Twelve-Month Evaluation of Temperature Effects of Radiotherapy in Patients after Mastectomy
Abstract
1. Introduction
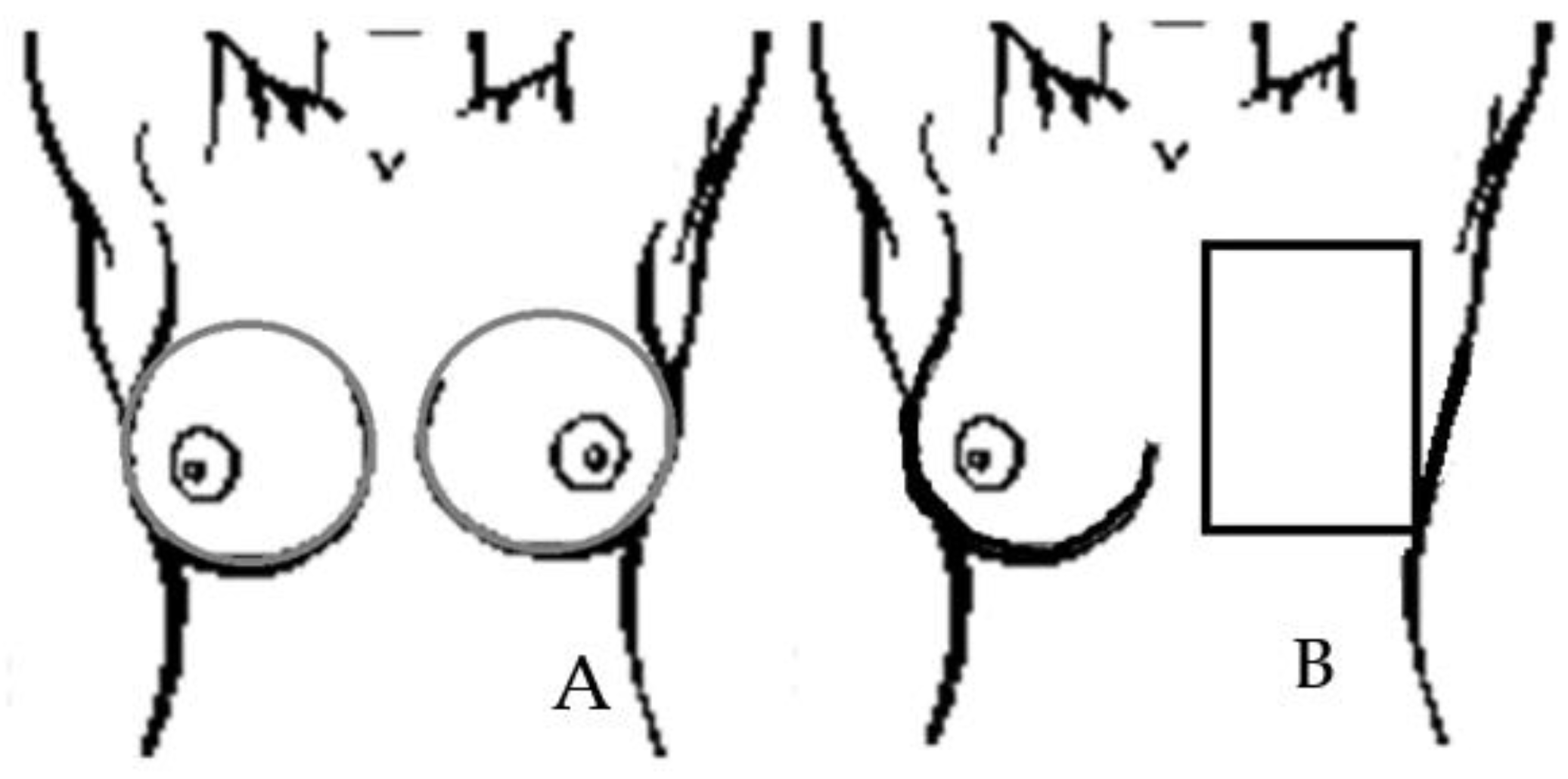
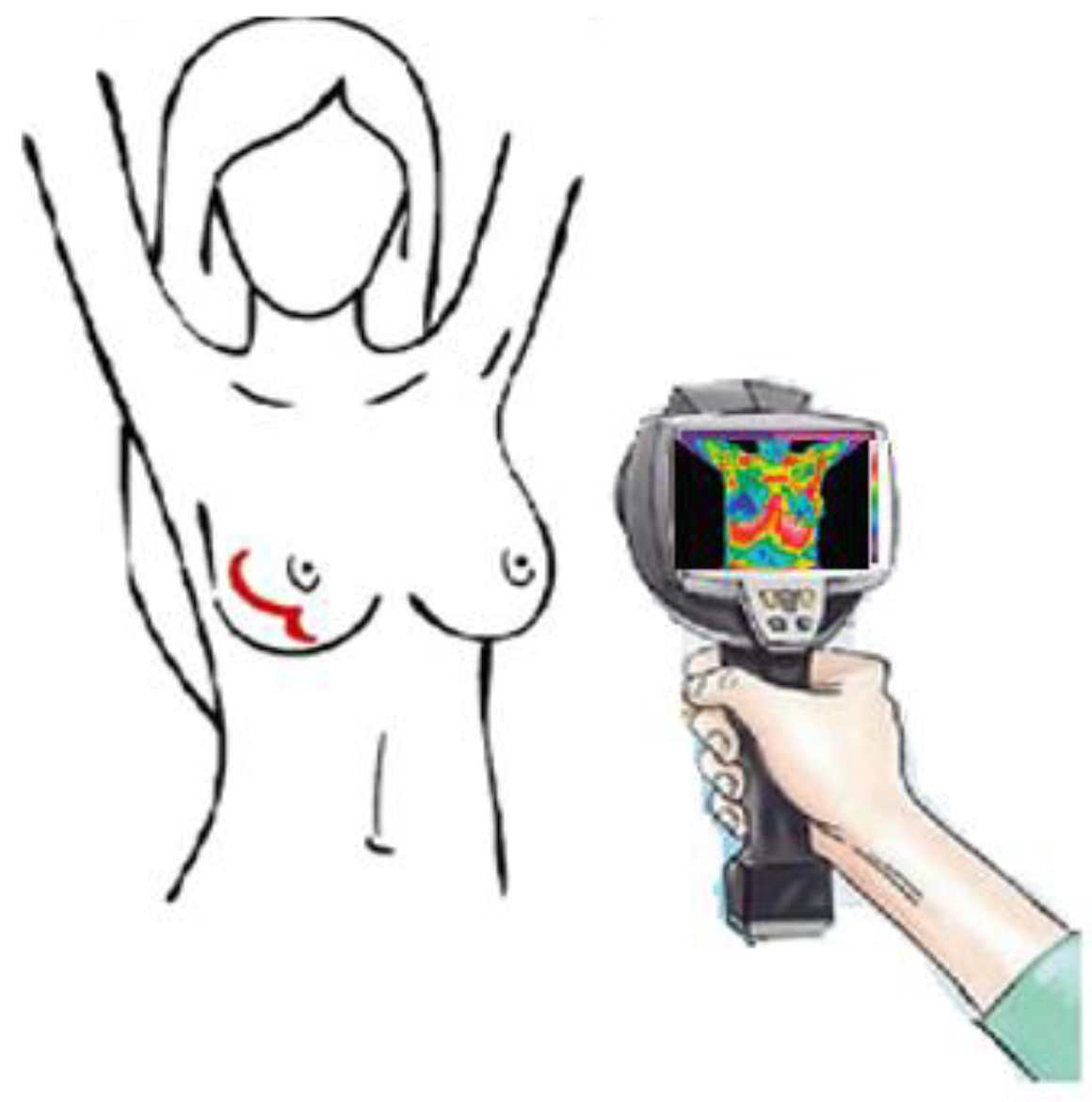
2. Materials and Methods
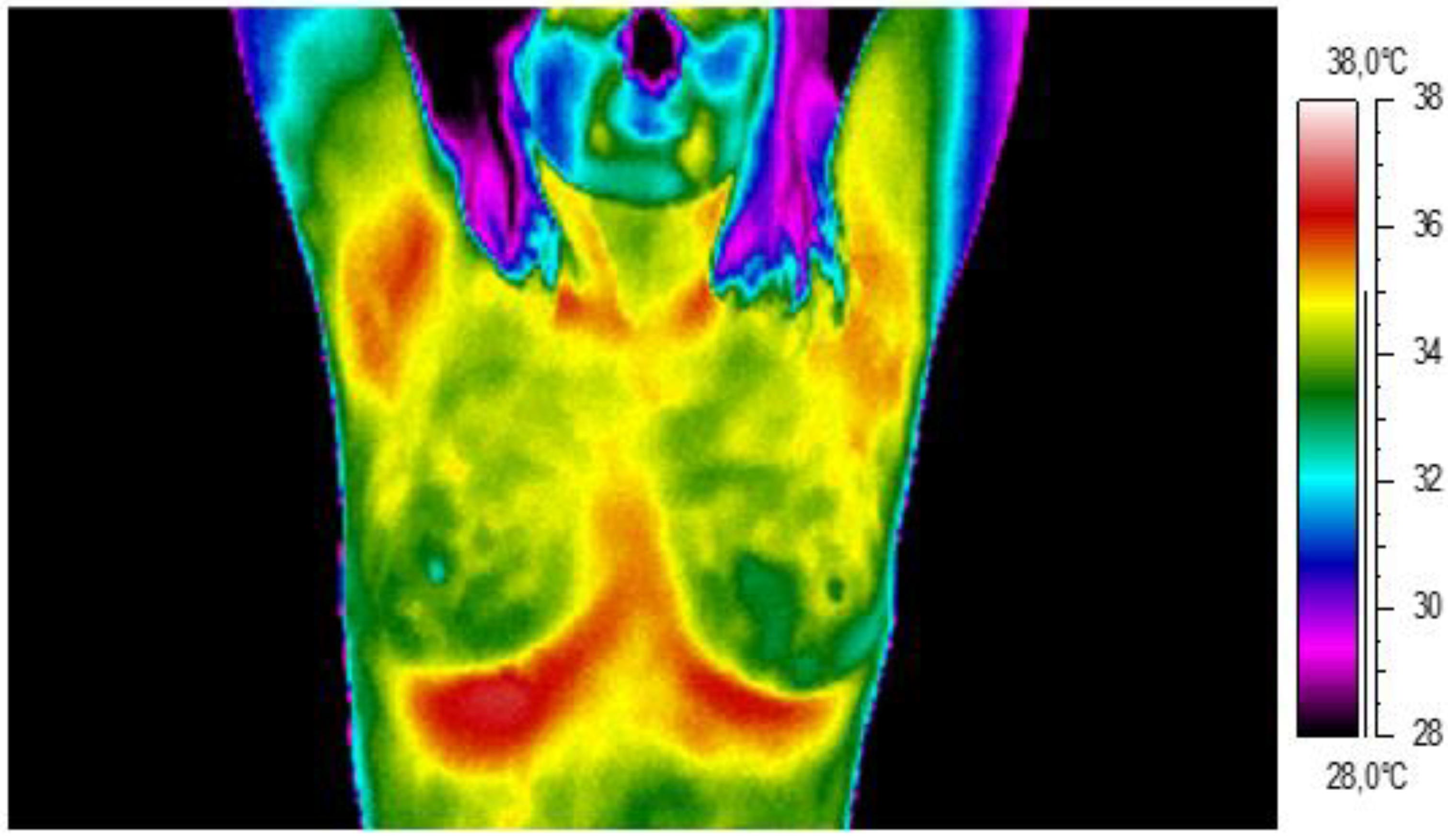
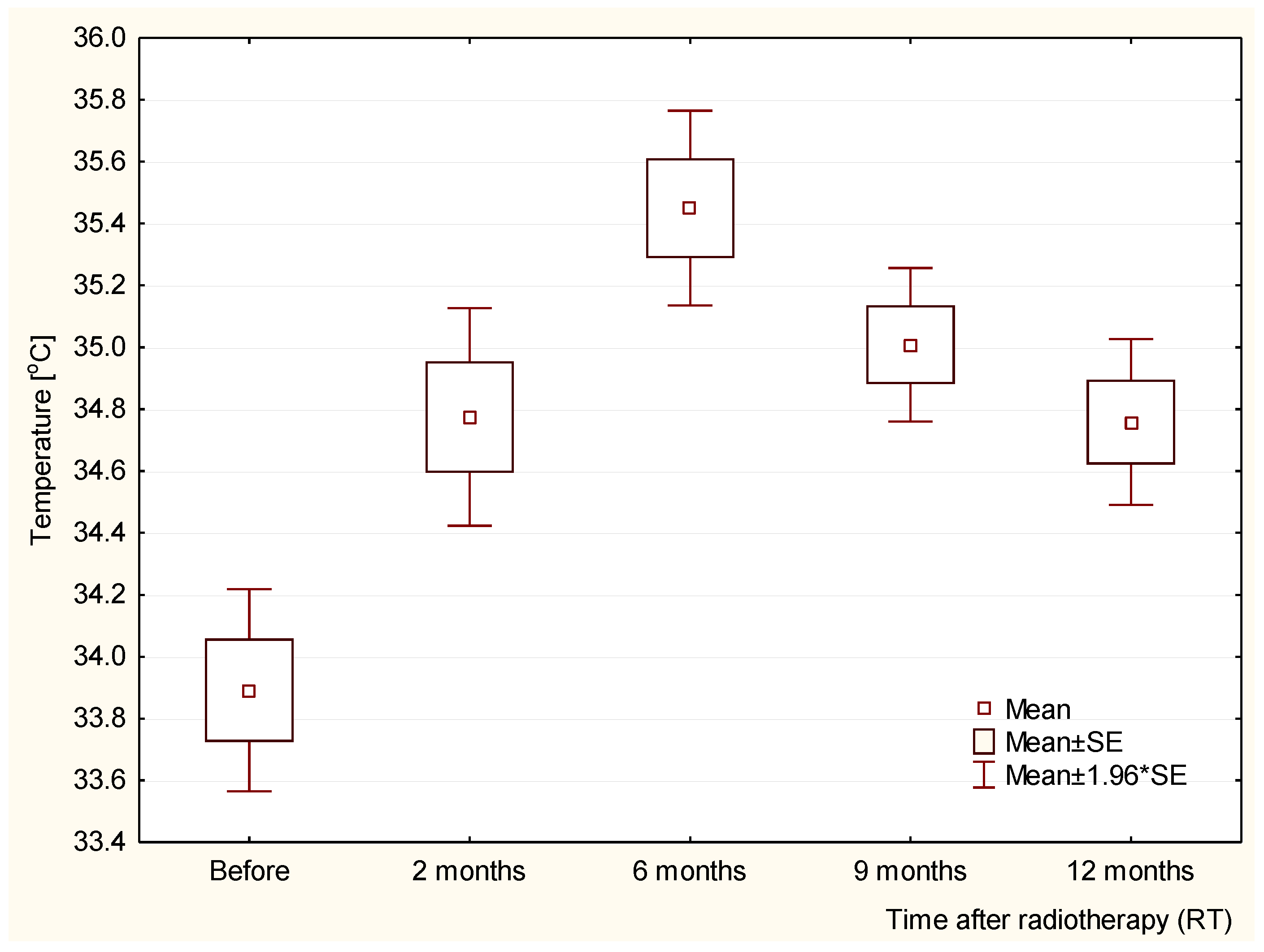
3. Results and Discussion
4. Discussion
5. Conclusions
- Thermography allows the assessment of the dynamics of temperature changes in the tissue after radiotherapy in the mastectomy area;
- The observed temperature fluctuations are the most probable effects of radiation changes in the tissues in the course of the radiation reaction and the healing process;
- The quantitative assessment of temperature fluctuations in the irradiated area, as one of the parameters of the intensification of the radiation reaction, may be used in clinical practice and in research studies that assess the effectiveness of pharmaceuticals used in the treatment of radiation symptoms;
- The small number of observations has limited the conclusions. The expansion of the research into the use of thermography in clinical practice is needed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podbielska, H.; Skrzek, A. Biomedyczne Zastosowania Termowizji; Oficyna Wydaw: Wrocław, Poland, 2014. [Google Scholar]
- Ammer, K.; Ring, E.F.J. The Thermal Image in Medicine and Biology; Uhlen-Verlag: Wien, Austria, 1995. [Google Scholar]
- Ring, E.F.J. Progress in the measurement of human body temperature. IEEE Eng. Med. Biol. Mag. 1998, 17, 19–24. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. The Technique of Infrared Imaging in Medicine. Thermol. Int. 2000, 10, 7–14. [Google Scholar]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef] [PubMed]
