Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Oversight
2.2. Patient Recruitment and Clinical Evaluation
2.3. Dietary Intake Assessment
2.4. Sample Acquisition and Handling
2.5. DNA Extraction and 16S Ribosomal rRNA Gene Amplification and Sequencing
2.6. DESeq2 Differential Abundance Analysis
2.7. Correlation between Nutritional Measures, Taxa Abundance and Clinical Indices
2.8. General Statistical Considerations
3. Results
3.1. Participant Characteristics
3.2. Dietary Assessment
3.3. Nutritional Supplements
3.4. Dietary Intake Is Not Correlated with the Severity of Fibromyalgia Symptoms
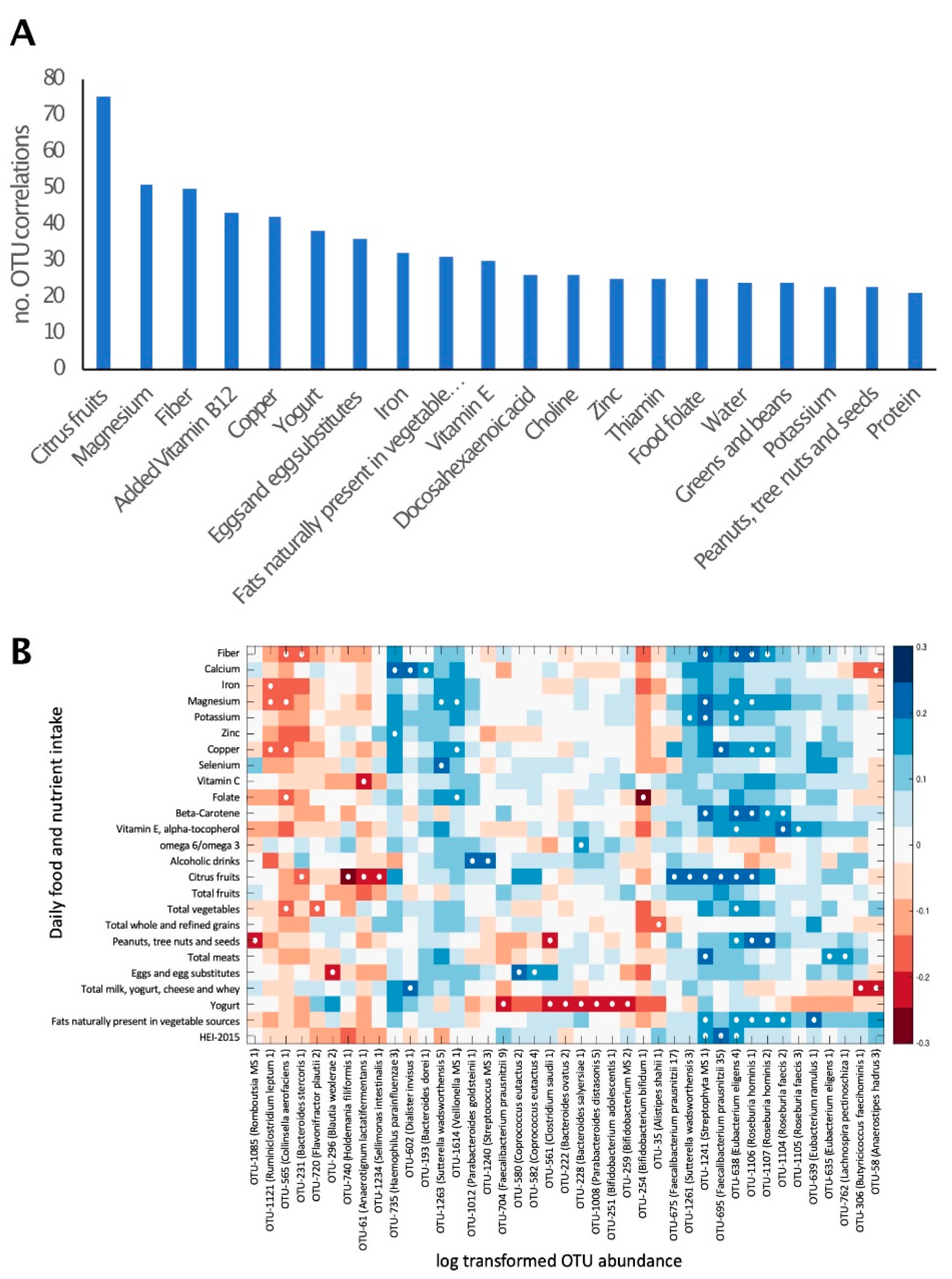
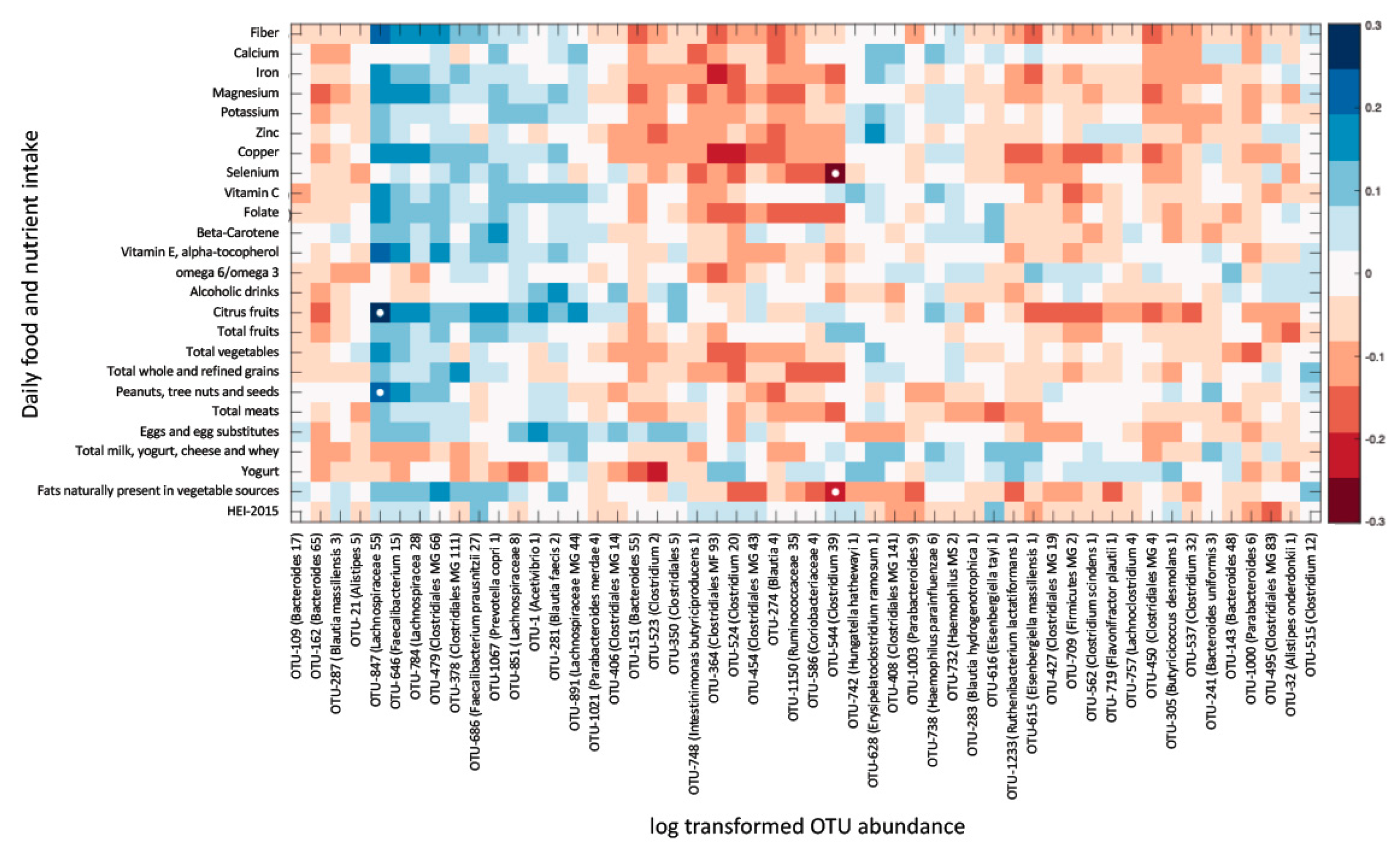
3.5. Overall Gut Microbiome Composition Is Correlated with Dietary Intake
3.6. Dietary Intake Is Not Associated with Most Fibromyalgia-Specific Microbiome Alterations
4. Discussion
5. Conclusions
Significance and Innovation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häuser, W.; Ablin, J.; Fitzcharles, M.-A.; Littlejohn, G.; Luciano, J.V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Primers 2015, 1, 15022. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Underwood, M.; Carnes, D. Fibromyalgia. BMJ 2014, 348, g1224. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of Fibromyalgia and Chronic Widespread Pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A Systematic Review and Meta-Analysis of the Prevalence of Chronic Widespread Pain in the General Population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A Clinical Review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise Therapy for Fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Häuser, W.; Ablin, J.; Perrot, S.; Fitzcharles, M.-A. Management of Fibromyalgia: Practical Guides from Recent Evidence-Based Guidelines. Pol. Arch. Intern. Med. 2017, 127, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, E.; Marley, J.; McVeigh, J.G.; McSorley, E.; Allsopp, P.; Kerr, D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients 2020, 12, 2664. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut Microbiome and Serum Metabolome Analyses Identify Molecular Biomarkers and Altered Glutamate Metabolism in Fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of Diet to the Composition of the Human Gut Microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtaza, N.; Cuív, P.