Systemic Lactate Elevation Induced by Tobacco Smoking during Rest and Exercise Is Not Associated with Nicotine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Testing Protocol
2.4. MMST (Maximal Multistage 20 m Shuttle Run Test)
2.5. Lactate, Glucose, and Heart Rate
2.6. Statistics
3. Results
3.1. Blood Lactate Response
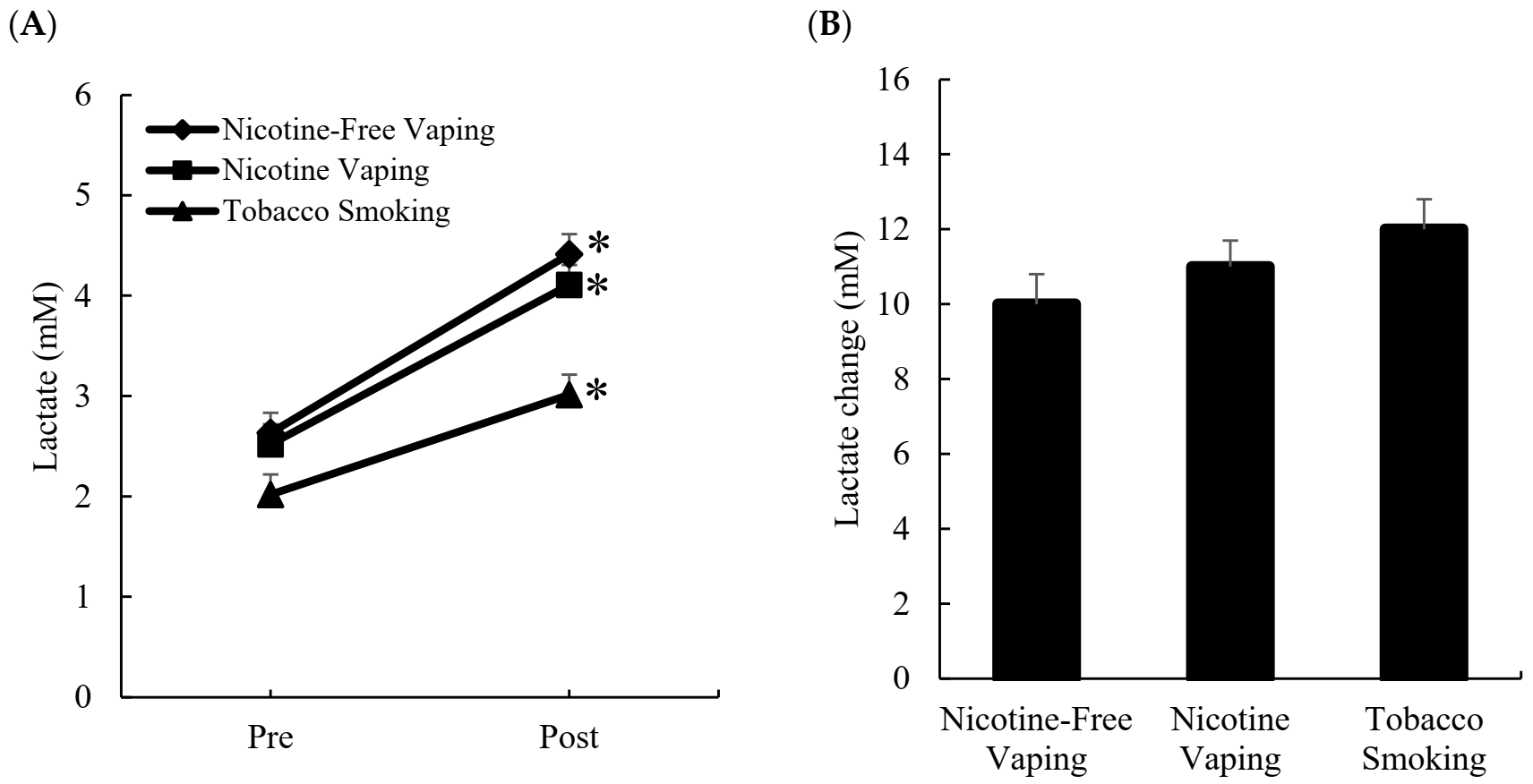
3.2. Blood Glucose Response
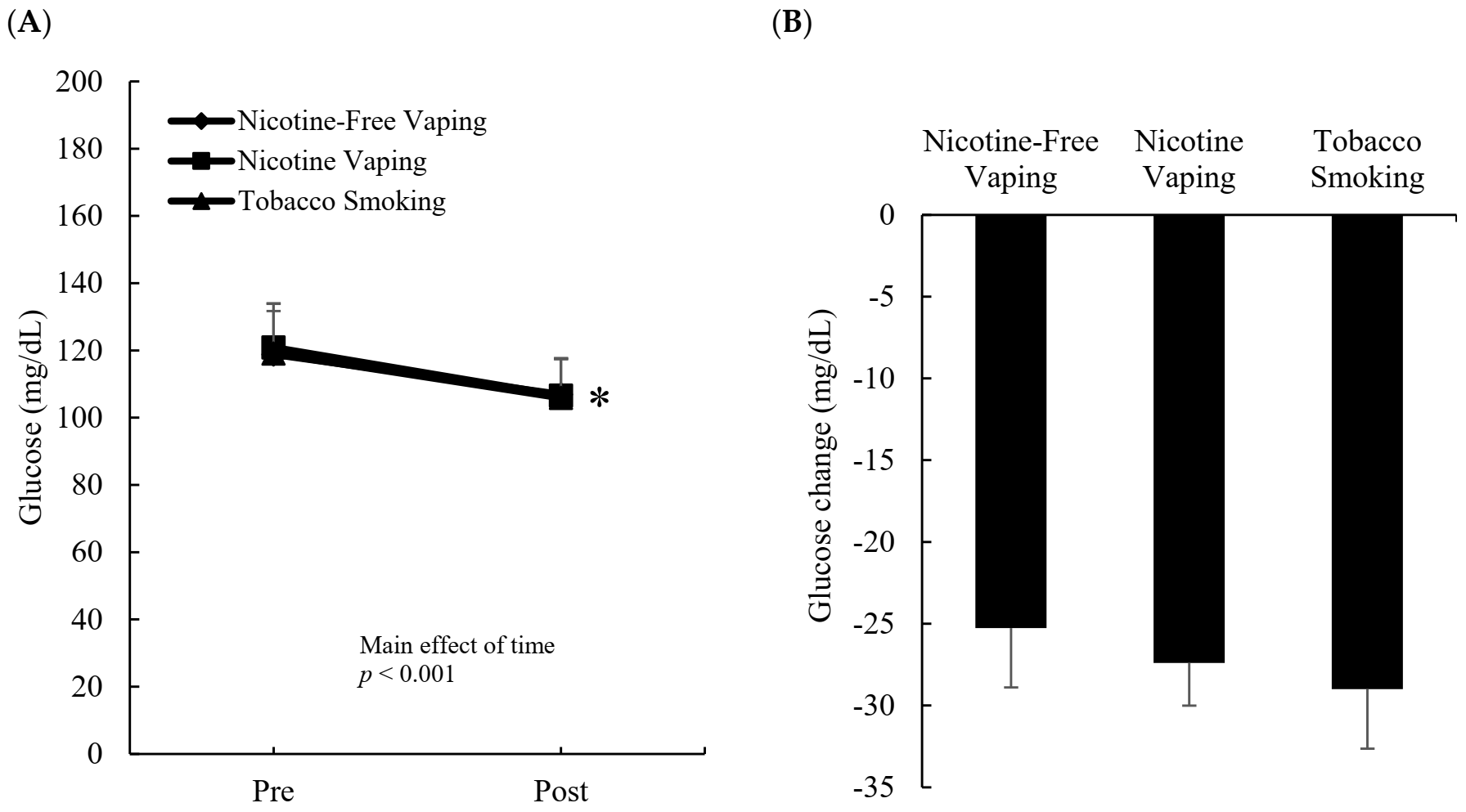
3.3. Heart Rate Response
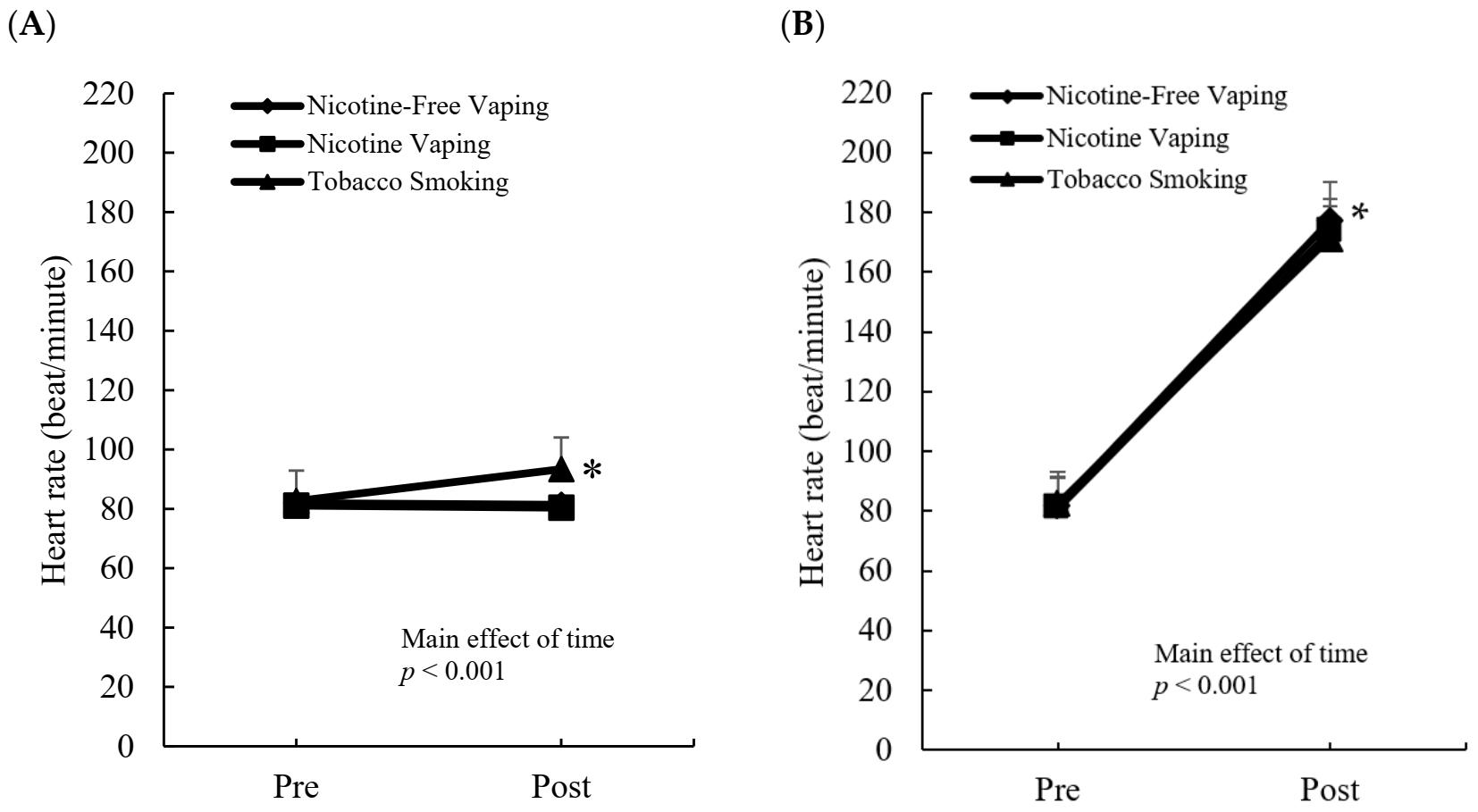
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.M.; De Falco, E.; Chimenti, I.; et al. Acute impact of tobacco vs. electronic cigarette smoking on oxidative stress and vascular function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Stevens, G.A.; Boerma, T.; White, R.A.; Tobias, M.I. Causes of international increases in older age life expectancy. Lancet 2015, 385, 540–548. [Google Scholar] [CrossRef]
- Mathers, C.D.; Boerma, T.; Ma Fat, D. Global and regional causes of death. Br. Med. Bull. 2009, 92, 7–32. [Google Scholar] [CrossRef]
- Ezzati, M.; Henley, S.J.; Thun, M.J.; Lopez, A.D. Role of smoking in global and regional cardiovascular mortality. Circulation 2005, 112, 489–497. [Google Scholar] [CrossRef]
- Bell, K.; Keane, H. Nicotine control: E-cigarettes, smoking and addiction. Int. J. Drug Policy 2012, 23, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Mrkaić, A.; Branković, S.; Ranđelović, P.; Veljković, M.; Pavlović, I.; Radenković, M. Acute effects of smoking on heart rate and peripheral resistance in young smokers. Acta Fac. Med. Naissensis 2015, 32, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Sumartiningsih, S.; Lin, H.F.; Lin, J.C. Cigarette smoking blunts exercise-induced heart rate response among young adult male smokers. Int. J. Environ. Res. Public Health 2019, 16, 1032. [Google Scholar] [CrossRef] [Green Version]
