Assessing Walking Programs in Fibromyalgia: A Concordance Study between Measures
Abstract
:1. Introduction
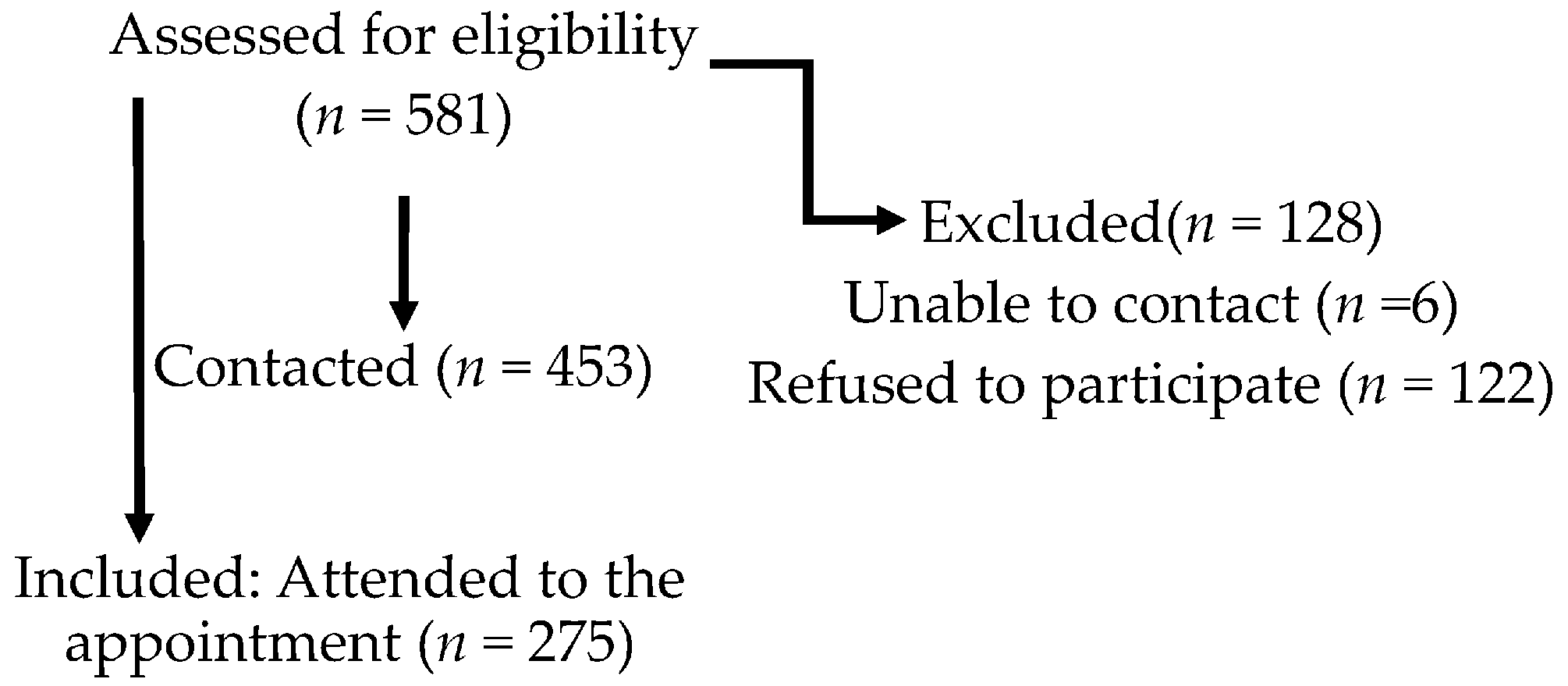
2. Methods
2.1. Population
2.2. Measures
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Aggarwal, A.; Lawrence, A. Performance of the American College of Rheumatology 2016 criteria for fibromyalgia in a referral care setting. Rheumatol. Int. 2019, 39, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Mathys, M.; Turk, D.C. Evidenced-based guidelines on the treatment of fibromyalgia patients: Are they consistent and if not, why not? Have effective psychological treatments been overlooked? J. Pain 2017, 18, 747–756. [Google Scholar] [CrossRef]
- O’Dwyer, T.; Maguire, S.; Mockler, D.; Durcan, L.; Wilson, F. Behaviour change interventions targeting physical activity in adults with fibromyalgia: A systematic review. Rheumatol. Int. 2019, 39, 805–817. [Google Scholar] [CrossRef]
- O’Connor, S.R.; Tully, M.A.; Ryan, B.; Bleakley, C.; Baxter, D.; Bradley, J.; McDonough, S.M. Walking exercise for chronic musculoskeletal pain: Systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 724–734.e3. [Google Scholar] [CrossRef] [Green Version]
- Loftus, N.; Dobbin, N.; Crampton, J.S. The effects of a group exercise and education programme on symptoms and physical fitness in patients with fibromyalgia: A prospective observational cohort study. Disabil. Rehabil. 2021, 1–8. [Google Scholar] [CrossRef]
- Bidonde, J.; Boden, C.; Foulds, H.; Kim, S.Y. Physical activity and exercise training for adults with fibromyalgia. In Fibromyalgia Syndrome; Ablin, J.N., Shoenfeld, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 59–72. [Google Scholar]
- Andrade, A.; Dominski, F.H.; Sieczkowska, S.M. What we already know about the effects of exercise in patients with fibromyalgia: An umbrella review. Semin. Arthritis Rheum. 2020, 50, 1465–1480. [Google Scholar] [CrossRef]
- WHO. Global Strategy on Diet, Physical Activity and Health 2017. Available online: http://www.who.int/dietphysicalactivity/pa/en/ (accessed on 30 March 2017).
