Toxic and Nutritional Optic Neuropathies—An Updated Mini-Review
Abstract
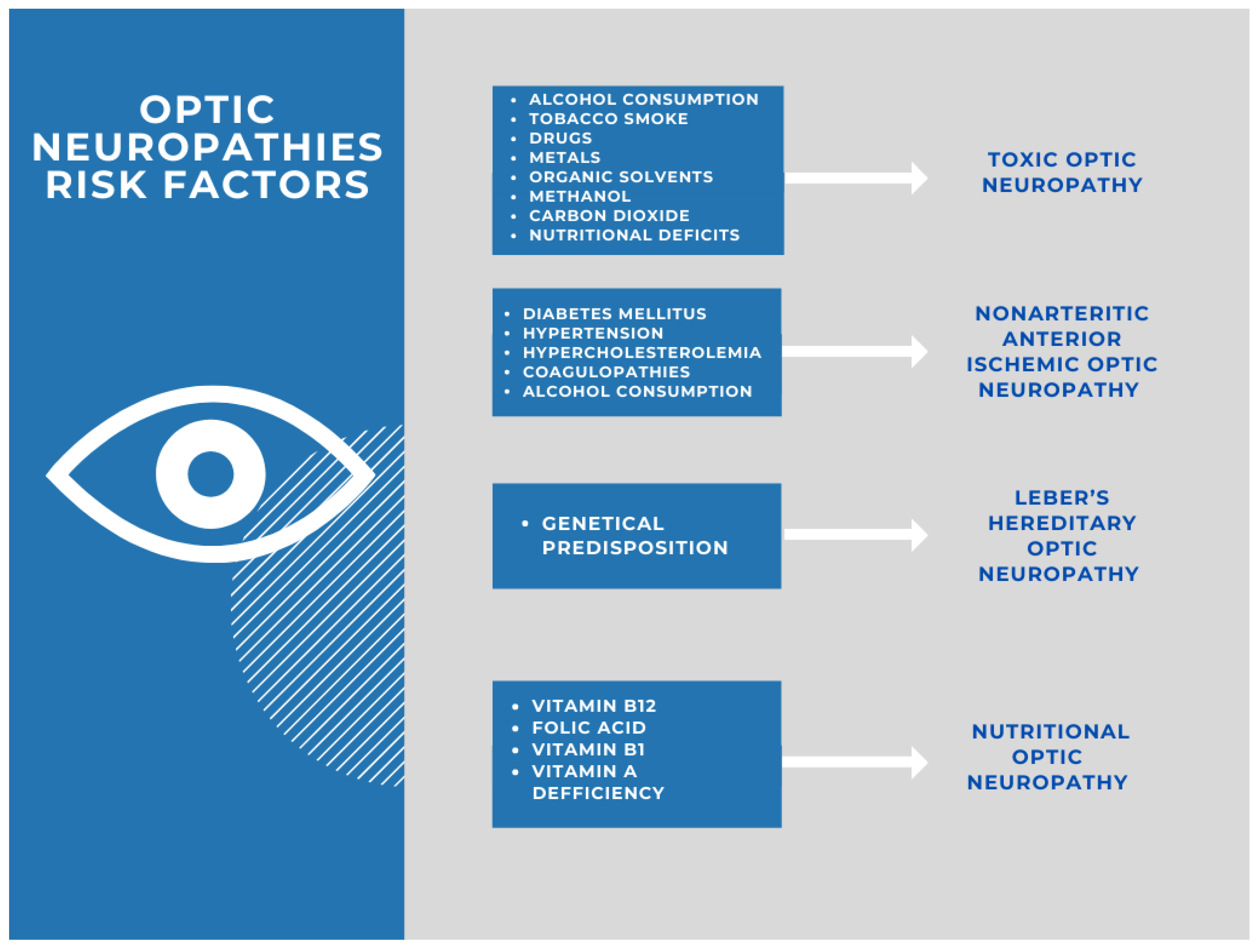
1. Introduction
2. The Effects of Alcohol on the Central and Peripheral Nervous Systems
3. Pathophysiology of Alcohol-Induced Neuropathies
4. Optic Neuropathy Induced by Toxic Substances
4.1. Methanol, Ethylene Glycol, and Diethylene Glycol
4.2. Cobalt, Lead, and Other Heavy Metals
4.3. Drugs
4.4. Other Toxic Substances
5. Nutritional Deficiencies as a Trigger of Optic Neuropathy
6. Treatment Options
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| EON | ethambutol-induced optic neuropathy |
| EPO | intravenous erythropoietin |
| LHON | Leber hereditary optic neuropathy |
| TON | toxic optic neuropathy |
| WHO | World Health Organization |
References
- Zhao, J.; Stockwell, T.; Roemer, A.; Naimi, T.; Chikritzhs, T. Alcohol Consumption and Mortality From Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. J. Stud. Alcohol Drugs 2017, 78, 375–386. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; INTERSTROKE Investigators; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Krenz, M.; Korthuis, R.J. Moderate ethanol ingestion and cardiovascular protection: From epidemiologic associations to cellular mechanisms. J. Mol. Cell. Cardiol. 2012, 52, 93–104. [Google Scholar] [CrossRef]
- Chen, G.; Luo, J. Anthocyanins: Are they beneficial in treating ethanol neurotoxicity? Neurotox. Res. 2010, 17, 91–101. [Google Scholar] [CrossRef]
- Patra, J.; Taylor, B.; Irving, H.; Roerecke, M.; Baliunas, D.; Mohapatra, S.; Rehm, J. Alcohol consumption and the risk of morbidity and mortality for different stroke types—A systematic review and meta-analysis. BMC Public Health 2010, 10, 158. [Google Scholar] [CrossRef]
- Cohen, J.I.; Nagy, L.E. Pathogenesis of alcoholic liver disease: Interactions between parenchymal and non-parenchymal cells. J. Dig. Dis. 2011, 12, 3–9. [Google Scholar] [CrossRef]
- Reidy, J.; McHugh, E.; Stassen, L. A review of the relationship between alcohol and oral cancer. Surgeon 2011, 9, 278–283. [Google Scholar] [CrossRef]
- Seitz, H.K.; Cho, C.H. Contribution of alcohol and tobacco use in gastrointestinal cancer development. Methods Mol. Biol. 2009, 472, 217–241. [Google Scholar]
- Xu, M.; Bower, K.A.; Chen, G.; Shi, X.; Dong, Z.; Ke, Z.; Luo, J. Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin. Alcohol. Clin. Exp. Res. 2010, 34, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Light alcohol drinking and cancer: A meta-analysis. Ann. Oncol. 2013, 24, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J. The risks associated with alcohol use and alcoholism. Alcohol. Res. Health 2011, 34, 135–143. [Google Scholar] [PubMed]