- Ammer, K. The Glamorgan Protocol for recording and evaluation of thermal images of the human body. Thermol. Int. 2008, 18, 125–129. [Google Scholar]
- Bauer, J.; Hurnik, P.; Zdziarski, J.; Mielczarek, W.; Podbielska, H. Thermovision and its applications in medicine. Acta Bio. Opt. Inf. Med. 1997, 3, 121–131. [Google Scholar]
- Chmielewski, L.; Kulikowski, L.J.; Nowakowski, A. Obrazowanie Biomedyczne; Akad. Oficyna Wydawnicza EXIT: Warszawa, Poland, 2003. [Google Scholar]
- Bojakowska, U.; Kalinowski, P.; Kowalska, M.E. Epidemiologia i profilaktyka raka piersi = Epidemiology and prophylaxis of breast cancer. J. Educ. Health Sport. 2016, 6, 701–710. [Google Scholar]
- Kopans, D.B. “Early” breast cancer detection using techniques other than mammography. Am. J. Roentgenol. 1984, 143, 465–468. [Google Scholar] [CrossRef][Green Version]
- Ng, E.Y.K. A review of thermography as promising non-invasive detection modality for breast tumor. Int. J. 2009, 48, 849–859. [Google Scholar] [CrossRef]
- Śniadecki, M. Kryteria Rzpoznawania I Wczesne Objawy Chorób Nowotworowych; Via Medica: Gdańsk, Poland, 2015. [Google Scholar]
- Ellis, L.M.; Fidler, I.J. Angiogenesis and breast cancer metastasis. Lancet 1995, 346, 388–390. [Google Scholar] [CrossRef]
- Fox, S.B.; Generali, D.G. Breast tumour angiogenesis. Breast Cancer Res. 2007, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Gamagami, P. Atlas of Mammography: New Early Signs in Breast Cancer; Blackwell Science: Oxford, UK, 1986. [Google Scholar]
- Niwińska, A.; Gałecki, J. Current indications and methods of postoperative radiation therapy—Repetition before the exam. Oncol. Clin Pract. 2016, 12, 18–24. [Google Scholar]
