Three-Month vs. One-Year Detraining Effects after Multicomponent Exercise Program in Hypertensive Older Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample and Ethical Procedures
2.2.1. Exercise Program and Detraining Period
2.2.2. Body Composition and Hemodynamic and Lipid Profiles
2.2.3. Functional Capacity
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanssen, H.; Boardman, H.; Deiseroth, A.; Moholdt, T.; Simonenko, M.; Kränkel, N.; Niebauer, J.; Tiberi, M.; Abreu, A.; Solberg, E.E.; et al. Personalized Exercise Prescription in the Prevention and Treatment of Arterial Hypertension: A Consensus Document from the European Association of Preventive Cardiology (EAPC) and the ESC Council on Hypertension. Eur. J. Prev. Cardiol. 2021, 29, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC): The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescatello, L.S.; Buchner, D.M.; Jakicic, J.M.; Powell, K.E.; Kraus, W.E.; Bloodgood, B.; Campbell, W.W.; Dietz, S.; Dipietro, L.; George, S.M.; et al. 2018 Physical Activity Guidelines Advisory Committee. Physical Activity to Prevent and Treat Hypertension: A Systematic Review: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Hollings, M.; Mavros, Y.; Freeston, J.; Fiatarone Singh, M. The Effect of Progressive Resistance Training on Aerobic Fitness and Strength in Adults with Coronary Heart Disease: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur. J. Prev. Cardiol. 2017, 24, 1242–1259. [Google Scholar] [CrossRef] [PubMed]
- Ceciliato, J.; Costa, E.C.; Azevêdo, L.; Sousa, J.C.; Fecchio, R.Y.; Brito, L.C. Effect of Resistance Training on Arterial Stiffness in Healthy Subjects: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Currie, K.D.; Floras, J.S.; La Gerche, A.; Goodman, J.M. Exercise Blood Pressure Guidelines: Time to Re-Evaluate What Is Normal and Exaggerated? Sports Med. 2018, 48, 1763–1771. [Google Scholar] [CrossRef]
- Fidalgo, A.S.F.; Farinatti, P.; Borges, J.P.; de Paula, T.; Monteiro, W. Institutional Guidelines for Resistance Exercise Training in Cardiovascular Disease: A Systematic Review. Sports Med. 2019, 49, 463–475. [Google Scholar] [CrossRef]
- Brook, R.D.; Appel, L.J.; Rubenfire, M.; Ogedegbe, G.; Bisognano, J.D.; Elliott, W.J.; Fuchs, F.D.; Hughes, J.W.; Lackland, D.T.; Staffileno, B.A.; et al. Beyond Medications and Diet: Alternative Approaches to Lowering Blood Pressure: A Scientific Statement from the American Heart Association: A Scientific Statement from the American Heart Association. Hypertension 2013, 61, 1360–1383. [Google Scholar] [CrossRef]
- Pescatello, L.S. What’s New in the ACSM Pronouncement on Exercise and Hypertension? 2019. Available online: https://www.acsm.org/home/featured-blogs—homepage/acsm-blog/2019/06/11/new-acsm-pronouncement-exercise-hypertension (accessed on 3 December 2019).