- Planas-Ballvé, A.; Grau-López, L.; Morillas, R.M.; Planas, R. Neurological manifestations of excessive alcohol consumption. Gastroenterol. Hepatol. 2017, 40, 709–717. [Google Scholar] [CrossRef]
- Rehm, J.; Room, R.; Monteiro, M.; Gmel, G.; Graham, K.; Rehn, N.; Sempos, C.T.; Frick, U.; Jernigan, D. Alcohol use. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; pp. 959–1109. [Google Scholar]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef]
- Roerecke, M.; Vafaei, A.; Hasan, O.S.; Chrystoja, B.; Cruz, M.; Lee, R.; Neuman, M.G.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586. [Google Scholar] [CrossRef]
- Xi, B.; Veeranki, S.P.; Zhao, M.; Ma, C.; Yan, Y.; Mi, J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 913–922. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018. World Health Orhanization 2018. Available online: https://www.who.int/publications/i/item/global-status-report-on-alcohol-and-health-2018 (accessed on 16 November 2020).
- Mellion, M.; Gilchrist, J.M.; De La Monte, S. Alcohol-related peripheral neuropathy: Nutritional, toxic, or both? Muscle Nerve 2011, 43, 309–316. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef]
- Brust, J.C. Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: A review. Int. J. Environ. Res. Public Health 2010, 7, 1540–1557. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kataria, A.; Kolla, B.P.; Thusius, N.; Loukianova, L.L. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin. Proc. 2019, 94, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Fox, V. Beyond Thiamine: Treatment for Cognitive Impairment in Korsakoff’s Syndrome. Psychosomatics 2018, 59, 311–317. [Google Scholar] [CrossRef]
- Chopra, K.; Tiwari, V. Alcoholic neuropathy: Possible mechanisms and future treatment possibilities. Br. J. Clin. Pharmacol. 2012, 73, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Patel, N. Approach to a case of autonomic peripheral neuropathy. J. Assoc. Physicians India 2006, 54, 727–732. [Google Scholar] [PubMed]
- Grochowski, C.; Blicharska, E.; Bogucki, J.; Proch, J.; Mierzwińska, A.; Baj, J.; Litak, J.; Podkowiński, A.; Flieger, J.; Teresiński, G.; et al. Increased Aluminum Content in Certain Brain Structures is Correlated with Higher Silicon Concentration in Alcoholic Use Disorder. Molecules 2019, 24, 1721. [Google Scholar] [CrossRef]
- Ansari, F.H.; Juergens, A.L. Saturday Night Palsy. [Updated 13 May 2020]; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557520/ (accessed on 15 August 2021).
