Soil Organic Carbon Distribution and Its Response to Soil Erosion Based on EEM-PARAFAC and Stable Carbon Isotope, a Field Study in the Rocky Desertification Control of South China Karst
Abstract
:1. Introduction
2. Materials and Methods
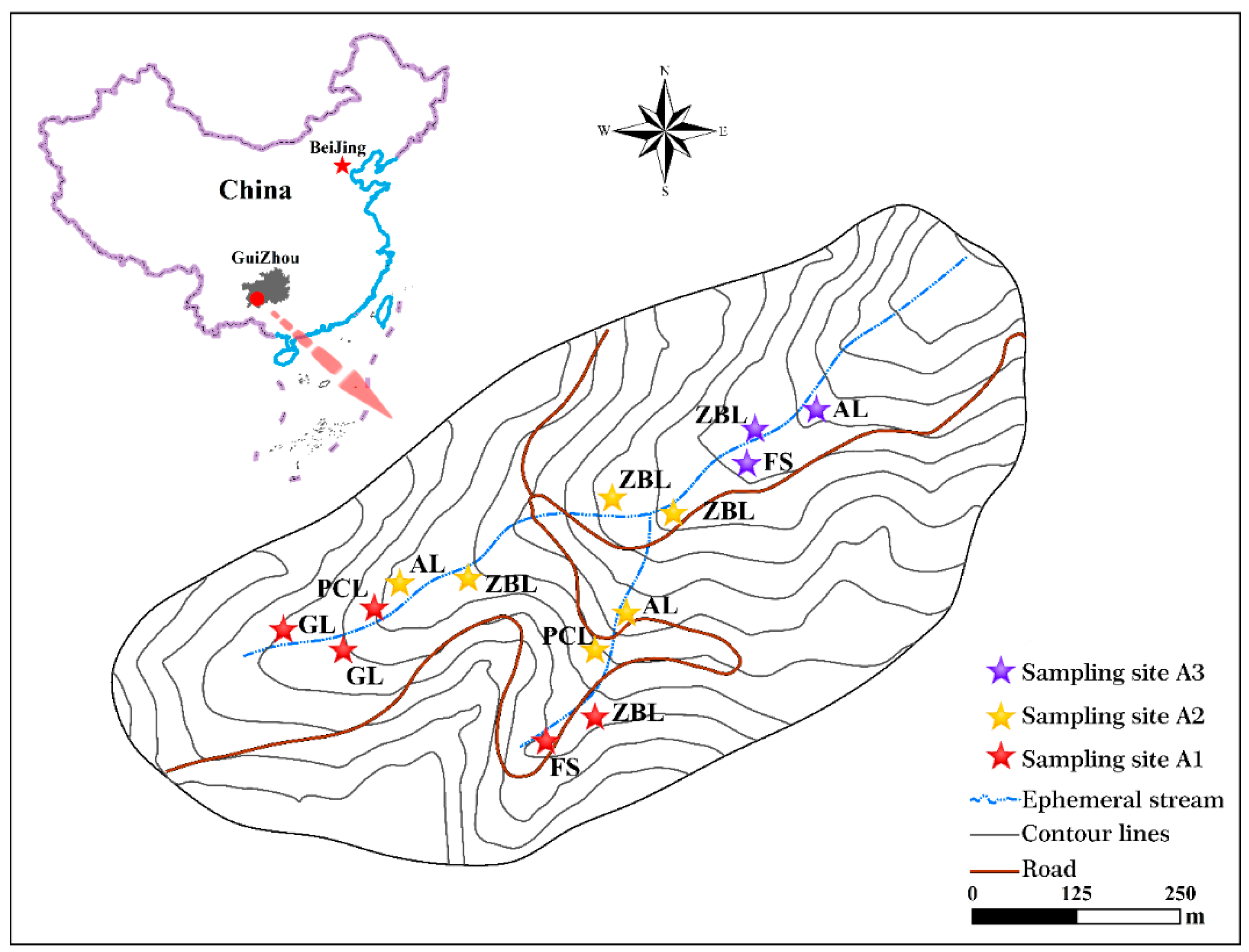
2.1. Study Area
2.2. Sample Collection and Preparation
2.2.1. Sample Collection
2.2.2. Laboratory Analysis
2.3. PARAFAC
2.4. δ13Csoc and Spectral Index
2.5. Statistical Analysis
3. Results and Analysis
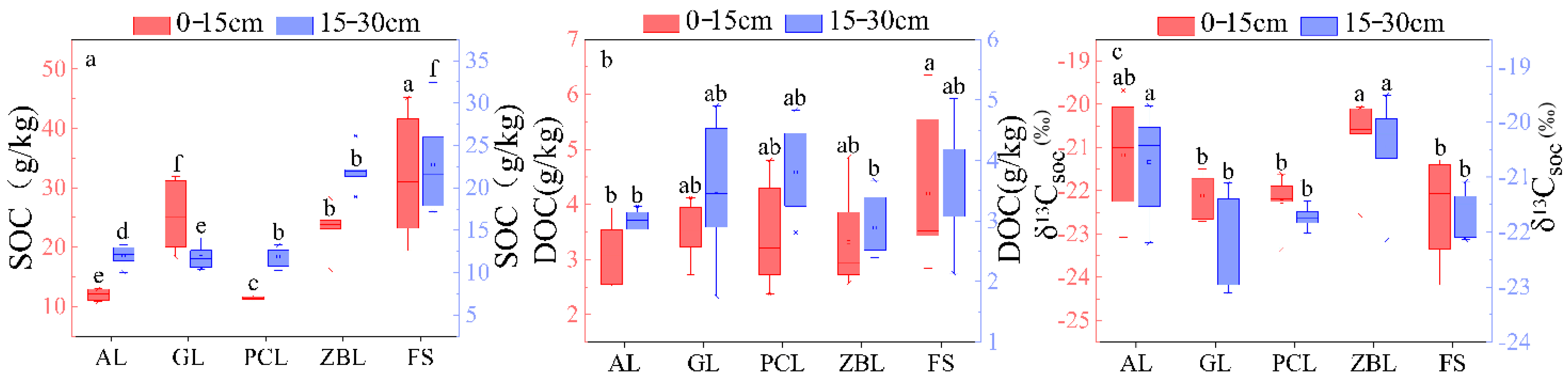
3.1. SOC and δ13Csoc Distribution Characteristics of Different Vegetation Restoration Types and Sites
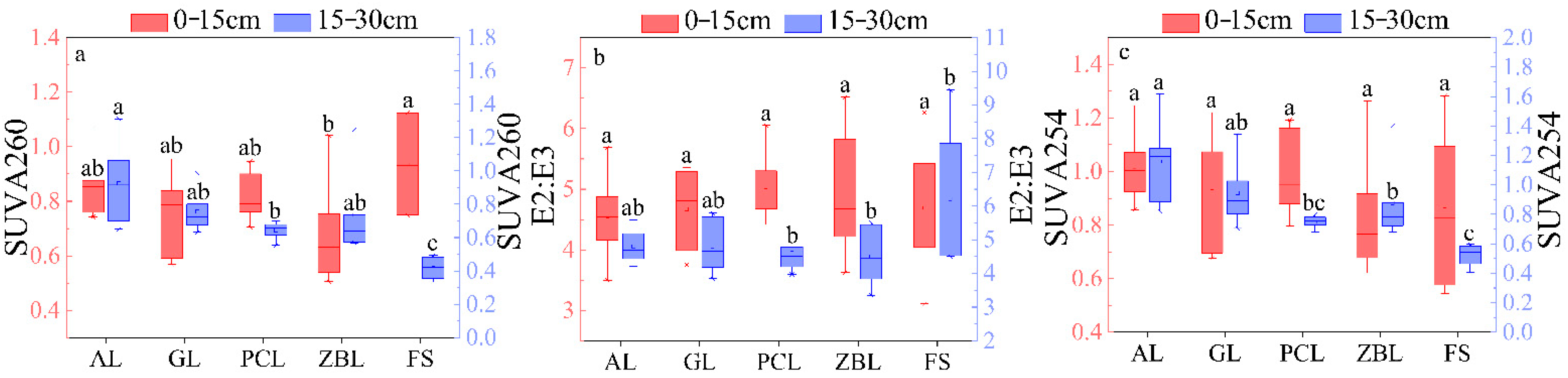
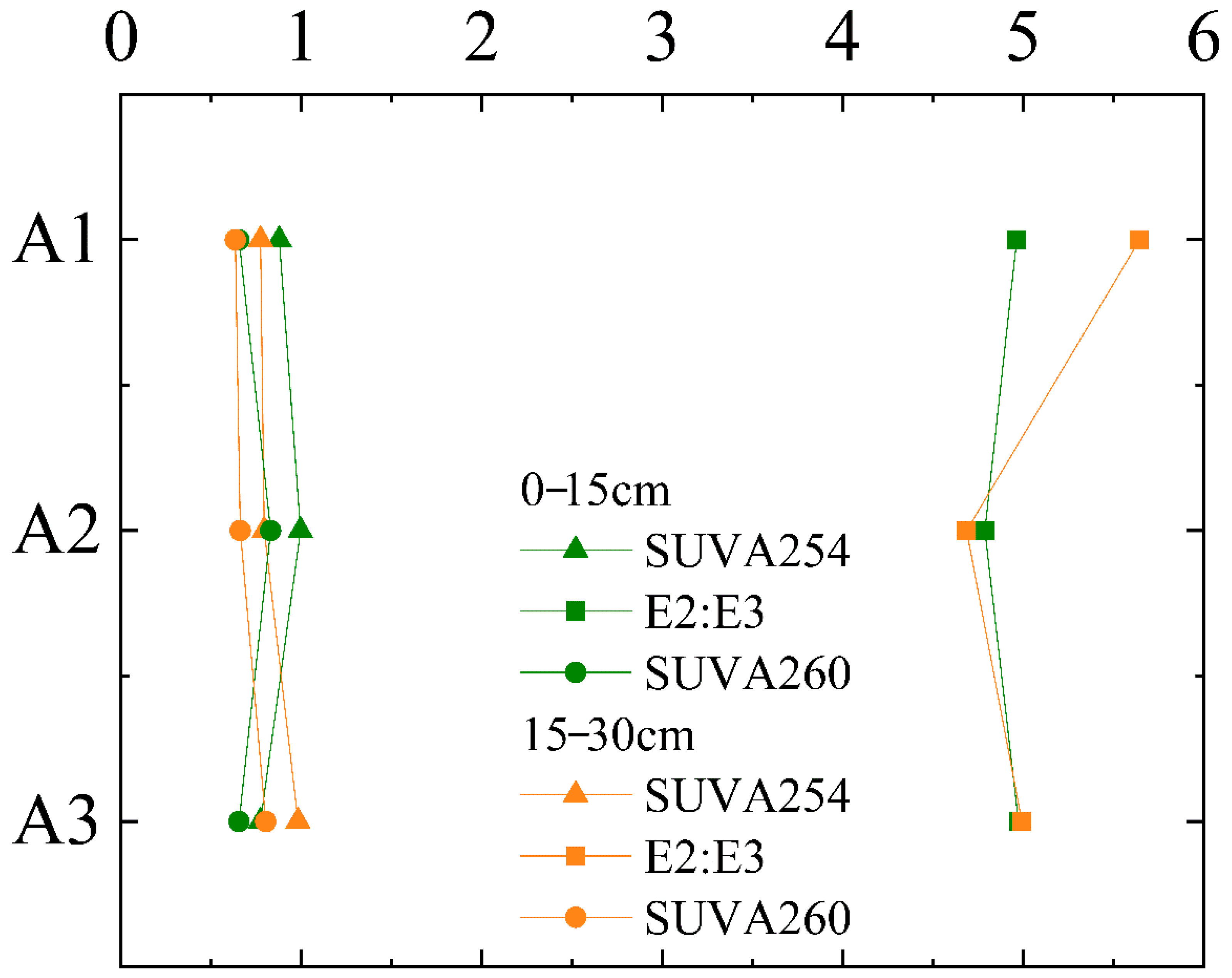
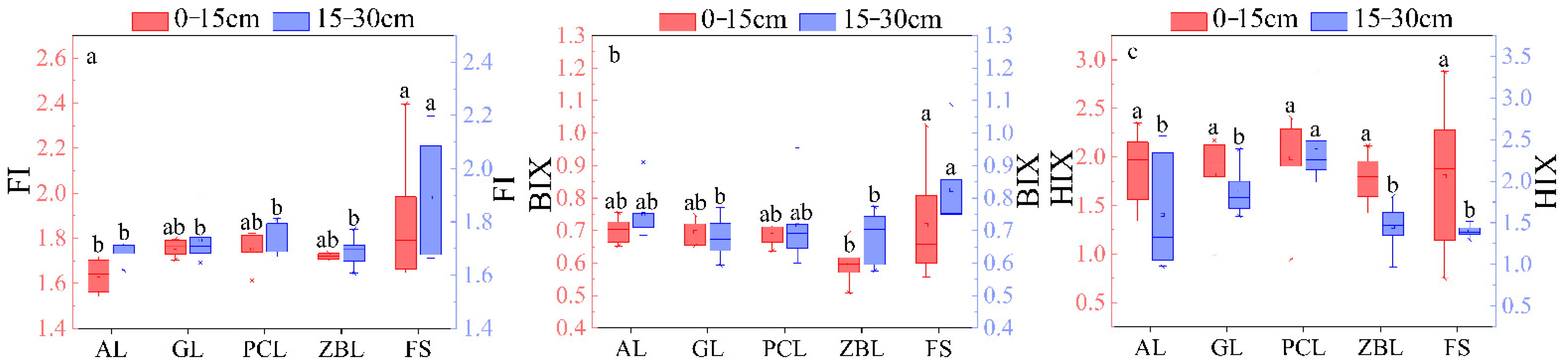
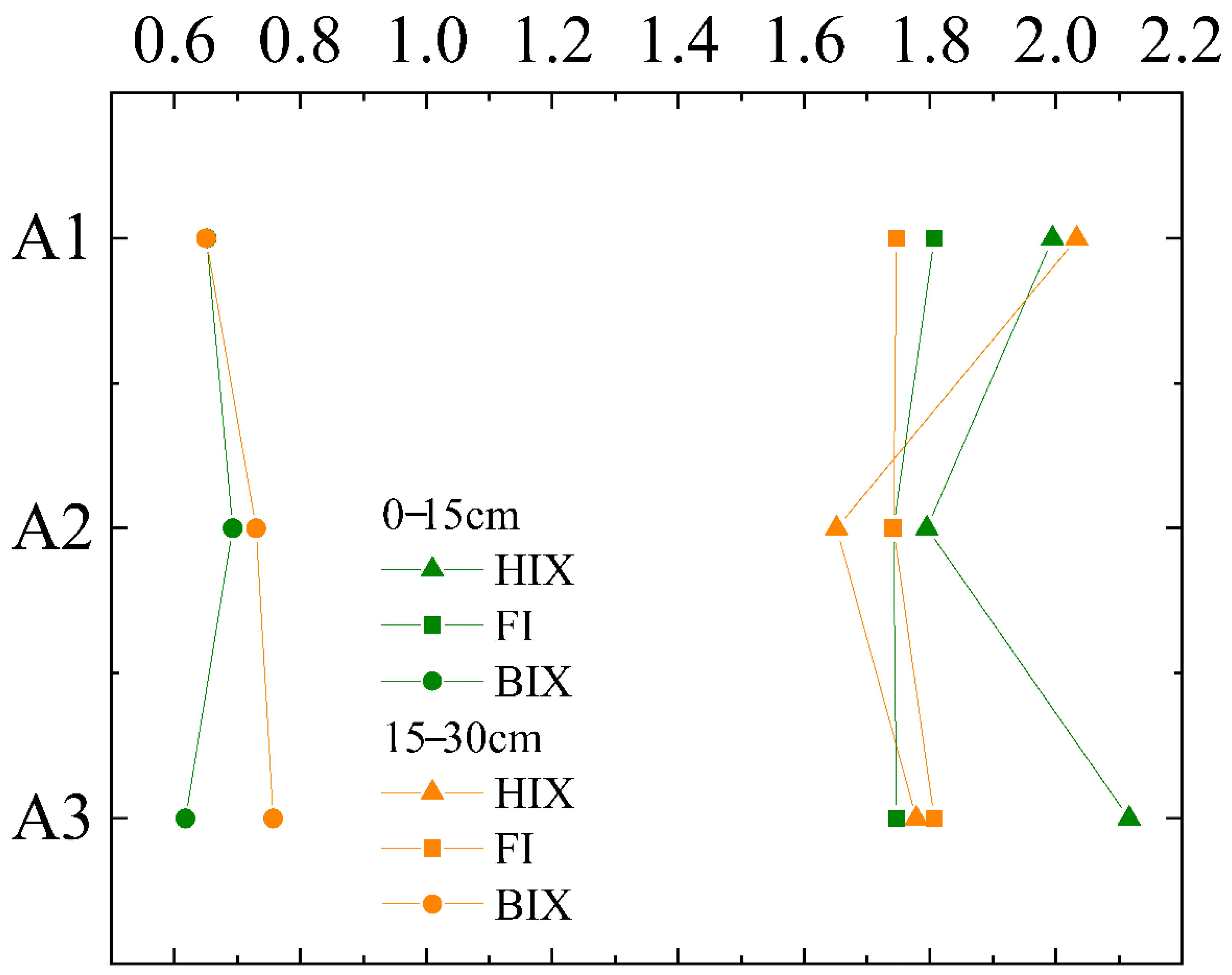
3.2. Spectral Parameter Characteristic
3.2.1. Characteristics of UV–Visible Absorption Spectrum
3.2.2. Characteristics of Fluorescence
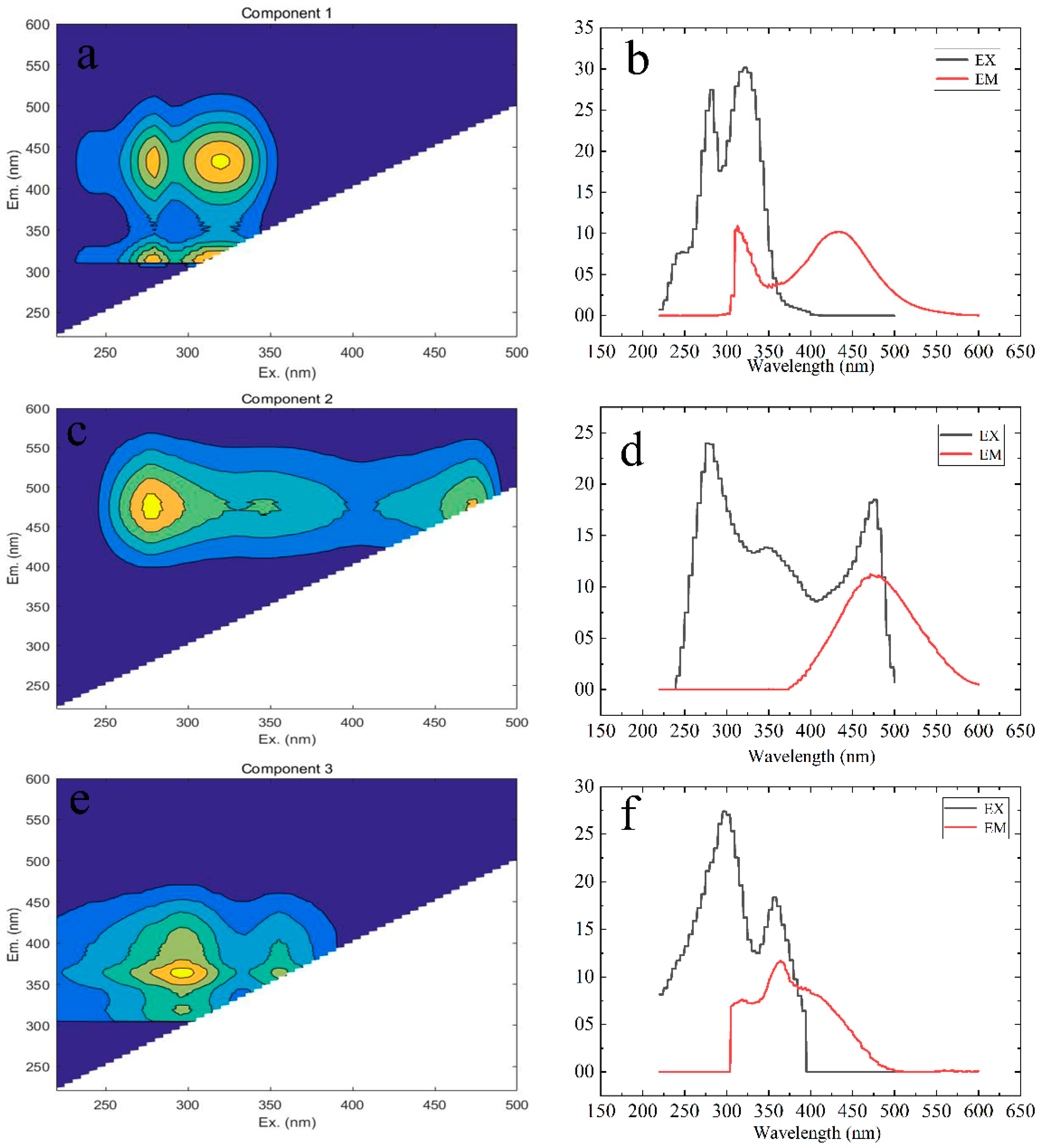
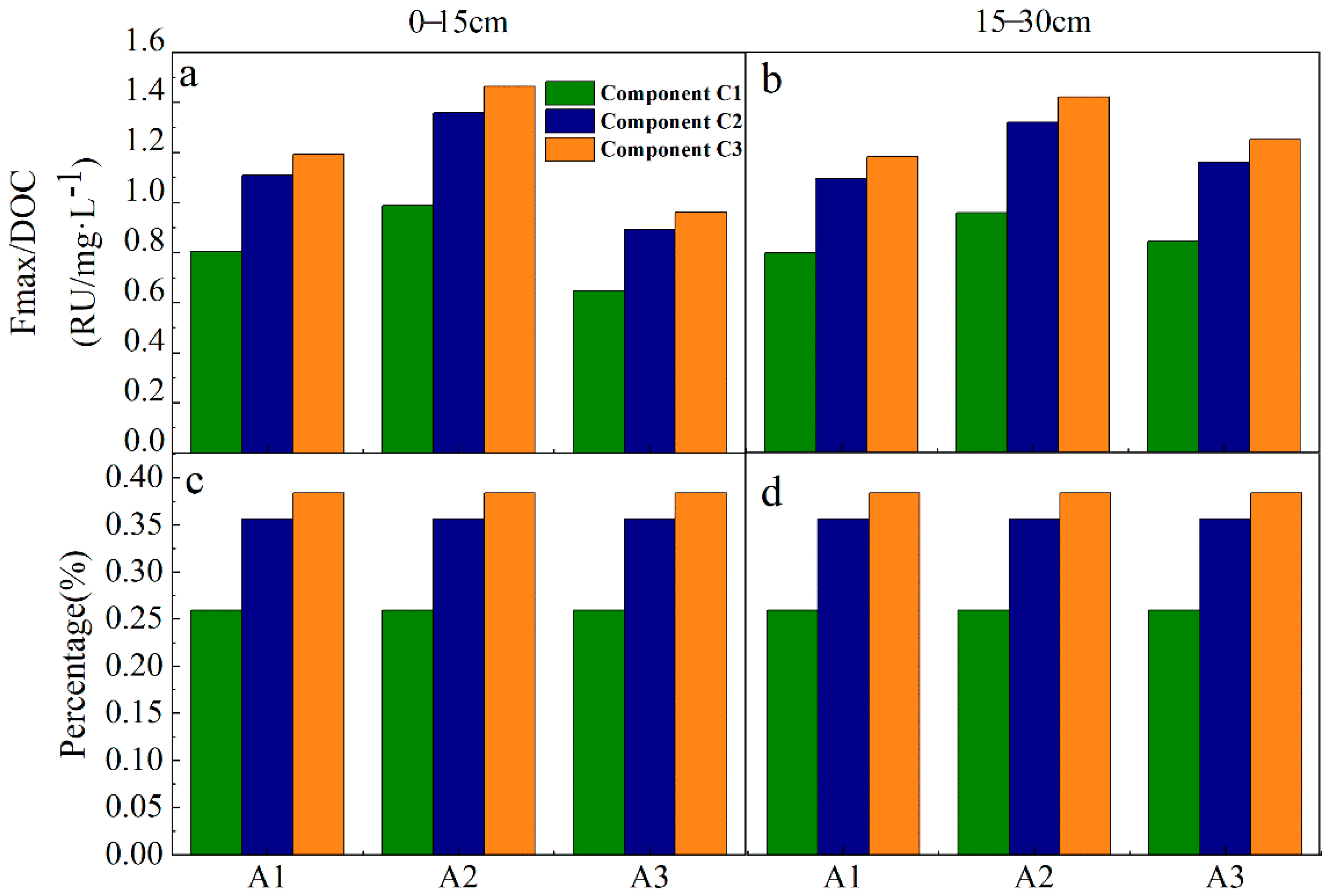
3.3. PARAFAC Component Analysis of Fluorescent Substances
4. Discussion
4.1. Spectral Characteristics and Isotopic Composition of Soils of Different Vegetation Restoration Types
4.2. Effects of Soil Erosion on the Distribution of Soil Organic Matter in the Drainage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil erosion and the global carbon budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Zhang, S. Improving the capacity of ecological carbon sink. People’s Daily, 10 June 2021; 13. (In Chinese) [Google Scholar]