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, CD010842. [Google Scholar] [CrossRef]
- Sanz-Baños, Y.; Pastor-Mira, M.A.; Lledó, A.; López-Roig, S.; Peñacoba, C.; Sánchez-Meca, J. Do women with fibromyalgia adhere to walking for exercise programs to improve their health? Systematic review and meta-analysis. Disabil. Rehabil. 2018, 40, 2475–2487. [Google Scholar] [CrossRef]
- Peñacoba, C.; Pastor, M.A.; López-Roig, S.; Velasco, L.; Lledo, A. Walking beliefs in women with fibromyalgia: Clinical profile and impact on walking behavior. Clin. Nurs. Res. 2016, 26, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Polaski, A.M.; Phelps, A.L.; Kostek, M.C.; Szucs, K.A.; Kolber, B.J. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS ONE 2019, 14, e0210418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaramonte, R.; Bonfiglio, M.; Chisari, S. Multidisciplinary protocol for the management of fibromyalgia associated with imbalance. Our experience and literature review. Rev. Assoc. Med. Bras. 2019, 65, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Lami, M.J.; Martínez, M.P.; Miró, E.; Sánchez, A.I.; Guzmán, M. Catastrophizing, acceptance, and coping as mediators between pain and emotional distress and disability in fibromyalgia. J. Clin. Psychol. Med. Settings 2018, 25, 80–92. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.A.; Danyliw, A.; Sawant, A.; Bello-Haas, V.D.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD010884. [Google Scholar] [CrossRef]
- Borges-Cosic, M.; Aparicio, V.A.; Estévez-López, F.; Soriano-Maldonado, A.; Acosta-Manzano, P.; Gavilán-Carrera, B.; Delgado-Fernández, M.; Geenen, R.; Segura-Jiménez, V. Sedentary time, physical activity, and sleep quality in fibromyalgia: The al-Ándalus project. Scand. J. Med. Sci. Sports 2019, 29, 266–274. [Google Scholar] [CrossRef]
- Sitthipornvorakul, E.; Klinsophon, T.; Sihawong, R.; Janwantanakul, P. The effects of walking intervention in patients with chronic low back pain: A meta-analysis of randomized controlled trials. Musculoskelet. Sci. Pract. 2018, 34, 38–46. [Google Scholar] [CrossRef]
- Cheng, S.-T.; Leung, C.; Chan, K.L.; Chen, P.P.; Chow, Y.F.; Chung, J.; Law, A.C.B.; Lee, J.S.W.; Leung, E.M.F.; Tam, C.W.C. The relationship of self-efficacy to catastrophizing and depressive symptoms in community-dwelling older adults with chronic pain: A moderated mediation model. PLoS ONE 2018, 13, e0203964. [Google Scholar] [CrossRef]
- Radunović, G.; Veličković, Z.; Rašić, M.; Janjić, S.; Marković, V.; Radovanović, S. Assessment of gait in patients with fibromyalgia during motor and cognitive dual task walking: A cross-sectional study. Adv. Rheumatol. 2021, 61, 53. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Tanai, G.; Buskila, D.; Amital, D.; Amital, H. Fibromyalgia and its consequent disability. Israel Med. Assoc. J. 2020, 7, 380–384. [Google Scholar]
- Cerón-Lorente, L.; Valenza, M.C.; Pérez-Mármol, J.M.; García-Ríos, M.D.C.; Castro-Sánchez, A.M.; Aguilar-Ferrándiz, M.E. The influence of balance, physical disability, strength, mechanosensitivity and spinal mobility on physical activity at home, work and leisure time in women with fibromyalgia. Clin. Biomech. 2018, 60, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hallam, K.T.; Peeters, A.; Gupta, A.; Bilsborough, S. Moving minds: Mental health and wellbeing benefits of a 50-day workplace physical activity program. Curr. Psychol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Nielson, W.R.; Jensen, M.P.; Heapy, A.; Czlapinski, R.; Kerns, R.D. Specific and general therapeutic mechanisms in cognitive behavioral treatment of chronic pain. J. Consult. Clin. Psychol. 2015, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nigg, C.R.; Fuchs, R.; Gerber, M.; Jekauc, D.; Koch, T.; Krell-Roesch, J.; Lippke, S.; Mnich, C.N. Assessing physical activity through questionnaires–A consensus of best practices and future directions. Psychol. Sport Exerc. 2020, 50, 101715. [Google Scholar] [CrossRef]