- College, A.; Sports, M.; Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine Position Stand. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Abreu, A.; Albus, C.; Ambrosetti, M.; Brotons, C.; Catapano, A.L.; Corra, U.; Cosyns, B.; Deaton, C.; Graham, I.; et al. Update on Cardiovascular Prevention in Clinical Prac-Tice: A Position Paper of the European Association of Preventive Cardiology of the. European Society of Cardiology. Eur. J. Prev. Cardiol. 2020, 27, 181–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.C.; Hay, J.L.; Kehler, D.S.; Boreskie, K.F.; Arora, R.C.; Umpierre, D.; Szwajcer, A.; Duhamel, T.A. Effects of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018, 48, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- Mariano, I.; Amaral, A.L.; Ribeiro, P.; Puga, G. Blood Pressure Responses to Stress after Chronic Physical Exercise: A Systematic Review with Meta-Analysis. J. Hypertens. 2021, 39 (Suppl. S1), e402. [Google Scholar] [CrossRef]
- Martínez-Aldao, D.; Diz, J.C.; Varela, S.; Sánchez-Lastra, M.A.; Ayán, C. Impact of a Five-Month Detraining Period on the Functional Fitness and Physical Activity Levels on Active Older People. Arch. Gerontol. Geriatr. 2020, 91, 104191. [Google Scholar] [CrossRef]
- Leitão, L.; Pereira, A.; Mazini, M.; Venturini, G.; Campos, Y.; Vieira, J.; Novaes, J.; Vianna, J.; da Silva, S.; Louro, H. Effects of Three Months of Detraining on the Health Profile of Older Women after a Multicomponent Exercise Program. Int. J. Environ. Res. Public Health 2019, 16, 3881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitão, L.; Marocolo, M.; de Souza, H.L.R.; Arriel, R.A.; Vieira, J.G.; Mazini, M.; Figueiredo, T.; Louro, H.; Pereira, A. Multicomponent Exercise Program for Improvement of Functional Capacity and Lipidic Profile of Older Women with High Cholesterol and High Triglycerides. Int. J. Environ. Res. Public Health 2021, 18, 10731. [Google Scholar] [CrossRef]
- Leitão, L.; Marocolo, M.; de Souza, H.L.R.; Arriel, R.A.; Vieira, J.G.; Mazini, M.; Louro, H.; Pereira, A. Can Exercise Help Regulate Blood Pressure and Improve Functional Capacity of Older Women with Hypertension against the Deleterious Effects of Physical Inactivity? Int. J. Environ. Res. Public Health 2021, 18, 9117. [Google Scholar] [CrossRef]
- Casas-Herrero, A.; Anton-Rodrigo, I.; Zambom-Ferraresi, F.; De Asteasu, M.L.S.; Martinez-Velilla, N.; Elexpuru-Estomba, J.; Ibañez, B. Effect of a Multicomponent Exercise Programme (VIVIFRAIL) on Functional Capacity in Frail Community El-Ders with Cognitive Decline: Study Protocol for a Randomized Multicentre Control Trial. Trials 2019, 20, 362–374. [Google Scholar] [CrossRef]
