The Effect of Low-intensity Aerobic Training Combined with Blood Flow Restriction on Maximal Strength, Muscle Mass, and Cycling Performance in a Cyclist with Knee Displacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject
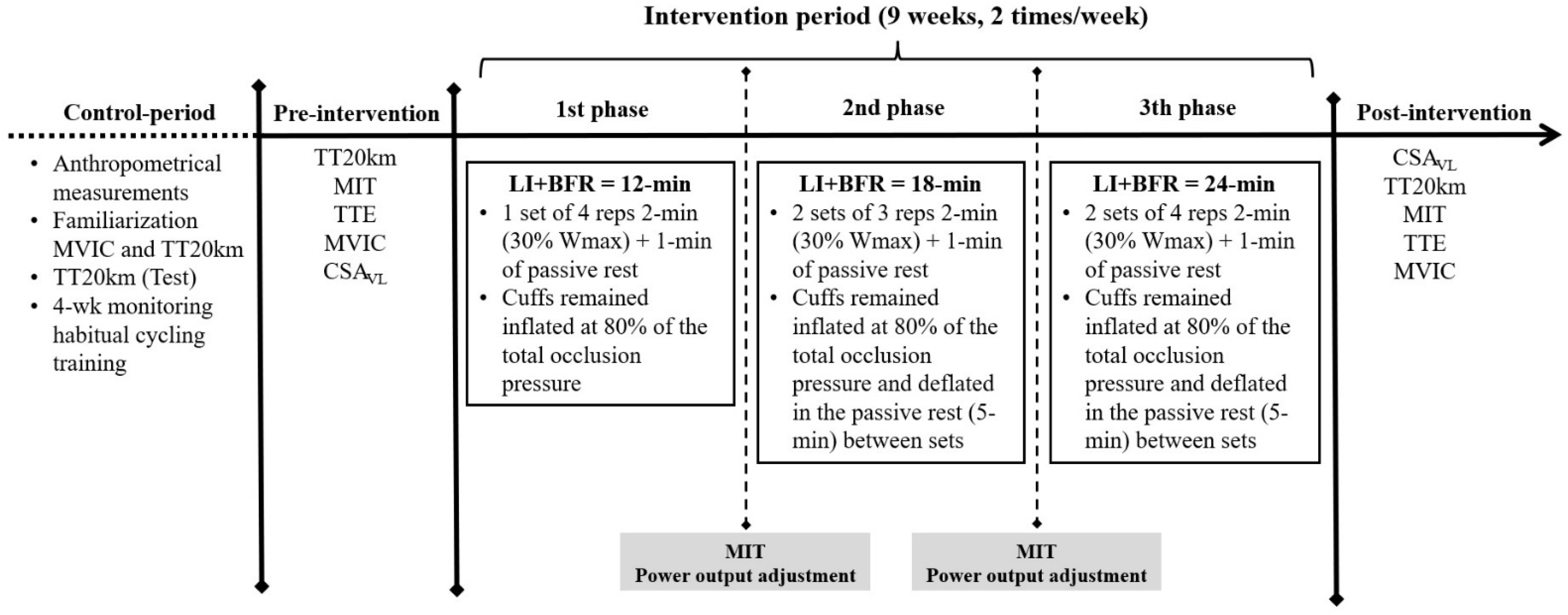
2.2. Experimental Overview
2.3. Cycling Performance Tests and Physiological Measurements
2.4. Maximal Voluntary Isometric Contraction and Muscle Cross-Sectional Area
2.5. Determination of Training Occlusion Pressure
2.6. Training Program
2.7. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions and Practical Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clarsen, B.; Krosshaug, T.; Bahr, R. Overuse Injuries in Professional Road Cyclists. Am. J. Sports Med. 2010, 38, 2494–2501. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.A.; Mitchell, E.A.; Taylor, C.W.; Bishop, D.J.; Christiansen, D. Blood-flow-restricted Exercise: Strategies for Enhancing Muscle Adaptation and Performance in the Endurance-trained Athlete. Exp. Physiol. 2021, 106, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Fujita, S.; Nakajima, T.; Sakamaki, M.; Ozaki, H.; Ogasawara, R.; Sugaya, M.; Kudo, M.; Kurano, M.; Yasuda, T.; et al. Effects of Low-Intensity Cycle Training with Restricted Leg Blood Flow on Thigh Muscle Volume and VO2MAX in Young Men. J. Sports Sci. Med. 2010, 9, 452–458. [Google Scholar] [PubMed]
- Mitchell, E.A.; Martin, N.R.W.; Turner, M.C.; Taylor, C.W.; Ferguson, R.A. The Combined Effect of Sprint Interval Training and Postexercise Blood Flow Restriction on Critical Power, Capillary Growth, and Mitochondrial Proteins in Trained Cyclists. J. Appl. Physiol. 2019, 126, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.W.; Ingham, S.A.; Ferguson, R.A. Acute and Chronic Effect of Sprint Interval Training Combined with Postexercise Blood-Flow Restriction in Trained Individuals. Exp. Physiol. 2016, 101, 143–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, M.F.; Caputo, F.; Corvino, R.B.; Denadai, B.S. Short-Term Low-Intensity Blood Flow Restricted Interval Training Improves Both Aerobic Fitness and Muscle Strength. Scand. J. Med. Sci. Sports 2016, 26, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Craig, N.P.; Hawley, J.A. The Bioenergetics of World Class Cycling. J. Sci. Med. Sport 2000, 3, 414–433. [Google Scholar] [CrossRef]