- Giles-Corti, B. Socioeconomic status differences in recreational physical activity levels and real and perceived access to a supportive physical environment. Prev. Med. 2002, 35, 601–611. [Google Scholar] [CrossRef]
- Reed, J.A.; Ainsworth, B.E.; Wilson, D.K.; Mixon, G.; Cook, A. Awareness and use of community walking trails. Prev. Med. 2004, 39, 903–908. [Google Scholar] [CrossRef]
- Heapy, A.A.; Tankha, H.; Higgins, D.M.; Driscoll, M.; LaChappelle, K.M.; Goulet, J.L.; Buta, E.; Piette, J.D.; Kerns, R.D.; Krein, S.L. Incorporating walking into cognitive behavioral therapy for chronic pain: Safety and effectiveness of a personalized walking intervention. J. Behav. Med. 2021, 44, 260–269. [Google Scholar] [CrossRef]
- Casey, M.; Cotter, N.; Kelly, C.; Mc Elchar, L.; Dunne, C.; Neary, R.; Lowry, D.; Hearty, C.; Doody, C. Exercise and acceptance and commitment therapy for chronic pain: A case series with one-year follow-up. Musculoskelet. Care 2019, 18, 64–73. [Google Scholar] [CrossRef]
- Hirase, T.; Kataoka, H.; Inokuchi, S.; Nakano, J.; Sakamoto, J.; Okita, M. Effects of exercise training combined with increased physical activity to prevent chronic pain in community-dwelling older adults: A preliminary randomized controlled trial. Pain Res. Manag. 2018, 2018, 2132039. [Google Scholar] [CrossRef]
- Salvat, I.; Zaldivar, P.; Monterde, S.; Montull, S.; Miralles, I.; Castel, A. Functional status, physical activity level, and exercise regularity in patients with fibromyalgia after Multidisciplinary treatment: Retrospective analysis of a randomized controlled trial. Rheumatol. Int. 2016, 37, 377–387. [Google Scholar] [CrossRef]
- Bravata, D.M.; Smith-Spangler, C.; Sundaram, V.; Gienger, A.L.; Lin, N.; Lewis, R.; Stave, C.D.; Olkin, I.; Sirard, J.R. Using pedometers to increase physical activity and improve health. JAMA 2007, 298, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Services, U.S.H. 2008 Physical Activity Guidelines for Americans. Createspace Independent Pub. 2014. Available online: https://books.google.es/books?id=IkbSoAEACAAJ (accessed on 24 August 2021).
- Richardson, C.R.; Newton, T.L.; Abraham, J.J.; Sen, A.; Jimbo, M.; Swartz, A.M. A Meta-analysis of pedometer-based walking interventions and weight loss. Ann. Fam. Med. 2008, 6, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Marshall, S.J.; Barreira, T.; Lee, J.-O. Effect of pedometer-based physical activity interventions. Res. Q. Exerc. Sport 2009, 80, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lawford, B.J.; Walters, J.; Ferrar, K. Does walking improve disability status, function, or quality of life in adults with chronic low back pain? A systematic review. Clin. Rehabil. 2015, 30, 523–536. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Franco, M.R.; Maher, C.G.; Lin, C.-W.C.; Morelhão, P.K.; Araújo, A.C.; Filho, R.F.N.; Pinto, R.Z. Physical activity interventions for increasing objectively measured physical activity levels in patients with chronic musculoskeletal pain: A systematic review. Arthritis Care Res. 2016, 68, 1832–1842. [Google Scholar] [CrossRef]
- Lang, A.E.; Hendrick, P.A.; Clay, L.; Mondal, P.; Trask, C.M.; Bath, B.; Penz, E.D.; Stewart, S.A.; Baxter, G.D.; Hurley, D.A.; et al. A randomized controlled trial investigating effects of an individualized pedometer driven walking program on chronic low back pain. BMC Musculoskelet. Disord. 2021, 22, 206. [Google Scholar] [CrossRef]
- Tudor-Locke, C. Taking steps toward increased physical activity: Using pedometers to measure and motivate. Pres. Counc. Phys. Fit. Sport Res. Dig. 2002, 3, 1–8. [Google Scholar]
- Lazaridou, A.; Koulouris, A.; Devine, J.K.; Haack, M.; Jamison, R.N.; Edwards, R.R.; Schreiber, K.L. Impact of daily yoga-based exercise on pain, catastrophizing, and sleep amongst individuals with fibromyalgia. J. Pain Res. 2019, 12, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. Behaviour Change: General Approaches. Public Health Guideline [PH6]. 2007. Available online: https://www.nice.org.uk/guidance/ph6 (accessed on 24 August 2021).