- Censabella, S.; Claes, S.; Orlandini, M.; Braekers, R.; Thijs, H.; Bulens, P. Retrospective study of radiotherapy-induced skin reactions in breast cancer patients: Reduced incidence of moist desquamation with a hydroactive colloid gel versus dexpanthenol. Eur. J. Oncol. Nurs. 2014, 18, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.G.; González, L.B.; Carazo, J.L.S.; Partearroyo, J.C.G.; Martínez, A.E.; González, A.V.; Torrecilla, J.L.L. Evaluation of acute skin toxicity in breast radiotherapy with a new quantitative approach. Radiother. Oncol. 2017, 122, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rodenberg, D.A.; Chaet, M.S.; Bass, R.C. Nitric Oxide: An Overview. Am. J. Surg. 1995, 170, 292. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yasuoka, H. Nitric Oxide in Breast Cancer. Clin. Cancer Res. 2006, 12, 1201. [Google Scholar] [CrossRef]
- Anbar, M. Hyperthermia of the cancerous breast: Analysis of mechanism. Cancer Lett. 1994, 84, 23. [Google Scholar] [CrossRef]
- Amalric, R.; Giraud, D.; Altschuler, C.; Amalric, F.; Spitalier, J.M.; Brandone, H.; Ayme, Y.; Gardiol, A.A. Does infrared thermography truly have a role in present day breast cancer management. Biomed. Thermol. 1982, 107, 269–278. [Google Scholar]
- Jones, C.H. Thermography of the female breast. In Diagnosis of Breast Disease; Parsons, C.A., Ed.; University Park Press: Baltimore, MD, USA, 1983; pp. 214–234. [Google Scholar]