- Munukutla, S.; Pan, G.; Deshpande, M.; Thandavarayan, R.A.; Krishnamurthy, P.; Palaniyandi, S.S. Alcohol Toxicity in Diabetes and Its Complications: A Double Trouble? Alcohol Clin. Exp. Res. 2016, 40, 686–697. [Google Scholar] [CrossRef]
- Julian, T.; Glascow, N.; Syeed, R.; Zis, P. Alcohol-related peripheral neuropathy: A systematic review and meta-analysis. J. Neurol. 2019, 266, 2907–2919. [Google Scholar] [CrossRef]
- Lacomis, D. Small-fiber neuropathy. Muscle Nerve 2002, 26, 173–188. [Google Scholar] [CrossRef]
- Mellion, M.L.; Silbermann, E.; Gilchrist, J.M.; Machan, J.; Leggio, L.; De La Monte, S. Small-fiber degeneration in alcohol-related peripheral neuropathy. Alcohol Clin. Exp. Res. 2014, 38, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Julian, T.H.; Syeed, R.; Glascow, N.; Zis, P. Alcohol-induced autonomic dysfunction: A systematic review. Clin. Auton. Res. 2020, 30, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, C.; Di Leo, R.; Girlanda, P.; Messina, C.; Vita, G. Is there a relationship between somatic and autonomic neuropathies in chronic alcoholics? J. Neurol. Sci. 2005, 228, 15–19. [Google Scholar] [CrossRef]
- Sadowski, A.; Houck, R.C. Alcoholic Neuropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Masaki, T.; Mochizuki, H.; Matsushita, S.; Yokoyama, A.; Kamakura, K.; Higuchi, S. Association of aldehyde dehydrogenase-2 polymorphism with alcoholic polyneuropathy in humans. Neurosci. Lett. 2004, 363, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Nevoret, M.L.; Casellini, C.; Parson, H. Diabetic neuropathy. Endocrinol. Metab. Clin. N. Am. 2013, 42, 747–787. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, N.; Jimenez-Shahed, J. Chronic Neurologic Effects of Alcohol. Clin. Liver Dis. 2019, 23, 141–155. [Google Scholar] [CrossRef]
- Nebuchennykh, M.; Løseth, S.; Mellgren, S.I. Aspects of peripheral nerve involvement in patients with treated hypothyroidism. Eur. J. Neurol. 2010, 17, 67–72. [Google Scholar] [CrossRef]
- Saylor, D.; Nakigozi, G.; Nakasujja, N.; Robertson, K.; Gray, R.H.; Wawer, M.J.; Sacktor, N. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology 2017, 89, 485–491. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Nevola, R.; Lus, G.; Restivo, L.; Guerrera, B.; Romano, C.; Zampino, R.; Rinaldi, L.; Sellitto, A.; Giordano, M.; et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: An overview. World J. Gastroenterol. 2015, 21, 2269–2280. [Google Scholar] [CrossRef]
- Kindstrand, E.; Nilsson, B.Y.; Hovmark, A.; Nennesmo, I.; Pirskanen, R.; Solders, G.; Asbrink, E. Polyneuropathy in late Lyme borreliosis—A clinical, neurophysiological and morphological description. Acta Neurol. Scand. 2000, 101, 47–52. [Google Scholar] [CrossRef]
- Antoine, J.C.; Camdessanché, J.P. Paraneoplastic neuropathies. Curr. Opin. Neurol. 2017, 30, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, R.; Liu, N.; Yi, N.; Zheng, H.; Zhang, Q.; Zhou, L.; Zhou, L.; Hu, R.; Lu, B. Liver fibrosis is independently associated with diabetic peripheral neuropathy in type 2 diabetes mellitus. J. Diabetes Investig. 2021, 12, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Berk, J.L. Neuropathy Associated with Systemic Amyloidosis. Semin. Neurol. 2019, 39, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Jokanović, M. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef]
- Goolsby, T.A.; Jakeman, B.; Gaynes, R.P. Clinical relevance of metronidazole and peripheral neuropathy: A systematic review of the literature. Int. J. Antimicrob. Agents 2018, 51, 319–325. [Google Scholar] [CrossRef]
- Badrinath, M.; John, S. Isoniazid Toxicity. [Updated 2 July 2020]; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531488/ (accessed on 15 August 2021).
- van den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef]
- Pareyson, D.; Saveri, P.; Pisciotta, C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr. Opin. Neurol. 2017, 30, 471–480. [Google Scholar] [CrossRef]
- Grzybowski, A.; Zülsdorff, M.; Wilhelm, H.; Tonagel, F. Toxic optic neuropathies: An updated review. Acta Ophthalmol. 2015, 93, 402–410. [Google Scholar] [CrossRef]
- Kesler, A.; Pianka, P. Toxic optic neuropathy. Curr. Neurol. Neurosci. Rep. 2003, 3, 410–414. [Google Scholar] [CrossRef]
- Margolin, E.; Shemesh, A. Toxic and Nutritional Optic Neuropathy. [Updated 5 July 2020]; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499979/ (accessed on 16 August 2021).