- De Cocker, K.; De Bourdeaudhuij, I.; Brown, W.; Cardon, G. The pedometer-based community intervention “10,000 Steps Ghent”: Who used a pedometer and who increased their steps? J. Sci. Med. Sport 2010, 12, e159. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Perez-Bravo, F.; Ibañez, L.; Salas, C.; Bailey, M.E.S.; Gill, J.M.R. Objective vs. Self-reported physical activity and sedentary time: Effects of measurement method on relationships with risk biomarkers. PLoS ONE 2012, 7, e36345. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Álvarez-Gallardo, I.C.; Romero-Zurita, A.; Camiletti-Moirón, D.; Munguía-Izquierdo, D.; Carbonell-Baeza, A.; Ruiz, J.R. Comparison of physical activity using questionnaires (Leisure time physical activity instrument and physical activity at home and work instrument) and accelerometry in fibromyalgia patients: The Al-Ándalus project. Arch. Phys. Med. Rehabil. 2014, 95, 1903–1911.e2. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Okifuji, A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J. Pain 2006, 7, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Healey, E.; Jinks, C.; Foster, N.; Chew-Graham, C.; Pincus, T.; Hartshorne, L.; Cooke, K.; Nicholls, E.; Proctor, J.; Lewis, M.; et al. The feasibility and acceptability of a physical activity intervention for older people with chronic musculoskeletal pain: The iPOPP pilot trial protocol. Musculoskelet. Care 2017, 16, 118–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor-Locke, C. A preliminary study to determine instrument responsiveness to change with a walking program: Physical activity logs versus pedometers. Res. Q. Exerc. Sport 2001, 72, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Allor, K.M.; Pivarnik, J.M. Stability and convergent validity of three physical activity assessments. Med. Sci. Sports Exerc. 2001, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Smit, J.H.; Pluijm, S.; Visser, M.; Deeg, D.J.; Lips, P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J. Clin. Epidemiol. 2004, 57, 252–258. [Google Scholar] [CrossRef]
- Bauman, A.; Ainsworth, B.E.; Sallis, J.F.; Hagströmer, M.; Craig, C.L.; Bull, F.C.; Pratt, M.; Venugopal, K.; Chau, J.; Sjöström, M.; et al. The descriptive epidemiology of sitting. Am. J. Prev. Med. 2011, 41, 228–235. [Google Scholar] [CrossRef]
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P. The IMAGE study group. systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011, 11, 11–12. [Google Scholar] [CrossRef]
- Zautra, A.J.; Davis, M.C.; Reich, J.W.; Nicassario, P.; Tennen, H.; Finan, P.; Kratz, A.; Parrish, B.; Irwin, M.R. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J. Consult. Clin. Psychol. 2008, 76, 408–421. [Google Scholar] [CrossRef]
- White, K.P.; Speechley, M.; Harth, M.; Ostbye, T. The London Fibromyalgia Epidemiology Study: Comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J. Rheumatol. 1999, 26, 1577–1585. [Google Scholar]