- Head, J.F.; Lipari, C.A.; Elliot, R.L. Comparison of mammography and breast infrared imaging: Sensitivity, specificity, false negatives, false positives, positive predictive value and negative predictive value. In Proceedings of the First Joint BMES/EMBS Conference. 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society, Atlanta, GA, USA, 13–16 October 1999. [Google Scholar] [CrossRef]
- Cholewka, A.; Kajewska, J.; Kawecki, M.; Sieroń-Stołtny, K.; Stanek, A. How to use thermal imaging in venous insufficiency? J. Therm. Anal. Calorimetry 2017, 130, 1317–1326. [Google Scholar] [CrossRef]
- Cholewka, A.; Drzazga, Z.; Sieroń, A.; Stanek, A. Thermovision diagnostics in chosen spine diseases treated by whole body cryotherapy. J. Therm. Anal. Calorim. 2010, 102, 113–119. [Google Scholar] [CrossRef]
- Cholewka, A.; Stanek, A.; Kwiatek, S.; Sieroń, A.; Drzazga, Z. Does the temperature gradient correlate with the photodynamic diagnosis parameter numerical colour value (NCV)? Photodiagn. Photodyn. Ther. 2013, 10, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cholewka, A.; Stanek, A.; Klimas, A.; Sieroń, A.; Drzazga, Z. Thermal imaging application in chronic venous disease: Pilot study. J. Anal. Calorim. 2013, 115, 1609–1618. [Google Scholar] [CrossRef][Green Version]
- Kajewska, J.; Cholewka, A.; Pająk, J.; Stanek, A. The thermal imaging parameters in correlation with USG duplex parameters used in chronic venous disease of lower extremities diagnosis. In Proceedings of the Quantitative InfraRed Thermography, Gdansk, Poland, 4–8 July 2016. [Google Scholar]
- Keyserlingk, J.R.; Ahlgren, P.D. Infrared imaging of the breast; initial reappraisal using high—Resolution digital technology in 100 successive cases of stage 1 and 2 breast cancer. Breast J. 1998, 4, 245–251. [Google Scholar] [CrossRef]