- Grzybowski, A.; Brona, P. Nutritional optic neuropathy instead of tobacco-alcohol amblyopia. Can. J. Ophthalmol. 2017, 52, 533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiotoroiu, S.; Noaghi, M.; Stefaniu, G.; Secureanu, F.; Purcarea, V.; Zemba, M. Tobacco-alcohol optic neuropathy--clinical challenges in diagnosis. J. Med. Life 2014, 7, 472–476. [Google Scholar] [PubMed]
- Kirkman, M.A.; Yu-Wai-Man, P.; Korsten, A.; Leonhardt, M.; Dimitriadis, K.; de Coo, I.; Klopstock, T.; Chinnery, P.F. Gene-environment interactions in Leber hereditary optic neuropathy. Brain 2009, 132 Pt 9, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, J.M.; Hickman, S.J. Treatment and Outcomes in Nutritional Optic Neuropathy. Curr. Treat. Options Neurol. 2019, 21, 5. [Google Scholar] [CrossRef]
- Sadun, A.A.; Carelli, V.; Salomao, S.R.; Berezovsky, A.; Quiros, P.A.; Sadun, F.; DeNegri, A.M.; Andrade, R.; Moraes, M.; Passos, A.; et al. Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am. J. Ophthalmol. 2003, 136, 231–238. [Google Scholar] [CrossRef]
- Mancinelli, R.; Barlocci, E.; Ciprotti, M.; Senofonte, O.; Fidente, R.M.; Draisci, R.; Attilia, M.L.; Vitali, M.; Fiore, M.; Ceccanti, M. Blood thiamine, zinc, selenium, lead and oxidative stress in a population of male and female alcoholics: Clinical evidence and gender differences. Ann. Ist. Super. Sanita 2013, 49, 65–72. [Google Scholar]
- Baj, J.; Flieger, W.; Teresiński, G.; Buszewicz, G.; Sitarz, E.; Forma, A.; Karakuła, K.; Maciejewski, R. Magnesium, Calcium, Potassium, Sodium, Phosphorus, Selenium, Zinc, and Chromium Levels in Alcohol Use Disorder: A Review. J. Clin. Med. 2020, 9, 1901. [Google Scholar] [CrossRef]
- Dudek, I.; Hajduga, D.; Sieńko, C.; Maani, A.; Sitarz, E.; Sitarz, M.; Forma, A. Alcohol-Induced Neuropathy in Chronic Alcoholism: Causes, Pathophysiology, Diagnosis, and Treatment Options. Curr. Pathobiol. Rep. 2020, 8, 87–97. [Google Scholar] [CrossRef]
- Singleton, C.K.; Martin, P.R. Molecular mechanisms of thiamine utilization. Curr. Mol. Med. 2001, 1, 197–207. [Google Scholar] [CrossRef]
- Madaan, P.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Yadav, S.; Kaur, S.; Bhatia, S.; Al-Harrasi, A.; Abdellatif, A.A.H.; et al. Exploring the Therapeutic Potential of Targeting Purinergic and Orexinergic Receptors in Alcoholic Neuropathy. Neurotox. Res. 2022. Epub ahead of printing. [Google Scholar] [CrossRef]
- Robins, M.T.; Heinricher, M.M.; Ryabinin, A.E. From Pleasure to Pain, and Back Again: The Intricate Relationship Between Alcohol and Nociception. Alcohol Alcohol. 2019, 54, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, A.; Tata, M.R.; Aurilio, C.; Ciccone, G.; Gemini, D.; Ugolini, G.; Argenzio, F. Peripheral neuropathy in chronic alcoholism: A retrospective cross-sectional study in 76 subjects. Alcohol Alcohol. 2001, 36, 271–275. [Google Scholar] [CrossRef]
- Narita, M.; Miyoshi, K.; Narita, M.; Suzuki, T. Involvement of microglia in the ethanol-induced neuropathic pain-like state in the rat. Neurosci. Lett. 2007, 414, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dina, O.A.; Barletta, J.; Chen, X.; Mutero, A.; Martin, A.; Messing, R.; Levine, J.D. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J. Neurosci. 2000, 20, 8614–8619. [Google Scholar] [CrossRef] [PubMed]
- Dina, O.A.; Gear, R.W.; Messing, R.O.; Levine, J.D. The severity of alcohol-induced painful peripheral neuropathy in female rats: Role of estrogen and protein kinase (A and C epsilon). Neuroscience 2007, 145, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Gerhard, U.; Gerlach, M.; Weijers, H.-G.; Boening, J.; Wiesbeck, G.A. Cortisol concentrations, stress-coping styles after withdrawal, and long-term abstinence in alcohol dependence. Addict. Biol. 2006, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Mullins, M.E. Toxic Alcohols. N. Engl. J. Med. 2018, 378, 270–280. [Google Scholar] [CrossRef]