- Tipping, E.; Woof, C.; Rigg, E.; Harrison, A.F.; Ineson, P.; Taylor, K.; Benham, D.; Poskitt, J.; Rowland, A.P.; Bol, R. Climatic influences on the leaching of dissolved organic matter from upland UK moorland soils, investigated by a field manipulation experiment. Environ. Int. 1999, 25, 83–96. [Google Scholar] [CrossRef]
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards-dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Berhe, A.A.; Xiao, H.; Liu, L.; Wang, D.; Peng, H.; Zeng, G. Characterizing dissolved organic matter in eroded sediments from a loess hilly catchment using fluorescence EEM-PARAFAC and UV–Visible absorption: Insights from source identification and carbon cycling. Geoderma 2019, 334, 37–48. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Jiang, T.; Kaal, J.; Liang, J.; Zhang, Y.; Wei, S.Q.; Wang, D.; Green, N.W. Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC-MS and thermally assisted hydrolysis and methylation. Sci. Total Environ. 2017, 603, 461–471. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Nie, X.; Huang, M.; Wang, D.; Xiao, H.; Liu, C.; Peng, H.; Jiang, J.; Zeng, G. The role of dissolved organic matter in soil organic carbon stability under water erosion. Environ. Int. 2019, 102, 724–733. [Google Scholar] [CrossRef]
- He, Q.; Xiao, Q.; Fan, J.; Zhao, H.; Cao, M.; Zhang, C.; Jiang, Y. The impact of heterotrophic bacteria on recalcitrant dissolved organic carbon formation in a typical karstic river. Sci. Total Environ. 2022, 152576. [Google Scholar] [CrossRef]