- da Silva Sobrinho, A.C.; de Almeida, M.L.; da Silva Rodrigues, G.; Finzeto, L.C.; Silva, V.R.R.; Bernatti, R.F.; Bueno Junior, C.R. Effect of Flexibility Training Associated with Multicomponent Training on Posture and Quality of Movement in Physically Inactive Older Women: A Randomized Study. Int. J. Environ. Res. Public Health 2021, 18, 10709. [Google Scholar] [CrossRef]
- Buendía-Romero, Á.; Vetrovsky, T.; Estévez-López, F.; Courel-Ibáñez, J. Effect of Physical Exercise Cessation on Strength, Functional, Metabolic and Structural Outcomes in Older Adults: A Protocol for Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e052913. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramirez-Vélez, R.; Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Valenzuela, P.L.; Lucia, A.; Izquierdo, M. Safety and Effectiveness of Long-Term Exercise Interventions in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Blocquiaux, S.; Gorski, T.; Van Roie, E.; Ramaekers, M.; Van Thienen, R.; Nielens, H.; Delecluse, C.; De Bock, K.; Thomis, M. The Effect of Resistance Training, Detraining and Retraining on Muscle Strength and Power, Myofibre Size, Satellite Cells and Myonuclei in Older Men. Exp. Gerontol. 2020, 133, 110860. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Lafarga, C.; Cordellat, A.; Forte, A.; Roldán, A.; Monteagudo, P. Short and Long-Term Trainability in Older Adults: Training and Detraining Following Two Years of Multicomponent Cognitive-Physical Exercise Training. Int. J. Environ. Res. Public Health 2020, 17, 5984. [Google Scholar] [CrossRef]
- Bosquet, L.; Berryman, N.; Dupuy, O.; Mekary, S.; Arvisais, D.; Bherer, L.; Mujika, I. Effect of Training Cessation on Muscular Performance: A Meta-Analysis: Training Cessation and Strength Performance. Scand. J. Med. Sci. Sports 2013, 23, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Boullosa, D.; McGuigan, M.; Fusco, A.; Cortis, C.; Arney, B.E.; Orton, B.; Dodge, C.; Jaime, S.; Radtke, K.; et al. 25 Years of Session Rating of Perceived Exertion: Historical Perspective and Development. Int. J. Sports Physiol. Perform. 2021, 16, 612–621. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Douda, H.T.; Kosmidou, K.V.; Smilios, I.; Volaklis, K.A.; Tokmakidis, S.P. Community-Based Training-Detraining Intervention in Older Women: A Five-Year Follow-up Study. J. Aging Phys. Act. 2015, 23, 496–512. [Google Scholar] [CrossRef]
- Monteagudo, P.; Cordellat, A.; Roldán, A.; Gómez-Cabrera, M.C.; Pesce, C.; Blasco-Lafarga, C. Exploring Potential Benefits of Accumulated Multicomponent-Training in Non-Active Older Adults: From Physical Fitness to Mental Health. Int. J. Environ. Res. Public Health 2021, 18, 9645. [Google Scholar] [CrossRef]