- Moon, C.S. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann. Occup. Environ. Med. 2017, 29, 44. [Google Scholar] [CrossRef]
- Kruse, J.A. Methanol and ethylene glycol intoxication. Crit. Care Clin. 2012, 28, 661–711. [Google Scholar] [CrossRef]
- Carelli, V. Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: Genetic and acquired optic neuropathies. Neurochem. Int. 2002, 40, 573–584. [Google Scholar] [CrossRef]
- Pakdel, F.; Sanjari, M.S.; Naderi, A.; Pirmarzdashti, N.; Haghighi, A.; Kashkouli, M.B. Erythropoietin in Treatment of Methanol Optic Neuropathy. J. Neuroophthalmol. 2018, 38, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Esfandiari, H.; Sanjari, N.; Ghahari, E. Erythropoietin as an adjunctive treatment for methanol-induced toxic optic neuropathy. Am. J. Drug Alcohol Abuse 2016, 42, 633–639. [Google Scholar] [CrossRef]
- Martin-Amat, G.; Tephly, T.R.; McMartin, K.E.; Makar, A.B.; Hayreh, M.S.; Hayreh, S.S.; Baumbach, G.; Cancilla, P. Methyl alcohol poisoning. II. Development of a model for ocular toxicity in methyl alcohol poisoning using the rhesus monkey. Arch. Ophthalmol. 1977, 95, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-induced optic neuropathy: A still-present problem. Arch. Toxicol. 2022, 96, 431–451. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.J.; Dargan, P.I.; Wood, D.M. Challenges in the diagnosis of ethylene glycol poisoning. Ann. Clin. Biochem. 2014, 51 Pt 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Froberg, K.; Dorion, R.P.; McMartin, K.E. The role of calcium oxalate crystal deposition in cerebral vessels during ethylene glycol poisoning. Clin. Toxicol. (Phila.) 2006, 44, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.H. Ethylene glycol poisoning: Quintessential clinical toxicology; analytical conundrum. Clin. Chim. Acta 2012, 413, 365–377. [Google Scholar] [CrossRef]
- Thanacoody, R.H.; Gilfillan, C.; Bradberry, S.M.; Davies, J.; Jackson, G.; Vale, A.J.; Thompson, J.P.; Eddleston, M.; Thomas, S.H. Management of poisoning with ethylene glycol and methanol in the UK: A prospective study conducted by the National Poisons Information Service (NPIS). Clin. Toxicol. (Phila.) 2016, 54, 134–140. [Google Scholar] [CrossRef]
- Brent, J. Fomepizole for the treatment of pediatric ethylene and diethylene glycol, butoxyethanol, and methanol poisonings. Clin. Toxicol. (Phila.) 2010, 48, 401–406. [Google Scholar] [CrossRef]
- Rietjens, S.J.; de Lange, D.W.; Meulenbelt, J. Ethylene glycol or methanol intoxication: Which antidote should be used, fomepizole or ethanol? Neth. J. Med. 2014, 72, 73–79. [Google Scholar]
- Brent, J. Fomepizole for ethylene glycol and methanol poisoning. N. Engl. J. Med. 2009, 360, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Sanaei-Zadeh, H.; Zamani, N.; Shadnia, S. Outcomes of visual disturbances after methanol poisoning. Clin. Toxicol. (Phila.) 2011, 49, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, S.; Pelclova, D.; Diblik, P.; Urban, P.; Kuthan, P.; Nurieva, O.; Kotikova, K.; Navratil, T.; Komarc, M.; Belacek, J.; et al. Long-term visual damage after acute methanol poisonings: Longitudinal cross-sectional study in 50 patients. Clin. Toxicol. (Phila.) 2015, 53, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Katavetin, P.; Tungsanga, K.; Eiam-Ong, S.; Nangaku, M. Antioxidative effects of erythropoietin. Kidney Int. 2007, 72, S10–S15. [Google Scholar] [CrossRef]
- Sun, Y.; Calvert, J.; Zhang, J.H. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke 2005, 36, 1672–1678. [Google Scholar] [CrossRef]
- King, C.E.; Rodger, J.; Bartlett, C.; Esmaili, T.; Dunlop, S.A.; Beazley, L.D. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp. Neurol. 2007, 205, 48–55. [Google Scholar] [CrossRef]
- DeVoti, E.; Marta, E.; Belotti, E.; Bregoli, L.; Liut, F.; Maiorca, P.; Mazzucotelli, V.; Cancarini, G. Diethylene glycol poisoning from transcutaneous absorption. Am. J. Kidney Dis. 2015, 65, 603–606. [Google Scholar] [CrossRef]