- Herman, C.; Cetingul, M.P. Quantitative visualization and detection of skin cancer using dynamic thermal imaging. J. Vis. Exp. 2011, 51, 2679. [Google Scholar] [CrossRef]
- Head, J.F.; Wang, F.; Elliott, R.L. Breast thermography is a noninvasive prognostic procedure that predicts tumor growth rate in breast cancer patients. Ann. N. Y. Acad. Sci. 1993, 698, 153–158. [Google Scholar] [CrossRef]
- Ng, E.Y.K.; Sudharsan, N.M. Numerical computation as a tool to aid thermographic Interpretation. J. Med. Eng. Technol. 2001, 25, 53–60. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Lee, T.; Seely, D. A Comparative Review of Thermography as a Breast Cancer Screening Technique. Integr. Cancer Ther. 2009, 8, 9–16. [Google Scholar] [CrossRef]
- Morales-Cervantes, A.; Kolosovas-Machuca, E.S.; Guevara, E.; Reducindo, M.M.; Hernández, A.B.B.; García, M.R.; González, F.J. An automated method for the evaluation of breast cancer using infrared thermography. EXCLI J. 2018, 17, 989–998. [Google Scholar]
- Brzezińska, D.; Baic, A.; Cholewka, A.; Stankiewicz, M.; Ślosarek, K.; Bzowski, J.; Stanek, A.; Sieroń, K. Zastosowanie obrazowania termicznego w diagnostyce nowotworów piersi. Inżynier I Fiz. Med. 2018, 7, 345–350. [Google Scholar]
- Plaza, D.; Baic, A.; Lange, B.; Stanek, A.; Szurko, A.; Sieroń, K.; Ślosarek, K.; Cholewka, A. Zastosowanie obrazowania termicznego w ocenie efektów radioterapii u pacjentek po mastektomii. Inżynier I Fiz. Med. 2020, 9, 277–280. [Google Scholar]