- Pastor, M.-Á.; López-Roig, S.; Lledó, A.; Peñacoba, C.; Velasco, L.; Schweiger-Gallo, I.; Cigarán, M.; Écija, C.; Limón, R.; Sanz, Y. Combining motivational and volitional strategies to promote unsupervised walking in patients with fibromyalgia: Study protocol for a randomized controlled trial. Trials 2014, 15, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, M.-Á.; López-Roig, S.; Sanz, Y.; Peñacoba, C.; Cigaran, M.; Lledó, A.; Velasco, L.; Ecija, C. Andar como forma de ejercicio físico en la Fibromialgia: Un estudio de identificación de creencias desde la Teoría de la Acción Planeada. An. Psicol. An. Psychol. 2015, 31, 433–446. [Google Scholar] [CrossRef] [Green Version]
- International Physical Activity Questionnaire (IPAQ). 2013. Available online: https://sites.google.com/site/theipaq/questionnaire_links (accessed on 14 December 2021).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogun, S.; Scott, D.; Cicuttini, F.; Jones, G.; Aitken, D. Longitudinal study of the relationship between physical activity and knee pain and functional limitation in community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 90, 104101. [Google Scholar] [CrossRef]
- Hirase, T.; Kataoka, H.; Nakano, J.; Inokuchi, S.; Sakamoto, J.; Okita, M. Effects of a psychosocial intervention programme combined with exercise in community-dwelling older adults with chronic pain: A randomized controlled trial. Eur. J. Pain 2017, 22, 592–600. [Google Scholar] [CrossRef] [PubMed]
- López-Roig, S.; Pastor, M.-Á.; Peñacoba, C.; Lledó, A.; Sanz, Y.; Velasco, L. Prevalence and predictors of unsupervised walking and physical activity in a community population of women with fibromyalgia. Rheumatol. Int. 2016, 36, 1127–1133. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2009, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Epskamp, S.; Rhemtulla, M.; Borsboom, D. Generalized network psychometrics: Combining network and latent variable models. Psychometrika 2017, 82, 904–927. [Google Scholar] [CrossRef] [Green Version]
- Epskamp, S.; Fried, E.I. A tutorial on regularized partial correlation networks. Psychol. Methods 2018, 23, 617–634. [Google Scholar] [CrossRef] [Green Version]
- Costantini, G.; Epskamp, S.; Borsboom, D.; Perugini, M.; Mõttus, R.; Waldorp, L.J.; Cramer, A. State of the aRt personality research: A tutorial on network analysis of personality data in R. J. Res. Pers. 2015, 54, 13–29. [Google Scholar] [CrossRef]
- Fried, E.I.; Van Borkulo, C.D.; Cramer, A.; Boschloo, L.; Schoevers, R.A.; Borsboom, D. Mental disorders as networks of problems: A review of recent insights. Soc. Psychiatry 2017, 52, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasp, T. Jasp (Version 0.8.6.0) [Computer software]; JASP Team: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Durante, R.; Ainsworth, B.E. The recall of physical activity: Using a cognitive model of the question-answering process. Med. Sci. Sports Exerc. 1996, 28, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Camiletti-Moirón, D.; Segura-Jiménez, V.; Álvarez-Gallardo, I.C.; Estévez-López, F.; Aparicio, V.A.; Carbonell-Baeza, A.; Ruiz, J.R.; Delgado-Fernández, M. Inter-accelerometer comparison to measure physical activity and sedentary time in female fibromyalgia patients: The al-Ándalus project. Clin. Exp. Rheumatol. 2015, 33, S46–S52. [Google Scholar] [PubMed]