Ó.; Morrison, M. Diet and the Microbiome. Gastroenterol. Clin. N. Am. 2017, 46, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota and Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut Microbiota Role in Irritable Bowel Syndrome: New Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The Low FODMAP Diet: Recent Advances in Understanding Its Mechanisms and Efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arranz, L.-I.; Canela, M.-Á.; Rafecas, M. Dietary Aspects in Fibromyalgia Patients: Results of a Survey on Food Awareness, Allergies, and Nutritional Supplementation. Rheumatol. Int. 2012, 32, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, P.; Soriano-Maldonado, A.; Delgado-Fernandez, M.; Alvarez-Gallardo, I.C.; Segura-Jimenez, V.; Estevez-Lopez, F.; Camiletti-Moirón, D.; Aparicio, V.A. Association of Dietary Habits with Psychosocial Outcomes in Women with Fibromyalgia: The al-Ándalus Project. J. Acad. Nutr. Diet. 2017, 117, 422–432.e1. [Google Scholar] [CrossRef]
- López-Rodríguez, M.M.; Granero Molina, J.; Fernández Medina, I.M.; Fernández Sola, C.; Ruiz Muelle, A. Patterns of Food Avoidance and Eating Behavior in Women with Fibromyalgia. Endocrinol. Diabetes Y Nutr. 2017, 64, 480–490. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The Fibromyalgia Impact Questionnaire: Development and Validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar] [PubMed]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993; ISBN 978-0-89862-210-2. [Google Scholar]
- Vol, S.; Bedouet, M.; Gusto, G.; Leglu, C.; Beslin, E.; Decou, P.; Nègre, E.; Planage, B.; Chazelle, E.; Mercier, F.; et al. Evaluating Physical Activity: The AQAP Questionnaire and Its Interpretation Software. Ann. Phys. Rehabil. Med. 2011, 54, 478–495. [Google Scholar] [CrossRef] [Green Version]
- Fitzcharles, M.-A.; Ste-Marie, P.A.; Panopalis, P.; Ménard, H.; Shir, Y.; Wolfe, F. The 2010 American College of Rheumatology Fibromyalgia Survey Diagnostic Criteria and Symptom Severity Scale Is a Valid and Reliable Tool in a French Speaking Fibromyalgia Cohort. BMC Musculoskelet. Disord. 2012, 13, 179. [Google Scholar] [CrossRef] [Green Version]
- Perrot, S.; Dumont, D.; Guillemin, F.; Pouchot, J.; Coste, J.; French Group for Quality of Life Research. Quality of Life in Women with Fibromyalgia Syndrome: Validation of the QIF, the French Version of the Fibromyalgia Impact Questionnaire. J. Rheumatol. 2003, 30, 1054–1059. [Google Scholar] [PubMed]