- Plaza, D.; Baic, A.; Lange, B.; Stanek, A.; Ślosarek, K.; Kowalczyk, A.; Cholewka, A. Correlation between Isotherms and Isodoses in Breast Cancer Radiotherapy—First Study. Int. J. Environ. Res. Public Health 2021, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Franklin, L.E.; Jenrette, J.M.; Aguero, E.G. Skin Toxicity During Breast Irradiation: Pathophysiology and Management. South. Med. J. 2004, 97, 989–993. [Google Scholar] [CrossRef]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation dermatitis: Clinical presentation. pathophysiology and treatment 2006. J. Am. Acad Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Maillot, O.; Leduc, N.; Atallah, V.; Escarmant, P.; Petit, A.; Belhomme, S.; Sargos, P.; Vinh-Hung, V. Evaluation of Acute Skin Toxicity of Breast Radiotherapy Using Thermography: Results of a Prospective Single-Centre Trial; Elsevier Masson SAS: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Prasada, S.S.; Ramachandraa, L.; Kumarb, V.; Davea, A.; Mesthac, L.K.; Venkatarmani, K. Evaluation of efficacy of thermographic breast imaging in breast cancer: A pilot study. Breast Dis. 2016, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lilla, C.; Ambrosone, C.B.; Kropp, S.; Helmbold, I.; Schmezer, P.; Von Fournier, D.; Haase, W.; Sautter-Bihl, M.-L.; Wenz, F.; Chang-Claude, J. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res. Treat. 2007, 106, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-Y.; Hou, M.-F.; Luo, K.-H.; Wei, S.-Y.; Huang, M.-Y.; Su, S.-J.; Kuo, H.-Y.; Yuan, S.-S.; Chen, G.-S.; Hu, S.C.-S. RTOG, CTCAE and WHO criteria for acute radiation dermatitis correlate with cutaneous blood flow measurements. Breast 2015, 24, 230–236. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, L.; Chen, G.; Li, X.; Meng, X.; Xing, L.; Zhao, H. Relationships between the changes of skin temperature and radiation skin injury. Int. J. Hyperth. 2019, 36, 1159–1166. [Google Scholar] [CrossRef]