- Weston, S.B.; Gray, A.B.; Schneider, D.A.; Gass, G.C. Effect of Ramp Slope on Ventilation Thresholds and V˙O2peak in Male Cyclists. Int. J. Sports Med. 2002, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Hoyos, J.; Perez, M.; Chicharro, J.L. Heart Rate and Performance Parameters in Elite Cyclists: A Longitudinal Study. Med. Sci. Sports Exerc. 2000, 32, 1777–1782. [Google Scholar] [PubMed] [Green Version]
- Lixandrão, M.E.; Ugrinowitsch, C.; Bottaro, M.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Min, L.L.; de Souza, E.O.; Laurentino, G.C.; Libardi, C.A. Vastus Lateralis Muscle Cross-Sectional Area Ultrasonography Validity for Image Fitting in Humans. J. Strength Cond. Res. 2014, 28, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A New Approach to Monitoring Exercise Training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar] [PubMed]
- Moreira, A.; Moreira, A.; Bilsborough, J.C.; Sullivan, C.J.; Cianciosi, M.; Aoki, M.S.; Coutts, A.J. Training Periodization of Professional Australian Football Players during an Entire Australian Football League Season. Int. J. Sports Physiol. Perform. 2015, 10, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, F.H.; Hawley, J.A.; Myburgh, K.H.; Schomer, H.H.; Noakes, T.D.; Dennis, S.C. Improved Athletic Performance in Highly Trained Cyclists after Interval Training. Med. Sci. Sports Exerc. 1996, 28, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Ronnestad, B.R.; Hansen, E.A.; Raastad, T. Effect of Heavy Strength Training on Thigh Muscle Cross-Sectional Area, Performance Determinants, and Performance in Well-Trained Cyclists. Eur. J. Appl. Physiol. 2010, 108, 965–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, L.M.; Klau, J.F.; Casa, D.J.; Kraemer, W.J.; Armstrong, L.E.; Maresh, C.M. The Effects of Resistance Training on Road Cycling Performance among Highly Trained Cyclists: A Systematic Review. J. Strength Cond. Res. 2010, 24, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Corvino, R.B.; de Oliveira, M.F.M.; dos Santos, R.P.; Denadai, B.S.; Caputo, F. Four Weeks of Blood Flow Restricted Training Increases Time to Exhaustion at Severe Intensity Cycling Exercise. Rev. Bras. Cineantropometria Desempenho Hum. 2014, 16, 570–578. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Rich, R.G. Effects of High- and Low-Intensity Exercise Training on Aerobic Capacity and Blood Lipids. Med. Sci. Sports Exerc. 1984, 16, 269–274. [Google Scholar] [CrossRef]

| Variables | Control Period | Pre- Intervention | Post- Intervention | Difference (%) (Pre vs. Post) |
|---|---|---|---|---|
| BW (kg) | 81 | 81.3 | 79.3 | −2.5 |