- Imam, Y.Z.B.; Kamran, S.; Karim, H.; Elalamy, O.; Sokrab, T.; Osman, Y.; Deleu, D. Neurological manifestation of recreational fatal and near-fatal diethylene glycol poisonings: Case series and review of literature. Medicine (Baltimore) 2014, 93, e62. [Google Scholar] [CrossRef]
- Reddy, N.J.; Sudini, M.; Lewis, L.D. Delayed neurological sequelae from ethylene glycol, diethylene glycol and methanol poisonings. Clin. Toxicol. (Phila.) 2010, 48, 967–973. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.; Temple, W.A.; Beasley, D.M.G. Diethylene glycol poisoning. Clin. Toxicol. (Phila.) 2009, 47, 525–535. [Google Scholar] [CrossRef]
- Scalzo, A.J. Diethylene glycol toxicity revisited: The 1996 Haitian epidemic. J. Toxicol. Clin. Toxicol. 1996, 34, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Drut, R.; Quijano, G.; Jones, M.C.; Scanferla, P. Hallazgospatológicosen la intoxicación por dietilenglicol [Pathologic findings in diethylene glycol poisoning]. Medicina (B Aires) 1994, 54, 1–5. [Google Scholar] [PubMed]
- Besenhofer, L.M.; Adegboyega, P.A.; Bartels, M.; Filary, M.J.; Perala, A.W.; McLaren, M.C.; McMartin, K.E. Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicol. Sci. 2010, 117, 25–35. [Google Scholar] [CrossRef]
- Catalani, S.; Rizzetti, M.C.; Padovani, A.; Apostoli, P. Neurotoxicity of cobalt. Hum. Exp. Toxicol. 2012, 31, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.D.; Hur, M.; Chen, J.J.; Bhatti, M.T. Cobalt toxic optic neuropathy and retinopathy: Case report and review of the literature. Am. J. Ophthalmol. Case Rep. 2020, 17, 100606. [Google Scholar] [CrossRef] [PubMed]
- Apostoli, P.; Catalani, S.; Zaghini, A.; Mariotti, A.; Poliani, P.L.; Vielmi, V.; Semeraro, F.; Duse, S.; Porzionato, A.; Macchi, V.; et al. High doses of cobalt induce optic and auditory neuropathy. Exp. Toxicol. Pathol. 2013, 65, 719–727. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Perez, J.; Peden, M. Optic Neuropathy from Cobalt Toxicity in a Patient who Ingested Cattle Magnets. Neuroophthalmology 2011, 35, 24–26. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Tan, P.; Zhou, W.; Zhu, X.; Cui, Y.; Zhu, L.; Feng, X.; Qi, H.; Zheng, J.; Gu, P.; et al. N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell. Mol. Neurobiol. 2012, 32, 1275–1285. [Google Scholar] [CrossRef]
- Baghdassarian, S.A. Optic neuropathy due to lead poisoning. Report of a case. Arch. Ophthalmol. 1968, 80, 721–723. [Google Scholar] [CrossRef]
- Abri Aghdam, K.; Zand, A.; SoltanSanjari, M. Bilateral Optic Disc Edema in a Patient with Lead Poisoning. J. Ophthalmic. Vis. Res. 2019, 14, 513–517. [Google Scholar] [CrossRef]
- Patel, A.; Athawale, A.M. Acute lead encephalopathy with optic neuropathy. Indian Pediatr. 2005, 42, 188–189. [Google Scholar] [PubMed]
- Ekinci, M.; Ceylan, E.; Çağatay, H.H.; Keleş, S.; Altınkaynak, H.; Kartal, B.; Koban, Y.; Hüseyinoğlu, N. Occupational exposure to lead decreases macular, choroidal, and retinal nerve fiber layer thickness in industrial battery workers. Curr. Eye Res. 2014, 39, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Pelclová, D.; Urban, P.; Ridzon, P.; Šenholdová, Z.; Lukáš, E.; Diblík, P.; Lacina, L. Two-year follow-up of two patients after severe thallium intoxication. Hum. Exp. Toxicol. 2009, 28, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Bahiga, L.M.; Mahmoud, L.; Kotb, N.A.; El-Dessoukey, E.A. Neurological syndromes produced by some toxic metals encountered industrially or environmentally. Z. Ernahr. 1978, 17, 84–88. [Google Scholar] [CrossRef]
- Cavalleri, A.; Belotti, L.; Gobba, F.; Luzzana, G.; Rosa, P.; Seghizzi, P. Colour vision loss in workers exposed to elemental mercury vapour. Toxicol. Lett. 1995, 77, 351–356. [Google Scholar] [CrossRef]
- Cavalleri, A.; Gobba, F. Reversible color vision loss in occupational exposure to metallic mercury. Environ Res. 1998, 77, 173–177. [Google Scholar] [CrossRef]