- De Bezerra, E.S.; Orssatto, L.B.R.; Oliveira, S.N.; Sakugawa, R.L.; Ribeiro, A.S.; Diefenthaeler, F.; Moro, A.R.P. One-Year Cessation Following Resistance Training Differently Affects Neuromuscular, Body Composition, and Functional Capacity in Older Adults. Sport Sci. Health 2021, 17, 347–355. [Google Scholar] [CrossRef]
- Ahn, N.; Kim, K. Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers? Int. J. Environ. Res. Public Health 2020, 17, 5910. [Google Scholar] [CrossRef]
- Marques, E.; Carvalho, J.; Soares, J.M.C.; Marques, F.; Mota, J. Effects of Resistance and Multicomponent Exercise on Lipid Profiles of Older Women. Maturitas 2009, 63, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The Metabolic Syndrome-What Is It and How Should It Be Managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Thom, J.M.; Rye, K.-A.; Parmenter, B.J. Aerobic, Resistance or Combined Training: A Systematic Review and Meta-Analysis of Exercise to Reduce Cardiovascular Risk in Adults with Metabolic Syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Esain, I.; Gil, S.M.; Bidaurrazaga-Letona, I.; Rodriguez-Larrad, A. Effects of 3 Months of Detraining on Functional Fitness and Quality of Life in Older Adults Who Regularly Exercise. Aging Clin. Exp. Res. 2019, 31, 503–510. [Google Scholar] [CrossRef]
- Lee, M.; Lim, T.; Lee, J.; Kim, K.; Yoon, B. Optimal Retraining Time for Regaining Functional Fitness Using Multicomponent Training after Long-Term Detraining in Older Adults. Arch. Gerontol. Geriatr. 2017, 73, 227–233. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Nascimento, D.; Tibana, R.A.; Benik, F.M.; Fontana, K.E.; Neto, F.R.; de Santana, F.S.; Santos-Neto, L.; Silva, R.A.S.; Silva, A.O.; Farias, D.L.; et al. Sustained Effect of Resistance Training on Blood Pressure and Hand Grip Strength Following a Detraining Period in Elderly Hypertensive Women: A Pilot Study. Clin. Interv. Aging 2014, 9, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Tofas, T.; Fatouros, I.G.; Draganidis, D.; Deli, C.K.; Chatzinikolaou, A.; Tziortzis, C.; Panayiotou, G.; Koutedakis, Y.; Jamurtas, A.Z. Effects of Cardiovascular, Resistance and Combined Exercise Training on Cardiovascular, Performance and Blood Redox Parameters in Coronary Artery Disease Patients: An 8-Month Training-Detraining Randomized Intervention. Antioxidants 2021, 10, 409. [Google Scholar] [CrossRef]