| Height (cm) | 173 | 173 | 173 | 0.0 |
| TT20 km (min) | 32.2 | 32.3 | 32.0 | −1.0 |
| TT20 km Wmean (W) | 265.5 | 261.7 | 272.0 | 3.9 |
| TT20 km Wmean (W·BW−1) | 3.3 | 3.2 | 4.4 | 37.5 |
| VO2peak (mL.kg−1·min−1) | n.a | 49.2 | 54.8 | 11.4 |
| Wmax (W) | n.a | 363.3 | 377.1 | 3.8 |
| Wmax (Wmax·BW−1) | n.a | 4.5 | 4.8 | 6.7 |
| WVT1 (W) | n.a | 225 | 225 | 0.0 |
| WVT2 (W) | n.a | 300 | 325 | 8.3 |
| TTE (s) | n.a | 238 | 275 | 15.5 |
| Volume/Intensity | Control Period | Intervention Period |
|---|---|---|
| Duration (h·week−1) | 6.2 ± 0.7 | 6.6 ± 1.3 |
| %RPEsession < 4 | 22.8 ± 3.4 | 23.4 ± 5.5 |
| %RPEsession > 4 and <7 | 38.8 ± 3.5 | 37.3 ± 2.1 |
| %RPEsession > 7 | 38.4 ± 5.8 | 39.4 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, F.A.; Pires, F.O.; Rønnestad, B.R.; Hardt, F.; Conceição, M.S.; Lixandrão, M.E.; Berton, R.; Tricoli, V. The Effect of Low-intensity Aerobic Training Combined with Blood Flow Restriction on Maximal Strength, Muscle Mass, and Cycling Performance in a Cyclist with Knee Displacement. Int. J. Environ. Res. Public Health 2022, 19, 2993. https://doi.org/10.3390/ijerph19052993
Pinheiro FA, Pires FO, Rønnestad BR, Hardt F, Conceição MS, Lixandrão ME, Berton R, Tricoli V. The Effect of Low-intensity Aerobic Training Combined with Blood Flow Restriction on Maximal Strength, Muscle Mass, and Cycling Performance in a Cyclist with Knee Displacement. International Journal of Environmental Research and Public Health. 2022; 19(5):2993. https://doi.org/10.3390/ijerph19052993
Chicago/Turabian StylePinheiro, Fabiano Aparecido, Flávio Oliveira Pires, Bent R. Rønnestad, Felipe Hardt, Miguel Soares Conceição, Manoel E. Lixandrão, Ricardo Berton, and Valmor Tricoli. 2022. "The Effect of Low-intensity Aerobic Training Combined with Blood Flow Restriction on Maximal Strength, Muscle Mass, and Cycling Performance in a Cyclist with Knee Displacement" International Journal of Environmental Research and Public Health 19, no. 5: 2993. https://doi.org/10.3390/ijerph19052993
APA StylePinheiro, F. A., Pires, F. O., Rønnestad, B. R., Hardt, F., Conceição, M. S., Lixandrão, M. E., Berton, R., & Tricoli, V. (2022). The Effect of Low-intensity Aerobic Training Combined with Blood Flow Restriction on Maximal Strength, Muscle Mass, and Cycling Performance in a Cyclist with Knee Displacement. International Journal of Environmental Research and Public Health, 19(5), 2993. https://doi.org/10.3390/ijerph19052993