- Chahoud, M.; Chahine, R.; Salameh, P.; Sauleau, E.A. Reliability, Factor Analysis and Internal Consistency Calculation of the Insomnia Severity Index (ISI) in French and in English among Lebanese Adolescents. ENeurologicalSci 2017, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Timon, C.M.; van den Barg, R.; Blain, R.J.; Kehoe, L.; Evans, K.; Walton, J.; Flynn, A.; Gibney, E.R. A Review of the Design and Validation of Web- and Computer-Based 24-h Dietary Recall Tools. Nutr. Res. Rev. 2016, 29, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Pitre, F.E.; Brereton, N.J.B. ANCHOR: A 16S RRNA Gene Amplicon Pipeline for Microbial Analysis of Multiple Environmental Samples. Environ. Microbiol. 2019, 21, 2440–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsen, J.; Brejnrod, A.; Mortensen, M.; Rasmussen, M.A.; Stokholm, J.; Al-Soud, W.A.; Sørensen, S.; Bisgaard, H.; Waage, J. Large-Scale Benchmarking Reveals False Discoveries and Count Transformation Sensitivity in 16S RRNA Gene Amplicon Data Analysis Methods Used in Microbiome Studies. Microbiome 2016, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; McCarthy, D.J.; Chen, Y.; Okoniewski, M.; Smyth, G.K.; Huber, W.; Robinson, M.D. Count-Based Differential Expression Analysis of RNA Sequencing Data Using R and Bioconductor. Nat. Protoc. 2013, 8, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Anders, S.; Kim, V.; Huber, W. RNA-Seq Workflow: Gene-Level Exploratory Analysis and Differential Expression. F1000Research 2015, 4, 1070. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, A.R.; Price, L.L.; Wang, C.; Chung, M. Dietary Quality is Associated with Better Self-Efficacy and Depression in Patients with Fibromyalgia from a Comparative Effectiveness Trial: A Small Pilot Study. J. Maine Med. Center 2020, 2, 3. [Google Scholar] [CrossRef]
- Steinemann, N.; Grize, L.; Ziesemer, K.; Kauf, P.; Probst-Hensch, N.; Brombach, C. Relative Validation of a Food Frequency Questionnaire to Estimate Food Intake in an Adult Population. Food Nutr. Res. 2017, 61, 1305193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Panizza, C.E.; Shvetsov, Y.B.; Harmon, B.E.; Wilkens, L.R.; Le Marchand, L.; Haiman, C.; Reedy, J.; Boushey, C.J. Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality? Nutrients 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Trotter, A.; Rodriguez-Palacios, A.; Cominelli, F. Mucosal Interactions between Genetics, Diet, and Microbiome in Inflammatory Bowel Disease. Front. Immunol. 2016, 7, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The Human Gut Firmicute Roseburia Intestinalis Is a Primary Degrader of Dietary β-Mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the Human Gut Microbiota by Dietary Fibres Occurs at the Species Level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Chassard, C.; Lawson, P.A.; Bernalier-Donadille, A. Bacteroides cellulosilyticus Sp. Nov., a Cellulolytic Bacterium from the Human Gut Microbial Community. Int. J. Syst. Evol. Microbiol. 2007, 57, 1516–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Matteuzzi, D.; Rossi, M. Folate Production by Bifidobacteria as a Potential Probiotic Property. Appl. Environ. Microbiol. 2007, 73, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut Microbiota Is Critical for the Induction of Chemotherapy-Induced Pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef] [Green Version]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A Low Fermentable Oligo-Di-Mono Saccharides and Polyols (FODMAP) Diet Reduced Pain and Improved Daily Life in Fibromyalgia Patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Bernardo, A.; Costa, J.; Cardoso, A.; Santos, P.; de Mesquita, M.F.; Vaz Patto, J.; Moreira, P.; Silva, M.L.; Padrão, P. Dietary Interventions in Fibromyalgia: A Systematic Review. Ann. Med. 2019, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slim, M.; Calandre, E.P.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Morillas-Arques, P. The Effects of a Gluten-Free Diet Versus a Hypocaloric Diet Among Patients With Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J. Clin. Gastroenterol. 2017, 51, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Hayes, C.; Hodson, F.J.; Collins, C.E. The Effect of a Pilot Dietary Intervention on Pain Outcomes in Patients Attending a Tertiary Pain Service. Nutrients 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Hu, X.; Feng, F.; Cai, L.; Chen, F. Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro. Nutrients 2020, 12, 3194. [Google Scholar] [CrossRef] [PubMed]

| FM (56) | First-Degree Relatives (10) | Household Members (18) | Unrelated Controls (40) | ANOVA p | |