- Feitosa-Santana, C.; Costa, M.F.; Lago, M.; Ventura, D.F. Long-term loss of color vision after exposure to mercury vapor. Braz. J. Med. Biol. Res. 2007, 40, 409–414. [Google Scholar] [CrossRef]
- Collins, C.; Saldana, M. Mercury exposure and its implications for visual health. Can. J. Ophthalmol. 2007, 42, 660–662. [Google Scholar] [CrossRef]
- Flieger, J.; Dolar-Szczasny, J.; Rejdak, R.; Majerek, D.; Tatarczak-Michalewska, M.; Proch, J.; Blicharska, E.; Flieger, W.; Baj, J.; Niedzielski, P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 9413. [Google Scholar] [CrossRef]
- Liu, E.M.; Rajagopal, R.; Grand, M.G. Optic Nerve Atrophy and Hair Loss in a Young Man. JAMA Ophthalmol. 2015, 133, 1469–1470. [Google Scholar] [CrossRef] [PubMed]
- Thery, J.-C.; Jardin, F.; Massy, N.; Massy, J.; Stamatoullas, A.; Tilly, H. Optical neuropathy possibly related to arsenic during acute promyelocytic leukemia treatment. Leuk. Lymphoma 2008, 49, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.; Al-Shafai, L.; Mankovskii, G.; Howarth, D.; Margolin, E. Clinicopathological Correlates: Chronic Arsenic Toxicity Causing Bilateral Symmetric Progressive Optic Neuropathy. J. Neuroophthalmol. 2020, 40, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Csaky, K.G.; Caruso, R.C. Gallium nitrate optic neuropathy. Am. J. Ophthalmol. 1997, 124, 567–568. [Google Scholar] [CrossRef]
- Li, J.; Tripathi, R.C.; Tripathi, B.J. Drug-induced ocular disorders. Drug Saf. 2008, 31, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.J.; Gilbar, P.J.; Grewal, G.D.; Holcombe, D.J. Optic neuritis induced by 5-fluorouracil chemotherapy: Case report and review of the literature. J. Oncol. Pharm. Pract. 2020, 26, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Yau, M.; Mezey, N.; Joarder, I.; Micieli, J.A. Neuro-ophthalmic Complications of Immune Checkpoint Inhibitors: A Systematic Review. Eye Brain 2020, 12, 139–167. [Google Scholar] [CrossRef]
- Sun, M.M.; Seleme, N.; Chen, J.J.; Zekeridou, A.; Sechi, E.; Walsh, R.D.; Beebe, J.D.; Sabbagh, O.; Mejico, L.J.; Gratton, S.; et al. Neuro-Ophthalmic Complications in Patients Treated With CTLA-4 and PD-1/PD-L1 Checkpoint Blockade. J. Neuroophthalmol. 2020, 41, 519–530. [Google Scholar] [CrossRef]
- Chamberlain, P.D.; Sadaka, A.; Berry, S.; Lee, A.G. Ethambutol optic neuropathy. Curr. Opin. Ophthalmol. 2017, 28, 545–551. [Google Scholar] [CrossRef]
- Song, W.; Si, S. The rare ethambutol-induced optic neuropathy: A case-report and literature review. Medicine (Baltimore) 2017, 96, e5889. [Google Scholar] [CrossRef]
- Simmons, I.G.; Good, P.A. Carbon monoxide poisoning causes optic neuropathy. Eye (Lond) 1998, 12 Pt 5, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Gobba, F.; Cavalleri, A. Color vision impairment in workers exposed to neurotoxic chemicals. Neurotoxicology 2003, 24, 693–702. [Google Scholar] [CrossRef]
- FC Lopes, A. Mitochondrial metabolism and DNA methylation: A review of the interaction between two genomes. Clin. Epigenetics 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.R.; Braun, J.M.; Papandonatos, G.; Greenberg, P.B. Occupational styrene exposure and acquired dyschromatopsia: A systematic review and meta-analysis. Am. J. Ind. Med. 2017, 60, 930–946. [Google Scholar] [CrossRef]
- Pilz, Y.L.; Bass, S.J.; Sherman, J. A Review of Mitochondrial Optic Neuropathies: From Inherited to Acquired Forms. J. Optom. 2017, 10, 205–214. [Google Scholar] [CrossRef]
- Koul, P.A. Ocular toxicity with ethambutol therapy: Timely recaution. Lung India Off. Organ Indian Chest Soc. 2015, 32, 1–3. [Google Scholar] [CrossRef]
- Purvin, V.; Kawasaki, A.; Borruat, F.-X. Optic neuropathy in patients using amiodarone. Arch Ophthalmol. 2006, 124, 696–701. [Google Scholar] [CrossRef]
- Behbehani, R. Clinical approach to optic neuropathies. Clin. Ophthalmol. 2007, 1, 233–246. [Google Scholar]