- Modaberi, S.; Saemi, E.; Federolf, P.A.; van Andel, S. A Systematic Review on Detraining Effects after Balance and Fall Prevention Interventions. J. Clin. Med. 2021, 10, 4656. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Yu, B.; Song, Q.; Mao, D. Effects of Exercise on Ankle Proprioception in Adult Women during 16 Weeks of Training and Eight Weeks of Detraining. Res. Sports Med. 2015, 23, 102–113. [Google Scholar] [CrossRef]
- Vogler, C.M.; Menant, J.C.; Sherrington, C.; Ogle, S.J.; Lord, S.R. Evidence of Detraining after 12-Week Home-Based Exercise Programs Designed to Reduce Fall-Risk Factors in Older People Recently Discharged from Hospital. Arch. Phys. Med. Rehabil. 2012, 93, 1685–1691. [Google Scholar] [CrossRef]
- He, L.; Van Roie, E.; Bogaerts, A.; Verschueren, S.; Delecluse, C.; Morse, C.I.; Thomis, M. The Genetic Effect on Muscular Changes in an Older Population: A Follow-up Study after One-Year Cessation of Structured Training. Genes 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Coetsee, C.; Terblanche, E. The Time Course of Changes Induced by Resistance Training and Detraining on Muscular and Physical Function in Older Adults. Eur. Rev. Aging Phys. Act. 2015, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Correa, C.S.; Baroni, B.M.; Radaelli, R.; Lanferdini, F.J.; Cunha, G.D.S.; Reischak-Oliveira, Á.; Vaz, M.A.; Pinto, R.S. Effects of Strength Training and Detraining on Knee Extensor Strength, Muscle Volume and Muscle Quality in Elderly Women. Age 2013, 35, 1899–1904. [Google Scholar] [CrossRef] [Green Version]
- Nolan, P.B.; Keeling, S.M.; Robitaille, C.A.; Buchanan, C.A.; Dalleck, L.C. The Effect of Detraining after a Period of Training on Cardiometabolic Health in Previously Sedentary Individuals. Int. J. Environ. Res. Public Health 2018, 15, 2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esain, I.; Rodriguez-Larrad, A.; Bidaurrazaga-Letona, I.; Gil, S.M. Exercise Cessation in Active Older Adults: Effects on Inflammatory Markers and Adiponectin. Geriatr. Gerontol. Int. 2020, 20, 494–499. [Google Scholar] [CrossRef]
- Moker, E.A.; Bateman, L.A.; Kraus, W.E.; Pescatello, L.S. The Relationship between the Blood Pressure Responses to Exercise Following Training and Detraining Periods. PLoS ONE 2014, 9, e105755. [Google Scholar] [CrossRef] [PubMed]

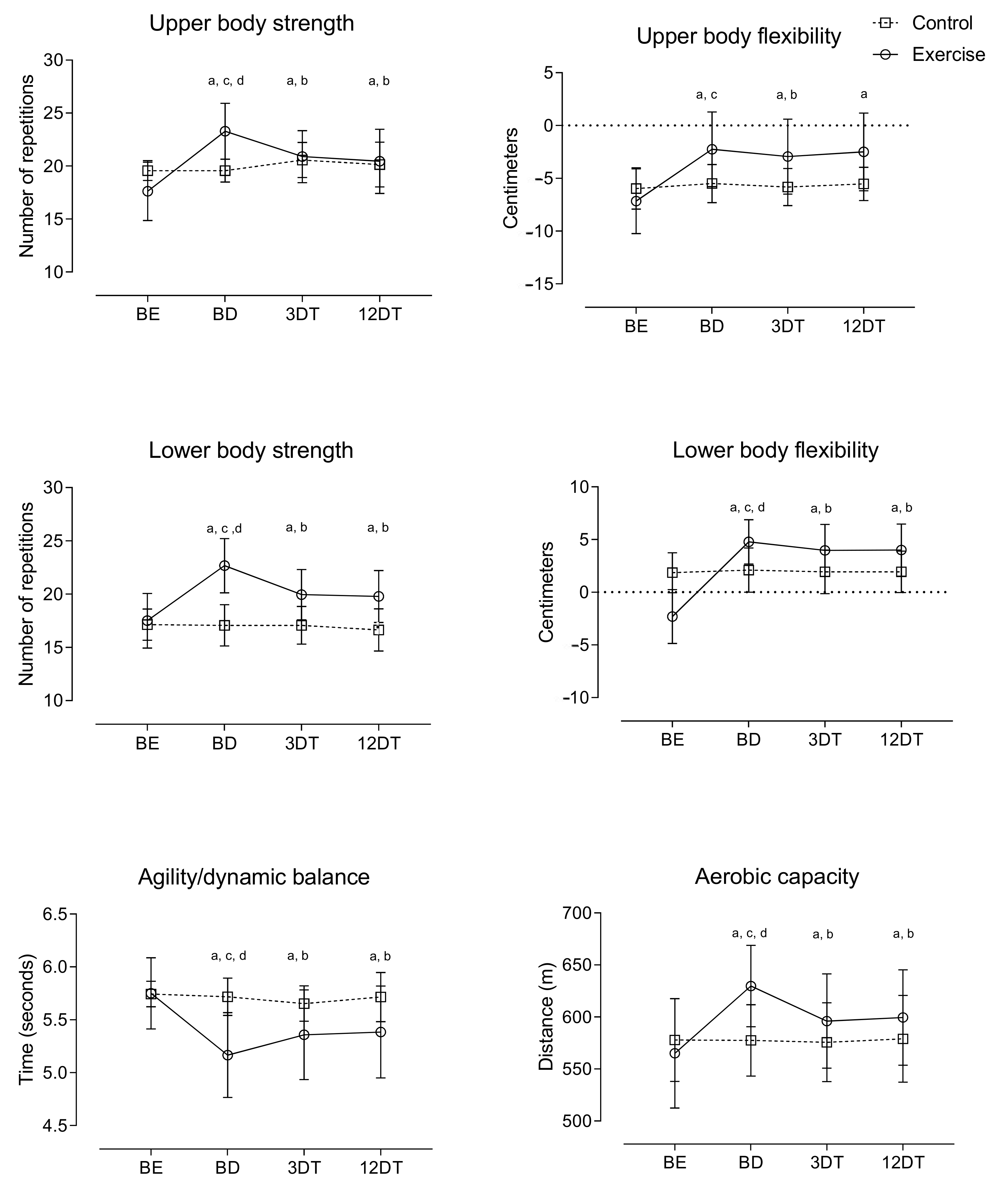
| Variable | CG (n = 14) | EG (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|
| BE | BD | 3DT | 12DT | BE | BD | 3DT | 12DT | |
| Height (cm) | 156.21 ± 3.10 | 156.21 ± 3.10 | 156.21 ± 3.10 | 156.21 ± 3.10 | 153.03 ± 3.42 | 153.03 ± 3.42 | 153.03 ± 3.42 | 153.03 ± 3.42 |
| Body weight (kg) | 69.38 ± 5.24 | 69.21 ± 5.55 | 69.79 ± 5.55 b | 69.86 ± 5.84 | 73.24 ± 7.30 | 71.84 ± 7.45 a | 72.24 ± 7.41 a,b | 72.28 ± 7.33 a |