- Lee, J.-W.; Lee, K.-E.; Park, D.-J.; Kim, S.-K.; Nah, S.-S.; Lee, J.H.; Lee, Y.-A.; Hong, S.-J.; Kim, H.-S.; Lee, H.-S.; et al. Determinants of quality of life in patients with fibromyalgia: A structural equation modeling approach. PLoS ONE 2017, 12, e0171186. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Amat, A.; Hita-Contreras, F.; Latorre-Román, P.A.; Gutierrez-López, M.D.L.C.; García-Pinillos, F.; Martínez-López, E.J. Association of the weekly practice of guided physical activity with the reduction of falls and symptoms of fibromyalgia in adult women. J. Strength Cond. Res. 2014, 28, 3146–3154. [Google Scholar] [CrossRef]
- Hassett, A.L.; Finan, P.H. The role of resilience in the clinical management of chronic pain. Curr. Pain Headache Rep. 2016, 20, 39. [Google Scholar] [CrossRef]
- Muntaner-Mas, A.; Martinez-Nicolas, A.; Lavie, C.J.; Blair, S.N.; Ross, R.; Arena, R.; Ortega, F.B. A systematic review of fitness apps and their potential clinical and sports utility for objective and remote assessment of cardiorespiratory fitness. Sports Med. 2019, 49, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Catala, P.; Lopez-Roig, S.; Ecija, C.; Suso-Ribera, C.; Puente, C.P. Why do some people with severe chronic pain adhere to walking prescriptions whilst others won’t? A cross-sectional study exploring clinical and psychosocial predictors in women with fibromyalgia. Rheumatol. Int. 2021, 41, 1479–1484. [Google Scholar] [CrossRef]
- Pulido-Martos, M.; Luque-Reca, O.; Segura-Jiménez, V.; Álvarez-Gallardo, I.C.; Soriano-Maldonado, A.; Acosta-Manzano, P.; Gavilán-Carrera, B.; McVeigh, J.G.; Geenen, R.; Delgado-Fernández, M.; et al. Physical and psychological paths toward less severe fibromyalgia: A structural equation model. Ann. Phys. Rehabil. Med. 2020, 63, 46–52. [Google Scholar] [CrossRef]
- Sosa-Reina, M.D.; Nunez-Nagy, S.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Monserrat, J.; Álvarez-Mon, M. Effectiveness of therapeutic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomized clinical trials. BioMed. Res. Int. 2017, 2017, 2356346. [Google Scholar] [CrossRef]
- Munguía-Izquierdo, D.; Pulido-Martos, M.; Acosta, F.M.; Acosta-Manzano, P.; Gavilán-Carrera, B.; Rodriguez-Ayllon, M.; Geenen, R.; Delgado-Fernández, M.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; et al. Objective and subjective measures of physical functioning in women with fibromyalgia: What type of measure is associated most clearly with subjective well-being? Disabil. Rehabil. 2021, 43, 1649–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcloughlin, M.J.; Colbert, L.H.; Stegner, A.J.; Cook, D.B. Are women with fibromyalgia less physically active than healthy women? Med. Sci. Sports Exerc. 2011, 43, 905–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutes, L.D.; Steinbaugh, E.K. Theoretical models for pedometer use in physical activity interventions. Phys. Ther. Rev. 2010, 15, 143–153. [Google Scholar] [CrossRef]
- Pastor-Mira, M.-A.; López-Roig, S.; Martínez-Zaragoza, F.; León, E.; Abad, E.; Lledó, A.; Peñacoba, C. Goal preferences, affect, activity patterns and health outcomes in women with fibromyalgia. Front. Psychol. 2019, 10, 10. [Google Scholar] [CrossRef]
- de la Coba, P.; Bruehl, S.; Galvez-Sánchez, C.M.; del Paso, G.A.R. Slowly repeated evoked pain as a marker of central sensitization in fibromyalgia: Diagnostic accuracy and reliability in comparison with temporal summation of pain. Psychosom. Med. 2018, 80, 573–580. [Google Scholar] [CrossRef]