- Roda, M.; Di Geronimo, N.; Pellegrini, M.; Schiavi, C. Nutritional Optic Neuropathies: State of the Art and Emerging Evidences. Nutrients 2020, 12, 2653. [Google Scholar] [CrossRef]
- Devalia, V.; Hamilton, M.S.; Molloy, A. British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, Y.; Lavin, P.J. Nutritional Optic Neuropathy Caused by Copper Deficiency After Bariatric Surgery. J. Neuroophthalmol. 2016, 36, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Naismith, R.T.; Shepherd, J.B.; Weihl, C.C.; Tutlam, N.T.; Cross, A. Acute and bilateral blindness due to optic neuropathy associated with copper deficiency. Arch. Neurol. 2009, 66, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007, 6, 442–455. [Google Scholar] [CrossRef]
- Zamani, N.; Hassanian-Moghaddam, H.; Shojaei, M.; Rahimian, S. Evaluation of the effect of erythropoietin + corticosteroid versus corticosteroid alone in methanol-induced optic nerve neuropathy. Cutan. Ocul. Toxicol. 2018, 37, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Alemzadeh-Ansari, M.; Karimian, F.; Esfandiari, H. Use of erythropoietin in ophthalmology: A review. Surv. Ophthalmol. 2021, 67, 427–439. [Google Scholar] [CrossRef]
- Acar, U.; Kucuk, B.; Sevinc, M.K.; Aykas, S.; Erdurmus, M.; Sobaci, G. Intravitreal erythropoietin injection in late-stage optic neuropathy: A safety study on human. Int. Ophthalmol. 2018, 38, 1021–1025. [Google Scholar] [CrossRef]
- Sadun, A.A.; Wang, M.Y. Ethambutol optic neuropathy: How we can prevent 100,000 new cases of blindness each year. J. Neuroophthalmol. 2008, 28, 265–268. [Google Scholar] [CrossRef]
- Fraunfelder, F.W.; Sadun, A.A.; Wood, T. Update on ethambutol optic neuropathy. Expert Opin. Drug Saf. 2006, 5, 615–618. [Google Scholar] [CrossRef]
- Kanaujia, V.; Jain, V.K.; Sharma, K.; Agarwal, R.; Mishra, P.; Sharma, R.K. Ethambutol-induced optic neuropathy in renal disorder: A clinico-electrophysiological study. Can. J. Ophthalmol. 2019, 54, 301–305. [Google Scholar] [CrossRef]
- Bouffard, M.A.; Nathavitharana, R.R.; Yassa, D.; Torun, N. Re-Treatment With Ethambutol After Toxic Optic Neuropathy. J. Neuroophthalmol. 2017, 37, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, S.-J.; Choung, H.K.; Kim, J.H.; Yu, Y.S. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. J. Neuroophthalmol. 2008, 28, 269–277. [Google Scholar] [CrossRef]
- Chan, R.Y.; Kwok, A.K. Ocular toxicity of ethambutol. Hong Kong Med. J. 2006, 12, 56–60. [Google Scholar] [PubMed]
- Addy, L.K.; Harrison, W.W. Case Report: Long-term Structural and Functional Effects of Ethambutol Optic Neuropathy. Optom. Vis. Sci. 2020, 97, 555–560. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baj, J.; Forma, A.; Kobak, J.; Tyczyńska, M.; Dudek, I.; Maani, A.; Teresiński, G.; Buszewicz, G.; Januszewski, J.; Flieger, J. Toxic and Nutritional Optic Neuropathies—An Updated Mini-Review. Int. J. Environ. Res. Public Health 2022, 19, 3092. https://doi.org/10.3390/ijerph19053092
Baj J, Forma A, Kobak J, Tyczyńska M, Dudek I, Maani A, Teresiński G, Buszewicz G, Januszewski J, Flieger J. Toxic and Nutritional Optic Neuropathies—An Updated Mini-Review. International Journal of Environmental Research and Public Health. 2022; 19(5):3092. https://doi.org/10.3390/ijerph19053092
Chicago/Turabian StyleBaj, Jacek, Alicja Forma, Joanna Kobak, Magdalena Tyczyńska, Iga Dudek, Amr Maani, Grzegorz Teresiński, Grzegorz Buszewicz, Jacek Januszewski, and Jolanta Flieger. 2022. "Toxic and Nutritional Optic Neuropathies—An Updated Mini-Review" International Journal of Environmental Research and Public Health 19, no. 5: 3092. https://doi.org/10.3390/ijerph19053092
APA StyleBaj, J., Forma, A., Kobak, J., Tyczyńska, M., Dudek, I., Maani, A., Teresiński, G., Buszewicz, G., Januszewski, J., & Flieger, J. (2022). Toxic and Nutritional Optic Neuropathies—An Updated Mini-Review. International Journal of Environmental Research and Public Health, 19(5), 3092. https://doi.org/10.3390/ijerph19053092