- Greco, F.; Quarta, L.G.; Grasso, R.F.; Beomonte Zobel, B.; Mallio, C.A. Increased visceral adipose tissue in clear cell renal cell carcinoma with and without peritumoral collateral vessels. Br. J. Radiol. 2020, 93, 20200334. [Google Scholar] [CrossRef]
- Vavassis, P.; Gelinas, M.; Chabot Tr, J.; Nguyen-Tân, P.F. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J. Otolaryngol. Head Neck Surg. 2008, 37, 124–129. [Google Scholar]
- Omidvari, S.; Saboori, H.; Mohammadianpanah, M.; Mosalaei, A.; Ahmadloo, N.; Mosleh-Shirazi, M.A.; Jowkar, F.; Namaz, S. Topical betamethasone for prevention of radiation dermatitis. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 213. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bentzen, S.M. The need for adverse effects reporting standards in oncology clinical trials. J. Clin. Oncol. 2004, 22, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.J.; Larsen, E.; Chan, P. Re-examining the Evidence in Radiation Dermatitis Management Literature: An Overview and a Critical Appraisal of Systematic Reviews. Int. J. Radiat. Oncol. 2012, 84, e357–e362. [Google Scholar] [CrossRef] [PubMed]


| Before RT [°C] | 2 Months after RT [°C] | 6 Months after RT [°C] | 9 Months after RT [°C] | 12 Months after RT [°C] | |
|---|---|---|---|---|---|
| Patient 1 | 34.0 | 34.5 | 36.3 | 34.7 | 34.5 |
| Patient 2 | 34.4 | 35.1 | 35.5 | 34.8 | 34.3 |
| Patient 3 | 34.1 | 34.7 | 35.0 | 34.6 | 34.2 |
| Patient 4 | 35.0 | 36.0 | 36.5 | 36.0 | 35.7 |
| Patient 5 | 34.4 | 35.9 | 36.1 | 35.7 | 35.4 |
| Patient 6 | 34.3 | 35.0 | 35.6 | 35.3 | 35.3 |
| Patient 7 | 33.5 | 34.4 | 35.0 | 34.9 | 34.7 |