- Antoniewicz, L.; Bosson, J.A.; Kuhl, J.; Abdel-Halim, S.M.; Kiessling, A.; Mobarrez, F.; Lundbäck, M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 2016, 255, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Kerr, D.M.; Brooksbank, K.J.; Taylor, R.G.; Pinel, K.; Rios, F.J.; Touyz, R.M.; Delles, C. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: A cross-over study. J. Hypertens. 2019, 37, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.A.; Guzzardi, L.J. Inhalation of products of combustion. Ann. Emerg. Med. 1983, 12, 628–632. [Google Scholar] [CrossRef]
- Huie, M.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Smoking increases conversion of lactate to glucose during submaximal exercise. J. Appl. Physiol. 1996, 80, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Increased dependence on blood glucose in smokers during rest and sustained exercise. J. Appl. Physiol. 1994, 76, 26–32. [Google Scholar] [CrossRef]
- Paradisis, G.P.; Zacharogiannis, E.; Mandila, D.; Smirtiotou, A.; Argeitaki, P.; Cooke, C.B. Multi-stage 20-m shuttle run fitness test, maximal oxygen uptake and velocity at maximal oxygen uptake. J. Hum. Kinet. 2014, 41, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1977; pp. 8–14. ISBN 0-12-179060-6. [Google Scholar]
- Sørensen, L.T.; Jørgensen, S.; Petersen, L.J.; Hemmingsen, U.; Bülow, J.; Loft, S.; Gottrup, F. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J. Surg. Res. 2009, 152, 224–230. [Google Scholar] [CrossRef]