- Farhad, K.; Oaklander, A.L. Fibromyalgia and small-fiber polyneuropathy: What’s in a name? Muscle Nerve 2018, 58, 611–613. [Google Scholar] [CrossRef]
- Newcomb, L.W.; Koltyn, K.F.; Morgan, W.P.; Cook, D.B. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med. Sci. Sports Exerc. 2011, 43, 1106–1113. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Mira, M.A.; López-Roig, S.; Martínez-Zaragoza, F.; Lledó, A.; Velasco, L.; León, E.; Gallardo, C.É.; Peñacoba, C. Promoting unsupervised walking in women with fibromyalgia: A randomized controlled trial. Psychol. Health Med. 2021, 26, 487–498. [Google Scholar] [CrossRef]
- McCracken, L.M.; Vowles, K.E.; Zhao-O’Brien, J. Further development of an instrument to assess psychological flexibility in people with chronic pain. J. Behav. Med. 2010, 33, 346–354. [Google Scholar] [CrossRef]
- Écija, C.; Luque-Reca, O.; Suso-Ribera, C.; Catala, P.; Peñacoba, C. Associations of cognitive fusion and pain catastrophizing with fibromyalgia impact through fatigue, pain severity, and depression: An exploratory study using structural equation modeling. J. Clin. Med. 2020, 9, 1763. [Google Scholar] [CrossRef]
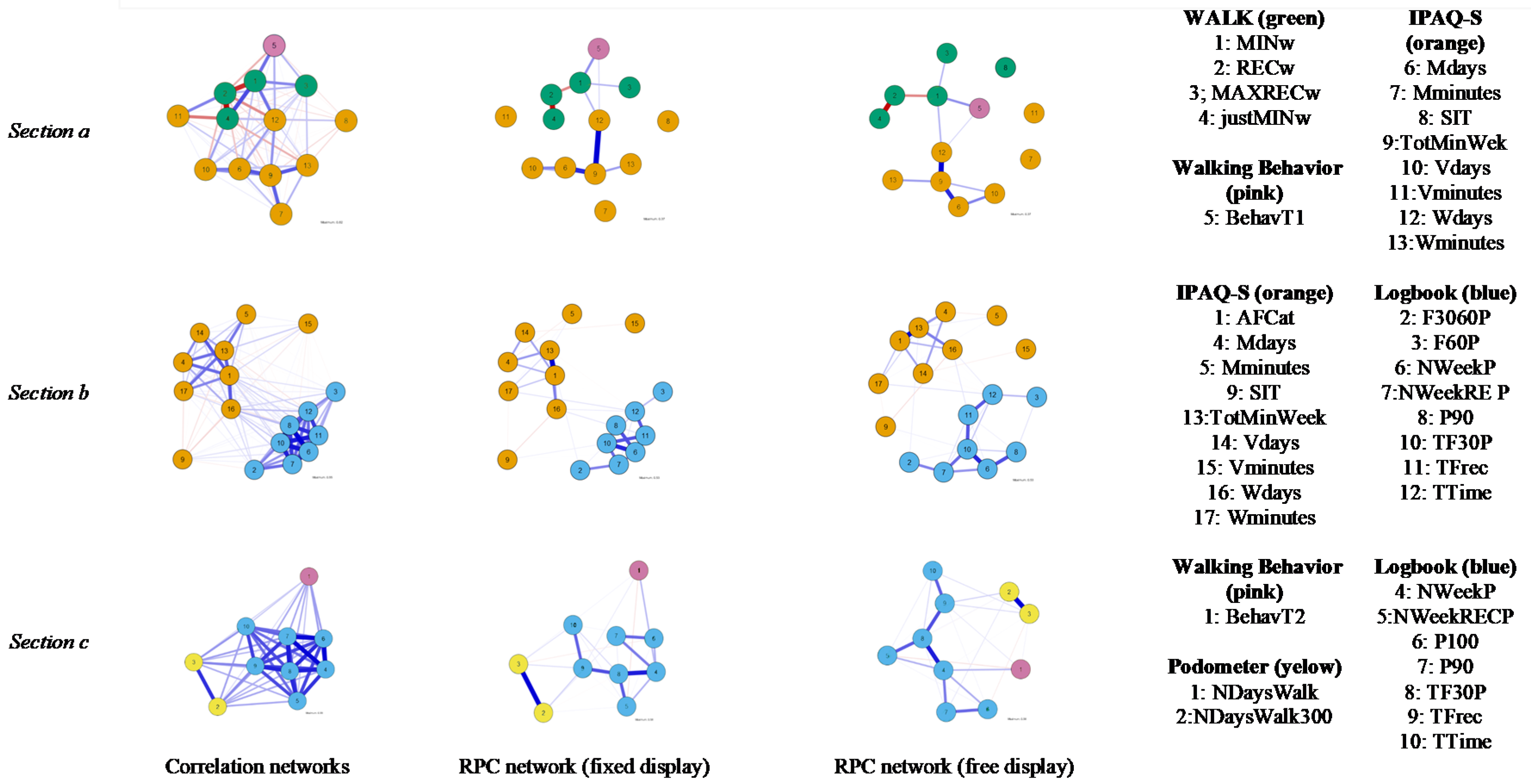
| Time 1 (T1) |
| Walking Behavior BehavT1: Mean score of self-reported adherence to minimum walking program |
| WALK questionnaire (WALK) MINw: Complete walking minimum program: 0 ‘NO’, 1 ‘YES’ justMINw: 0 ‘Complete more than minimum program’, 1 ‘Just the minimum program’ RECw: Complete walking recommended program: 0 ‘NO’, 1 ‘YES’ MAXRECw: Complete maximum recommended program: 0 ‘NO’, 1 ‘YES’ |
| IPAQ-S Questionnaire (IPAQ-S) Wdays: ‘Walk (days/week)’ Wminutes: ‘Walk (minutes/day)’ Mdays: ‘Moderate-intensity activity (days/week)’ Mminutes: ‘Moderate-intensity activity (minutes/day)’ Vdays: ’Vigorous-intensity activities (days/week)’ Vminutes: ‘Vigorous-intensity activities (minutes/day)’ TotMinWeek: ‘Total activity (minutes/week)’ SIT: ‘Sit (minutes/week)’ AFCat: Categorical variable; low, moderate and high level of physical activity |
| Time 2 (T2) |
| Walking Behavior BehavT2: Mean score of self-reported adherence to minimum walking program |
| T1–T2 |
| Logbook TFrec: Total times walking TTime: Total minutes walked F3060P: Number of times/week between 30–60 min F60P: Number of times/week > 60 min TF30P: Total times walking minimum program (30 min) NWeekP: Number of weeks minimum program completed NWeekRECP: Number of weeks of walking recommended program P90: Completed 90% of walking minimum program (4 weeks) P100: Completed 100% of walking minimum program (6 weeks) |
| Data of Pedometers: Steps NDaysWalk: Average steps per walking day for exercising NDaysWalk3000: Number of days that patient walked 3000 steps or more |
| Age | Medical Status |
| Mean age: 51.85 (95% CI [50.75, 52.93]) | 78% had the medical recommendation |
| SD = 9.16, Mdn = 52.69 | to walk (n = 212) |
| Employment status | Education |
| 31% working away from home (n = 85) | 12% university education (n = 33) |
| 26% housewives (n = 71) | 12.8% literate (n = 35) |
| 21.6% unemployed (n = 59) | 47% primary education (n = 129) |