| Patient 8 | 33.0 | 34.5 | 35.1 | 34.7 | 34.5 |
| Patient 9 | 33.5 | 34.3 | 34.9 | 34.7 | 34.4 |
| Patient 10 | 33.7 | 34.4 | 35.2 | 35.0 | 34.9 |
| Patient 11 | 33.6 | 34.5 | 35.1 | 34.8 | 34.5 |
| Patient 12 | 33.2 | 34.0 | 35.1 | 34.9 | 34.7 |
| 2 Months after RT [°C] | 6 Months after RT [°C] | 9 Months after RT [°C] | 12 Months after RT [°C] |
|---|---|---|---|
| 0.88 | 1.56 | 1.12 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baic, A.; Plaza, D.; Lange, B.; Michalecki, Ł.; Stanek, A.; Ślosarek, K.; Cholewka, A. Twelve-Month Evaluation of Temperature Effects of Radiotherapy in Patients after Mastectomy. Int. J. Environ. Res. Public Health 2022, 19, 2834. https://doi.org/10.3390/ijerph19052834
Baic A, Plaza D, Lange B, Michalecki Ł, Stanek A, Ślosarek K, Cholewka A. Twelve-Month Evaluation of Temperature Effects of Radiotherapy in Patients after Mastectomy. International Journal of Environmental Research and Public Health. 2022; 19(5):2834. https://doi.org/10.3390/ijerph19052834
Chicago/Turabian StyleBaic, Agnieszka, Dominika Plaza, Barbara Lange, Łukasz Michalecki, Agata Stanek, Krzysztof Ślosarek, and Armand Cholewka. 2022. "Twelve-Month Evaluation of Temperature Effects of Radiotherapy in Patients after Mastectomy" International Journal of Environmental Research and Public Health 19, no. 5: 2834. https://doi.org/10.3390/ijerph19052834
APA StyleBaic, A., Plaza, D., Lange, B., Michalecki, Ł., Stanek, A., Ślosarek, K., & Cholewka, A. (2022). Twelve-Month Evaluation of Temperature Effects of Radiotherapy in Patients after Mastectomy. International Journal of Environmental Research and Public Health, 19(5), 2834. https://doi.org/10.3390/ijerph19052834