- Derrien, M.; Kim, M.S.; Ock, G.; Hong, S.; Cho, J.; Shin, K.H.; Hur, J. Estimation of different source contributions to sediment organic matter in an agricultural-forested watershed using end member mixing analyses based on stable isotope ratios and fluorescence spectroscopy. Sci. Total Environ. 2018, 618, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Bauer, J.E.; Canuel, E.A.; Chambers, R.M.; Yamashita, Y.; Jaffé, R.; Barrett, A. Effects of land use on sources and ages of inorganic and organic carbon in temperate headwater streams. Biogeochemistry 2014, 119, 275–292. [Google Scholar] [CrossRef]
- Yang, L.; Hur, J. Critical evaluation of spectroscopic indices for organic matter source tracing via end member mixing analysis based on two contrasting sources. Water Res. 2014, 59, 80–89. [Google Scholar] [CrossRef]
- Che, L.; Cheng, M.; Xing, L.; Cui, Y.; Wan, L. Effects of permafrost degradation on soil organic matter turnover and plant growth. Catena 2022, 208, 105721. [Google Scholar] [CrossRef]
- He, W.; Jung, H.; Lee, J.H.; Hur, J. Differences in spectroscopic characteristics between dissolved and particulate organic matters in sediments: Insight into distribution behavior of sediment organic matter. Sci. Total Environ. 2016, 547, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W.; Wang, H.J. A potentially important CO2 sink produced by the global water cycle Hui. Chin. Sci. Bull. 2008, 20, 2418–2422, (In Chinese with English abstract). [Google Scholar]
- Jiang, Z.; Yuan, D.; Cao, J.; Qin, X.; He, S.; Zhang, C. Research on Karst carbon sequestration potential in China. Acta Geogr. Sin. 2012, 33, 129–134, (In Chinese with English abstract). [Google Scholar]