|---|---|---|---|---|---|
| Sex | Women | Women | Men * | Women | <0.0001 |
| Age (years) | 47 ± 8 | 44 ± 17 | 47 ± 10 | 44 ± 9 | 0.58 |
| Married (%) | 54% | 40% | 94% * | 58% | 0.01 |
| No. of children | 1.5 ± 1.2 | 1.1 ± 1.45 | 1.0 ± 1.1 | 1.4 ± 1.3 | 0.38 |
| No. of household members | 2.6 ± 1.4 | 3.2 ± 1.6 | 2.7 ± 1.1 | 3.0 ± 1.6 | 0.74 |
| Academic education | 77% | 70% | 72% | 88% | 0.42 |
| Ethnicity, maternal (%caucasian) | 96% | 100% | 100% | 90% | 0.27 |
| Ethnicity, paternal (%caucasian) | 93% | 100% | 100% | 88% | 0.29 |
| Occupational status (% working) | 64% | 60% | 83% | 73% | 0.41 |
| Smoking | 9% | 0% | 6% | 8% | 0.74 |
| BMI | 29.6 ± 7.4 | 28.3 ± 7.1 | 28.7 ± 5.4 | 28.5 ± 7.3 | 0.36 |
| FM (56) | First-Degree Relatives (10) | Household Members (18) | Unrelated Controls (40) | |
|---|---|---|---|---|
| Energy (kcal) | 1940 ± 460 | 1936 ± 322 | 2180 ± 552 | 2008 ± 600 |
| Energy (kcal/kg) | 28 ± 11 | 24 ± 5 * | 34 ± 11 | 31 ± 12 |
| Protein (g) | 76 ± 25 | 83 ± 24 | 89 ± 31 | 81 ± 24 |
| Protein (g/kg) | 1.1 ± 0.5 | 1.1 ± 0.4 | 1.4 ± 0.6 | 1.2 ± 0.4 |
| Protein (% of E) | 16 ± 4 | 17 ± 4 | 16 ± 4 | 15 ± 3 |
| Carbohydrates (% of E) | 47 ± 10 | 48 ± 6 | 46 ± 5 | 49 ± 9 |
| Sugars (g) 1 | 108 ± 45 | 91 ± 36 | 98 ± 36 | 110 ± 62 |
| Fibers (g) 2 | 19 ± 7 | 16 ± 5 | 18 ± 5 | 23 ± 12 |
| Lipids (% of E) | 36 ± 7 | 35 ± 5 | 37 ± 5 | 35 ± 7 |
| Saturated (% of E) | 11 ± 4 | 12 ± 2 | 12 ± 3 | 11± 4 |
| Monounsaturated (% of E) | 14 ± 4 | 13 ± 2 | 14 ± 2 | 13 ± 4 |
| Polyunsaturated (% of E) | 8 ± 2 | 7 ± 2 | 8 ± 2 | 8 ± 3 |
| Omega-6/omega-3 ratio | 7.3 ± 2.5 | 7.0 ± 1.9 | 8.1 ± 2.2 | 7.7 ± 2.3 |
| FM (56) | First-Degree Relatives (10) | Household Members (18) | Unrelated Controls (40) | |
|---|---|---|---|---|
| Vitamins | ||||
| Vitamin C (mg) | 114 ± 77 | 97 ± 62 | 92 ± 59 | 132 ± 112 |
| Folate (μg) | 371 ± 180 | 354 ± 110 | 375 ± 111 | 424 ± 161 |
| ß-carotene (mg) | 4.3 ± 3.5 | 2.5 ± 1.9 | 3.0 ± 1.9 | 4.5 ± 4.9 |
| Vitamin D (μg) | 5.6 ± 3.5 | 4.8 ± 3.3 | 4.6 ± 4.1 | 4.5 ± 3.1 |
| Vitamin E (mg) | 10 ± 5 | 8 ± 3 | 10 ± 4 | 12 ± 7 |
| Vitamin K (μg) | 176 ± 201 | 121 ± 70 | 157 ± 127 | 243 ± 279 |
| Minerals | ||||
| Calcium (mg) | 872 ± 358 | 926 ± 337 | 944 ± 458 | 906 ± 378 |
| Iron (mg) | 13 ± 4 | 12 ± 3 | 14 ± 4 | 14 ± 5 |
| Magnesium (mg) | 317 ± 94 | 290 ± 61 | 334 ± 97 | 362 ± 138 |