| %BF (%) | 38.71 ± 1.12 | 39.05 ± 1.43 | 39.09 ± 1.14 a | 39.06 ± 1.34 | 39.00 ± 1.36 | 38.06 ± 1.28 a | 38.34 ± 1.33 a | 38.50 ± 1.34 a |
| BMI (kg/m2) | 28.46 ± 2.34 | 28.39 ± 2.50 | 28.63 ± 2.49 b | 28.65 ± 2.56 | 31.28 ± 3.04 | 30.68 ± 3.10 a | 30.85 ± 3.08 a,b | 30.87 ± 3.05 a |
| (∆ METP) | (∆ 3DT) | (∆ 12DT) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | CG | EG | p Value | CG | EG | p Value | CG | EG | p Value |
| Body weight (%) | −0.27 ± 1.44 | −1.95 ± 0.76 | <0.01 | 0.84 ± 0.52 | 0.57 ± 0.22 | 0.09 | 0.91 ± 1.19 | 0.64 ± 1.27 | 0.82 |
| BMI (%) | −0.27 ± 1.44 | −1.95 ± 0.76 | <0.01 | 0.84 ± 0.52 | 0.57 ± 0.22 | 0.09 | 0.90 ± 1.18 | 0.64 ± 1.27 | 0.88 |
| BF% (%) | 0.88 ± 2.13 | −2.42 ± 0.49 | <0.01 | 0.16 ± 2.42 | 0.76 ± 0.80 | 0.39 | 0.05 ± 2.29 | 1.18 ± 1.65 | 0.14 |
| SBP (%) | −0.42 ± 1.85 | −5.17 ± 2.70 | <0.01 | 0.17 ± 1.91 | 8.19 ± 5.17 | <0.01 | −0.12 ± 2.46 | 7.86 ± 4.62 | <0.01 |
| DBP (%) | −0.32 ± 1.78 | −5.23 ± 1.36 | <0.01 | 0.26 ± 1.13 | −2.10 ± 6.13 | 0.13 | 0.67 ± 1.27 | −2.27 ± 6.74 | 0.09 |
| RHR (%) | −0.35 ± 1.90 | −8.72 ± 3.96 | <0.01 | −0.25 ± 1.29 | 7.21 ± 3.30 | <0.01 | 0.18 ± 1.76 | 8.19 ± 3.86 | <0.01 |
| TG (%) | −1.81 ± 4.29 | −16.41 ± 3.39 | <0.01 | 1.46 ± 3.00 | 5.66 ± 4.46 | <0.01 | 3.45 ± 6.48 | 7.25 ± 7.21 | 0.08 |
| TC (%) | −0.19 ± 1.37 | −18.71 ± 1.99 | <0.01 | −1.16 ± 3.16 | 10.42 ± 3.09 | <0.01 | −0.17 ± 4.43 | 10.38 ± 4.72 | <0.01 |
| GL (%) | 0.77 ± 2.34 | −15.25 ± 6.34 | <0.01 | 0.73 ± 3.12 | 17.12 ± 7.59 | <0.01 | 1.72 ± 4.12 | 19.36 ± 6.01 | <0.01 |
| LBS (%) | −0.58 ± 5.11 | 30.35 ± 8.67 | <0.01 | 0.46 ± 8.55 | −12.03 ± 2.99 | <0.01 | −2.39 ± 6.01 | −12.75 ± 4.73 | <0.01 |
| UBS (%) | 0.00 ± 2.86 | 33.62 ± 12.87 | <0.01 | 5.43 ± 10.57 | −9.86 ± 8.84 | <0.01 | 3.16 ± 11.67 | −11.68 ± 12.55 | <0.01 |
| 2TUG (%) | −0.45 ± 2.18 | −10.13 ± 5.01 | <0.01 | −1.10 ± 2.12 | 3.78 ± 3.87 | <0.01 | −0.02 ± 3.70 | 4.37 ± 5.67 | 0.02 |
| 6MWT(%) | 0.02 ± 2.22 | 11.93 ± 6.83 | <0.01 | −0.34 ± 1.48 | −5.38 ± 3.49 | <0.01 | 0.21 ± 2.79 | −4.82 ± 4.38 | <0.01 |
| UBF (cm) | 0.46 ± 0.63 | 4.92 ± 1.65 | <0.01 | −0.32 ± 0.58 | −0.69 ± 0.35 | 0.03 | −0.04 ± 1.42 | −0.25 ± 1.23 | 0.65 |
| LBF (cm) | 0.25 ± 1.28 | 7.08 ± 2.43 | <0.01 | −0.18 ± 0.54 | −0.81 ± 1.20 | 0.05 | −0.17 ± 0.69 | −0.77 ± 1.26 | 0.10 |
| Study | N | Type of Training | Intensity and Duration of Training | Effects of Training | Detraining Duration | Effects of Detraining |
| Bezerra et al. [30]. | 15 | Strength training | 3 sessions/week 9 weeks | Functional capacity and strength improved | 1 year | Strength and functional capacity benefits maintained |