- Xia, Y.; Warshaw, J.B.; Haddad, G.G. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 273, R1734–R1741. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.A.; DellaValle, B.; Gesser, H.; Rodnick, K.J. Limited effects of exogenous glucose during severe hypoxia and a lack of hypoxia-stimulated glucose uptake in isolated rainbow trout cardiac muscle. J. Exp. Biol. 2013, 216, 3422–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronow, W.S.; Cassidy, J.; Vangrow, J.S.; March, H.; Kern, J.C.; Goldsmith, J.R.; Khemka, M.; Pagano, J.; Vawter, M. Effect of cigarette smoking and breathing carbon monoxide on cardiovascular hemodynamics in anginal patients. Circulation 1974, 50, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.T.; Morice, A.H. Breath carbon monoxide as an indication of smoking habit. Chest 2000, 117, 758–763. [Google Scholar] [CrossRef]
- Low, E.; Ong, M.C.; Tan, M. Breath carbon monoxide as an indication of smoking habit in the military setting. Singap. Med. J. 2004, 45, 578–582. [Google Scholar]
- Javors, M.A.; Hatch, J.P.; Lamb, R.J. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction 2005, 100, 159–167. [Google Scholar] [CrossRef]
- Dorey, A.; Scheerlinck, P.; Nguyen, H.; Albertson, T. Acute and chronic carbon monoxide toxicity from tobacco smoking. Mil. Med. 2020, 185, e61–e67. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Qian, L.; Cheng, Z.; Chen, C.; Wang, K.; Hu, S.; Zhang, X.; Wu, T. Lactate and Myocadiac Energy Metabolism. Front. Physiol. 2021, 12, 715081. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Schieveld, S.J.; Brinkert, W. Serum lactate level as a indicator of tissue hypoxia in severely ill patients. Ned. Tijdschr. Geneeskd. 2000, 144, 737–741. [Google Scholar] [PubMed]
- Mu, J.; Brozinick, J.T., Jr.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Robinson, B.F.; Epstein, S.E.; Beiser, G.D.; Braunwald, E. Control of heart rate by the autonomic nervous system: Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ. Res. 1966, 19, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Vora, R.; Zareba, W.; Utell, M.J.; Pietropaoli, A.P.; Chalupa, D.; Little, E.L.; Oakes, D.; Bausch, J.; Wiltshire, J.; Frampton, M.W. Inhalation of ultrafine carbon particles alters heart rate and heart rate variability in people with type 2 diabetes. Part. Fibre Toxicol. 