| Potassium (g) | 3.0 ± 0.9 | 2.8 ± 0.7 | 2.9 ± 0.9 | 3.2 ± 1.3 |
| Zinc (mg) | 11 ± 4 | 11 ± 4 | 13 ± 4 | 11 ± 4 |
| Copper (mg) | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.3 ± 0.4 | 1.6 ± 0.6 |
| Non-nutrients | ||||
| Caffeine (mg) | 128 ± 156 | 116 ± 132 | 159 ± 130 | 128 ± 114 |
| Food Groups (servings) | ||||
| Fruits 1 | 1.6 ± 1.1 | 1.2 ± 0.9 | 1.1 ± 0.7 | 2.0 ± 3.1 |
| Vegetables 1 | 1.9 ± 1.0 | 1.9 ± 0.7 | 1.8 ± 0.8 | 2.3 ± 1.3 |
| Grains 2 | 5.0 ± 2.3 | 6.3 ± 2.1 | 7.1 ± 2.3 | 6.1 ± 2.4 |
| Whole grains 2 | 0.9 ± 0.8 | 0.5 ± 0.5 | 1.3 ± 1.1 | 1.2 ± 1.4 |
| Protein foods (animal) 2 | 4.4 ± 3.0 | 4.4 ± 2.0 | 4.8 ± 2.8 | 4.0 ± 2.2 |
| Protein foods (plant) 2 | 1.6 ± 1.5 | 1.2 ± 1.2 | 1.6 ± 1.6 | 2.0 ± 2.2 |
| Dairy 1 | 1.7 ± 1.2 | 2.2 ± 1.1 | 2.0 ± 1.4 | 1.7 ± 1.1 |
| Yogurt 1 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.1 ± 0.2 | 0.2 ± 0.3 |
| Alcoholic drinks 3 | 0.6 ± 1.4 | 0.3 ± 0.4 | 0.5 ± 0.9 | 0.5 ± 1.1 |
| Diet quality (HEI 2015) | 58 ± 16 | 52 ± 8 | 53 ± 12 | 55 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minerbi, A.; Brereton, N.J.B.; Anjarkouchian, A.; Moyen, A.; Gonzalez, E.; Fitzcharles, M.-A.; Shir, Y.; Chevalier, S. Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2022, 19, 3254. https://doi.org/10.3390/ijerph19063254
Minerbi A, Brereton NJB, Anjarkouchian A, Moyen A, Gonzalez E, Fitzcharles M-A, Shir Y, Chevalier S. Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia. International Journal of Environmental Research and Public Health. 2022; 19(6):3254. https://doi.org/10.3390/ijerph19063254
Chicago/Turabian StyleMinerbi, Amir, Nicholas J. B. Brereton, Abraham Anjarkouchian, Audrey Moyen, Emmanuel Gonzalez, Mary-Ann Fitzcharles, Yoram Shir, and Stéphanie Chevalier. 2022. "Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia" International Journal of Environmental Research and Public Health 19, no. 6: 3254. https://doi.org/10.3390/ijerph19063254
APA StyleMinerbi, A., Brereton, N. J. B., Anjarkouchian, A., Moyen, A., Gonzalez, E., Fitzcharles, M.-A., Shir, Y., & Chevalier, S. (2022). Dietary Intake Is Unlikely to Explain Symptom Severity and Syndrome-Specific Microbiome Alterations in a Cohort of Women with Fibromyalgia. International Journal of Environmental Research and Public Health, 19(6), 3254. https://doi.org/10.3390/ijerph19063254






