19, no. 6: 3413. https://doi.org/10.3390/ijerph19063413
APA StyleVan Dyne, A., Moy, J., Wash, K., Thompson, L., Skow, T., Roesch, S. C., & Cronan, T. (2022). Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis. International Journal of Environmental Research and Public Health, 19(6), 3413. https://doi.org/10.3390/ijerph19063413