- Jiang, Z.; Qin, X.; Cao, J.; Jiang, X.; He, S.; Luo, W. Zonal calculation of atmospheric CO2 carbon sink generated by karstification in China. Carsologica Sin. 2011, 30, 363–367. (In Chinese) [Google Scholar]
- Yang, H.; Xie, Y.; Zhu, T.; Zhou, M. Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region. Forests 2021, 12, 221. [Google Scholar] [CrossRef]
- Qiu, S.; Peng, J.; Zheng, H.; Xu, Z.; Meersmans, J. How can massive ecological restoration programs interplay with social-ecological systems? A review of research in China’s karst region. Sci. Total Environ. 2021, 807, 150723. [Google Scholar] [CrossRef]
- Xiong, K.; Chen, Q. Discussion on karst rocky desert evolution trend based on ecologically comprehensive treatment. Caisologica Sin. 2010, 29, 50–56. (In Chinese) [Google Scholar]
- Liu, Z.; Li, K.; Xiong, K.; Li, Y.; Wang, J.; Sun, J.; Cai, L. Effects of Zanthoxylum bungeanum planting on soil hydraulic properties and soil moisture in a karst area. Agr. Water Manag. 2021, 257, 107125. [Google Scholar] [CrossRef]
- Lan, J. Responses of soil organic carbon components and their sensitivity to karst rocky desertification control measures in Southwest China. J. Soils Sediments 2020, 21, 978–989. [Google Scholar] [CrossRef]
- Xie, L.; Zhong, J.; Chen, F.; Cao, F.; Li, J.; Wu, L. Evaluation of soil fertility in the succession of karst rocky desertification using principal component analysis. Solid Earth 2015, 6, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Wall, A.; Hytönen, J. Soil fertility of afforested arable land compared to continuously. Plant Soil 2005, 275, 247–260. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, G.; Xiong, K.; Cai, L. Dynamic variations in soil moisture in an epikarst fissure in the karst rocky desertification area. J. Hydrol. 2020, 591, 125587. [Google Scholar] [CrossRef]
- Xiong, K.; Li, J.; Long, M. Characteristics and key problems of soil erosion in typical karst rocky desertification control area. Acta Geogr. Sin. 2012, 67, 878–888. (In Chinese) [Google Scholar]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.L.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating binding characteristics of cadmium and copper to DOM derived from compost and rice straw using EEM-PARAFAC combined with two-dimensional FTIR correlation analyses. J. Hazard. Mater. 2017, 344, 539–548. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr.-Meth. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Rontani, J.F.; Volkman, J.K. Lipid characterization of coastal hypersaline cyanobacterial mats from the Camargue (France). Org. Geochem. 2005, 36, 251–272. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Cory, R.M.; Mcknight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Cory, R.M.; Miller, M.P.; Mcknight, D.M.; Guerard, J.J.; Miller, P.L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr.-Meth. 2010, 8, 67–78. [Google Scholar]
- Huguet, A.; Vacher, L.; Relexans, S.; Froidefond, J.M.; Parlanti, E. Properties of Fluorescent Dissolved Organic Matter in the Gironde Estuary. Org. Geochem. 2008, 40, 706–719. [Google Scholar] [CrossRef]