2014, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Franzen, K.F.; Willig, J.; Cayo Talavera, S.; Meusel, M.; Sayk, F.; Reppel, M.; Dalhoff, K.; Mortensen, K.; Droemann, D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc. Med. 2018, 23, 419–425. [Google Scholar] [CrossRef]
- Jensen, K.P.; Valentine, G.; Gueorguieva, R.; Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 2020, 237, 1359–1369. [Google Scholar] [CrossRef]
- Mündel, T. Nicotine: Sporting friend or foe? A review of athlete use, performance consequences and other considerations. Sports Med. 2017, 47, 2497–2506. [Google Scholar] [CrossRef] [Green Version]
- Morente-Sánchez, J.; Zandonai, T.; Mateo-March, M.; Sanabria, D.; Sánchez-Muñoz, C.; Chiamulera, C.; Zabala Diaz, M. Acute effect of S nus on physical performance and perceived cognitive load on amateur footballers. Scand. J. Med. Sci. Sports 2015, 25, e423–e431. [Google Scholar] [CrossRef]
- Richter, P.A.; Bishop, E.E.; Wang, J.; Swahn, M.H. Tobacco smoke exposure and levels of urinary metals in the US youth and adult population: The National Health and Nutrition Examination Survey (NHANES) 1999–2004. Int. J. Environ. Res. Public Health 2009, 6, 1930–1946. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Das, S.; Young-Wolff, K.C. Smoking, mental illness, and public health. Annual Rev. Public Health 2017, 38, 165–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, M.; Brozek, G.; Lawson, J.; Skoczynski, S.; Zejda, J.E. E-smoking: Emerging public health problem? Int. J. Occup. Med. Environ. Health 2017, 30, 329–344. [Google Scholar] [CrossRef]