| Douda et al. [28] | 42 | METP | 3 × 45 min/week 9 months every year over 5 years | Functional capacity and strength improved | 3 months every year over 5 years | Functional capacity and strength benefits maintained |
| Essain et al. [35] | 38 | METP | 2 × 50 min/week 9 months | - | 3 months | TG, TC, strength and cardiorespiratory benefits maintained |
| Lee et al. [36] | 18 | METP | 2 s 60 min/week 12 months | Functional capacity improved | 12 months | Functional capacity benefits maintained except with upper-body flexibility |
| Leitão et al. [18] | 17 | METP | 2 × 45 min/week 9 months | Functional capacity, TG and TC improved | 3 months | Functional capacity, TG and TC benefits maintained |
| Martinez-Aldao et al. [15] | 65 | METP | 2 × 45 min/week 8 months | - | 5 months | Strength, upper body flexibility and agility decreased |
| Nascimento et al. [37] | 12 | Strength training | 2 sessions/week | Strength and BP improved | 14 weeks | Strength and BP benefits maintained |
| Sobrinho et al. [20] | 52 | METP | 2 × 90 min/week 14 weeks | Flexibility and BP improved | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, L.; Marocolo, M.; de Souza, H.L.R.; Arriel, R.A.; Campos, Y.; Mazini, M.; Junior, R.P.; Figueiredo, T.; Louro, H.; Pereira, A. Three-Month vs. One-Year Detraining Effects after Multicomponent Exercise Program in Hypertensive Older Women. Int. J. Environ. Res. Public Health 2022, 19, 2871. https://doi.org/10.3390/ijerph19052871
Leitão L, Marocolo M, de Souza HLR, Arriel RA, Campos Y, Mazini M, Junior RP, Figueiredo T, Louro H, Pereira A. Three-Month vs. One-Year Detraining Effects after Multicomponent Exercise Program in Hypertensive Older Women. International Journal of Environmental Research and Public Health. 2022; 19(5):2871. https://doi.org/10.3390/ijerph19052871
Chicago/Turabian StyleLeitão, Luis, Moacir Marocolo, Hiago L. R. de Souza, Rhai André Arriel, Yuri Campos, Mauro Mazini, Ricardo Pace Junior, Teresa Figueiredo, Hugo Louro, and Ana Pereira. 2022. "Three-Month vs. One-Year Detraining Effects after Multicomponent Exercise Program in Hypertensive Older Women" International Journal of Environmental Research and Public Health 19, no. 5: 2871. https://doi.org/10.3390/ijerph19052871
APA StyleLeitão, L., Marocolo, M., de Souza, H. L. R., Arriel, R. A., Campos, Y., Mazini, M., Junior, R. P., Figueiredo, T., Louro, H., & Pereira, A. (2022). Three-Month vs. One-Year Detraining Effects after Multicomponent Exercise Program in Hypertensive Older Women. International Journal of Environmental Research and Public Health, 19(5), 2871. https://doi.org/10.3390/ijerph19052871