- Leenheer, J.A.; Croué, J.P. Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef] [Green Version]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Res. II 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Li, Y.; Yao, X.L.; Ding, W.H.; Zhang, Y.B.; Jeppesen, E.; Zhang, Y.L.; Podgorski, D.C.; Chen, C.M.; Ding, Y.; et al. Response of chromophoric dissolved organic matter dynamics to tidal oscillations and anthropogenic disturbances in a large subtropical estuary. Sci. Total Environ. 2019, 662, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Shangguan, Z.P.; Deng, L. Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession. Forests 2021, 12, 1195. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhu, W.X.; Zhou, Y.B.; Yin, Y. Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef] [Green Version]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Frouz, J. Effects of soil macro-and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2017, 332, 161–172. [Google Scholar] [CrossRef]
- Grandy, A.S.; Strickland, M.S.; Lauber, C.L.; Bradford, M.A.; Fierer, N. The influence of microbial communities, management, and soil texture on soil organic matter chemistry. Geoderma 2009, 150, 278–286. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Tropical tree species composition affects the oxidation of dissolved organic matter from litter. Biogeochemistry 2008, 88, 127–138. [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Yan, J.; Guo, N.; Wen, S.; Wang, D.; Gao, J.; Zhao, Z. UV Vis spectral characteristics of dissolved organic matter (DOM) in soil and sediment of typical fluctuating zone in Three Gorges Reservoir Area. Environ. Sci. 2014, 35, 933–941. (In Chinese) [Google Scholar]