| 9.9% retired due to pain (n = 27) | 28% secondary education (n = 77) |
| 6.6% on sick leave (n = 18) | |
| 4.8% retired (n = 13) |
| Betweenness | Centrality Closeness | Degree | ||
|---|---|---|---|---|
| (a) | Walking Behavior | |||
| BehavT1 | −0.667 | 0.000 | −0.377 | |
| WALK questionnaire | ||||
| MINw | 2.00 * | 0.000 | 0.768 | |
| RECw | 0.189 | 0.000 | 0.768 | |
| MAXRECw | −0.667 | 0.000 | −0.77 | |
| justMINw | −0.667 | 0.000 | 0.162 | |
| IPAQ−S | ||||
| Mdays | 0.189 | 0.000 | 0.621 | |
| Mminutes | −0.667 | 0.000 | −1.13 * | |
| SIT | −0.667 | 0.000 | −1.13 * | |
| TotMinWeek | 1.47 * | 0.000 | 2.199 * | |
| Vdays | −0.667 | 0.000 | −0.107 | |
| Vminutes | −0.667 | 0.000 | −1.13 * | |
| Wdays | 1.47 * | 0.000 | 0.766 | |
| Wminutes | −0.667 | 0.000 | −0.637 | |
| (b) | Logbook | |||
| NWeekP | −0.137 | 0.367 | 1.08 * | |
| NWeekRECP | −0.396 | 0.191 | 0.660 | |
| F3060P | −0.862 | −0.061 | −0.696 | |
| F60P | −0.862 | −0.613 | −1.00 * | |
| TF30P | 1.41 * | 0.765 | 1.82 * | |
| TFrec | 1.88 * | 1.00 * | 0.640 | |
| TTime | 1.62 * | 0.970 | 1.31 * | |
| IPAQ−S | ||||
| AFCat | 0.640 | 0.743 | 1.23 * | |
| Mdays | −0.758 | 0.211 | −0.075 | |
| Mminutes | −0.862 | −2.04 * | −1.26 * | |
| P90 | −0.862 | −0.129 | −0.161 | |
| SIT | −0.862 | −1.00 * | −1.17 * | |
| TotMinWeek | 0.174 | 0.562 | 0.356 | |
| Vdays | −0.085 | −0.053 | −0.467 | |
| Vminutes | −0.862 | −2.35 | −1.40 * | |
| Wdays | 1.364 * | 1.05 * | −0.098 | |
| Wminutes | −0.551 | 0.392 | −0.763 | |
| (c) | Walking Behavior | |||
| BehavT2 | −0.702 | −0.890 | −1.54 * | |
| Pedometer | ||||
| NDaysWalk | 0.000 | −1.09 * | −0.177 | |
| NDaysWalk3000 | −0.702 | −1.34 * | −0.062 | |
| Logbook | ||||
| NWeekP | 1.30 * | 1.18 * | 1.50 * | |
| NWeekRECP | −0.702 | 0.158 | −0.321 | |
| P100 | −0.702 | −0.554 | −0.611 | |
| P90 | −0.702 | 0.013 | −0.279 | |
| TF30P | 1.70 * | 1.49 * | 1.74 * | |
| TFrec | 1.20 * | 1.10 * | 0.488 | |
| TTime | −0.702 | −0.083 | −0.733 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Roig, S.; Ecija, C.; Peñacoba, C.; Ivorra, S.; Nardi-Rodríguez, A.; Lecuona, O.; Pastor-Mira, M.A. Assessing Walking Programs in Fibromyalgia: A Concordance Study between Measures. Int. J. Environ. Res. Public Health 2022, 19, 2995. https://doi.org/10.3390/ijerph19052995
López-Roig S, Ecija C, Peñacoba C, Ivorra S, Nardi-Rodríguez A, Lecuona O, Pastor-Mira MA. Assessing Walking Programs in Fibromyalgia: A Concordance Study between Measures. International Journal of Environmental Research and Public Health. 2022; 19(5):2995. https://doi.org/10.3390/ijerph19052995
Chicago/Turabian StyleLópez-Roig, Sofía, Carmen Ecija, Cecilia Peñacoba, Sofía Ivorra, Ainara Nardi-Rodríguez, Oscar Lecuona, and María Angeles Pastor-Mira. 2022. "Assessing Walking Programs in Fibromyalgia: A Concordance Study between Measures" International Journal of Environmental Research and Public Health 19, no. 5: 2995. https://doi.org/10.3390/ijerph19052995
APA StyleLópez-Roig, S., Ecija, C., Peñacoba, C., Ivorra, S., Nardi-Rodríguez, A., Lecuona, O., & Pastor-Mira, M. A. (2022). Assessing Walking Programs in Fibromyalgia: A Concordance Study between Measures. International Journal of Environmental Research and Public Health, 19(5), 2995. https://doi.org/10.3390/ijerph19052995