- Jacobs, D., Jr.; Adachi, H.; Mulder, I.; Kromhout, D.; Menotti, A.; Nissinen, A.; Blackburn, H. Cigarette smoking and mortality risk: Twenty-five-year follow-up of the Seven Countries Study. Arch. Intern. Med. 1999, 159, 733–740. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. Morb. Mortal. Wkly. Rep. 2008, 57, 1226–1228. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumartiningsih, S.; Rahayu, S.; Handoyo, E.; Lin, J.-C.; Lim, C.L.; Starczewski, M.; Fuchs, P.X.; Kuo, C.-H. Systemic Lactate Elevation Induced by Tobacco Smoking during Rest and Exercise Is Not Associated with Nicotine. Int. J. Environ. Res. Public Health 2022, 19, 2902. https://doi.org/10.3390/ijerph19052902
Sumartiningsih S, Rahayu S, Handoyo E, Lin J-C, Lim CL, Starczewski M, Fuchs PX, Kuo C-H. Systemic Lactate Elevation Induced by Tobacco Smoking during Rest and Exercise Is Not Associated with Nicotine. International Journal of Environmental Research and Public Health. 2022; 19(5):2902. https://doi.org/10.3390/ijerph19052902
Chicago/Turabian StyleSumartiningsih, Sri, Setya Rahayu, Eko Handoyo, Jung-Charng Lin, Chin Leong Lim, Michal Starczewski, Philip X. Fuchs, and Chia-Hua Kuo. 2022. "Systemic Lactate Elevation Induced by Tobacco Smoking during Rest and Exercise Is Not Associated with Nicotine" International Journal of Environmental Research and Public Health 19, no. 5: 2902. https://doi.org/10.3390/ijerph19052902
APA StyleSumartiningsih, S., Rahayu, S., Handoyo, E., Lin, J.-C., Lim, C. L., Starczewski, M., Fuchs, P. X., & Kuo, C.-H. (2022). Systemic Lactate Elevation Induced by Tobacco Smoking during Rest and Exercise Is Not Associated with Nicotine. International Journal of Environmental Research and Public Health, 19(5), 2902. https://doi.org/10.3390/ijerph19052902