- Chen, Y.; Cheng, X.; Zhang, Q. Recovery approach affects soil quality in the water level fluctuation zone of the Three Gorges Reservoir, China: Implications for revegetation. Environ. Sci. Pollut. Res. 2014, 21, 2018–2031. [Google Scholar]
- Yao, X.; Zou, S.; Yuan, X.; Xu, D.; Yao, M. Migration characteristics of dissolved organic matter in typical karst water system. Environ. Sci. 2014, 35, 1766–1772. (In Chinese) [Google Scholar]
| Site | Vegetation Restoration Type | Rock Outcrop Ratio | Slope | Vegetation Coverage |
|---|---|---|---|---|
| A1 | GL | 0% | 3° | 95% |
| A1 | GL | 5% | 2° | 95% |
| A1 | FS | 50% | 5° | 80% |
| A1 | PCL | 0% | 5° | 99% |
| A1 | ZBL | 50% | 5° | 50% |
| A2 | AL | 20% | 3° | 70% |
| A2 | ZBL | 50% | 5° | 50% |
| A2 | PCL | 15% | 2° | 30% |
| A2 | GL | 5% | 2° | 95% |
| A2 | ZBL | 10% | 2° | 40% |
| A2 | ZBL | 40% | 2° | 38% |
| A3 | ZBL | 40% | 2° | 38% |
| A3 | GL | 5% | 2° | 95% |
| A3 | FS | 55% | 5° | 75% |
| Sites | Depth (cm) | pH | SOC (g/kg) | DOC (mg/kg) | δ13Csoc (‰) |
|---|---|---|---|---|---|
| A1 | 0–15 | 7.30 ± 0.07 a | 31.93 ± 1.73 a | 96.95 ± 7.40 ab | −21.53 ± 0.28 b |
| A1 | 15–30 | 7.36 ± 0.07 a | 27.06 ± 1.19 a | 85.79 ± 7.20 ab | −21.97 ± 0.36 ab |
| A2 | 0–15 | 7.34 ± 0.07 a | 28.27 ± 1.69 a | 79.36 ± 4.13 b | −21.06 ± 0.23 b |
| A2 | 15–30 | 7.41 ± 0.05 a | 25.35 ± 1.37 a | 75.88 ± 3.06 a | −21.79 ± 0.27 a |
| A3 | 0–15 | 7.46 ± 0.09 a | 27.73 ± 3.05 b | 125.99 ± 13.29 a | −22.44 ± 0.30 b |
| A3 | 15–30 | 7.52 ± 0.09 a | 26.09 ± 2.82 b | 89.76 ± 7.83 a | −22.64 ± 0.29 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Z.; Xiong, K.; Li, Y.; Cheng, K. Soil Organic Carbon Distribution and Its Response to Soil Erosion Based on EEM-PARAFAC and Stable Carbon Isotope, a Field Study in the Rocky Desertification Control of South China Karst. Int. J. Environ. Res. Public Health 2022, 19, 3210. https://doi.org/10.3390/ijerph19063210
Wang X, Liu Z, Xiong K, Li Y, Cheng K. Soil Organic Carbon Distribution and Its Response to Soil Erosion Based on EEM-PARAFAC and Stable Carbon Isotope, a Field Study in the Rocky Desertification Control of South China Karst. International Journal of Environmental Research and Public Health. 2022; 19(6):3210. https://doi.org/10.3390/ijerph19063210
Chicago/Turabian StyleWang, Xinwen, Ziqi Liu, Kangning Xiong, Yuan Li, and Kun Cheng. 2022. "Soil Organic Carbon Distribution and Its Response to Soil Erosion Based on EEM-PARAFAC and Stable Carbon Isotope, a Field Study in the Rocky Desertification Control of South China Karst" International Journal of Environmental Research and Public Health 19, no. 6: 3210. https://doi.org/10.3390/ijerph19063210
APA StyleWang, X., Liu, Z., Xiong, K., Li, Y., & Cheng, K. (2022). Soil Organic Carbon Distribution and Its Response to Soil Erosion Based on EEM-PARAFAC and Stable Carbon Isotope, a Field Study in the Rocky Desertification Control of South China Karst. International Journal of Environmental Research and Public Health, 19(6), 3210. https://doi.org/10.3390/ijerph19063210






