Bone Quality in Patients with Parkinson’s Disease Determined by Quantitative Ultrasound (QUS) of the Calcaneus: Influence of Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Benito-Leon, J. Epidemiologia de la enfermedad de Parkinson en España y su contextualizacion mundial [Epidemiology of Parkinson’s disease in Spain and its contextualisation in the world]. Rev. Neurol. 2018, 66, 125–134. (In Spanish) [Google Scholar] [PubMed]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996, 14, 317–335. [Google Scholar] [CrossRef]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, J.J.; Alegre, J.; Gomez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B.; et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Chung, K.K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef]
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/ta160/documents/ta160-technologies-for-the-primary-and-secondary-prevention-of-osteoporotic-fractures-appendix-b-proposal-paper-presented-to-the-institutes-guidance-executive2 (accessed on 24 August 2021).
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Torsney, K.M.; Noyce, A.; Doherty, K.M.; Bestwick, J.P.; Dobson, R.; Lees, A.J. Bone health in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torres, M.; Varsavsky, M.; Avilés Pérez, M.D. Osteoporosis. Definición. Epidemiología. Rev. Osteoporos. Metab. Miner. 2010, 2 (Suppl. 3), S5–S7. [Google Scholar]
- Dennison, E.M.; Compston, J.E.; Flahive, J.; Siris, E.S.; Gehlbach, S.H.; Adachi, J.D.; Boonen, S.; Chapurlat, R.; Diez-Perez, A.; Anderson, F.A.; et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 2012, 50, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Yarnall, A.; Noyce, A.J.; Giovannoni, G. Bone health in chronic neurological diseases: A focus on multiple sclerosis and parkinsonian syndromes. Pract. Neurol. 2013, 13, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Cheng, P.-Y.; Wu, S.-L.; Lai, C.-H. Parkinson’s disease and risk of hip fracture: An 8-year follow-up study in Taiwan. Parkinsonism Relat. Disord. 2012, 18, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, L.; Ji, H.F. Osteoporosis risk and bone mineral density levels in patients with Parkinson’s disease: A meta-analysis. Bone 2013, 52, 498–505. [Google Scholar] [CrossRef]
- Rison, R.A.; Richardson, K. Idiopathic Parkinson’s disease, osteoporosis, and hip fractures: A case report. Case Rep. Neurol. 2011, 14, 14–17. [Google Scholar] [CrossRef]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Park, K.; Oeda, T.; Kohsaka, M.; Tomita, S.; Umemura, A.; Sawada, H. Low body mass index and life prognosis in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 55, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Parashos, S.A.; Bloem, B.R.; Browner, N.M.; Giladi, N.; Gurevich, T.; Hausdorff, J.M.; He, Y.; Lyons, K.E.; Mari, Z.; Morgan, J.C.; et al. What predicts falls in Parkinson disease?: Observations from the Parkinson’s Foundation registry. Neurol. Clin. Pract. 2018, 8, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Barberán, M.; Campusano, C.; Trincado, P.; Oviedo, S.; Brantes, S.; Sapunar, J.; Canessa, J.; Cid, P.; Escobar, F.; Eugenin, D.; et al. Recomendaciones para el uso correcto de densitometría ósea en la práctica clínica. Consenso de la Sociedad Chilena de Endocrinología y Diabetes [Guidelines of the Chilean Endocrinology Society for the correct clinical use of bone densitometry]. Rev. Med. Chil. 2018, 146, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Baim, S. Quantitative Ultrasound (QUS) in the Management of Osteoporosis and Assessment of Fracture Risk. J. Clin. Densitom. 2017, 20, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.Q.; Alfonso, F.J.N.; Castelló, J.; Moral, J.V.C.; Ribas, J.M.; Montoro, A.M.F. Capacidad predictiva de la densitometría de calcáneo en mujeres climatéricas. Rev. Esp. Enf. Met. Óseas 2007, 16, 119–123. [Google Scholar] [CrossRef]
- Yamada, M.; Ito, M.; Hayashi, K.; Ohki, M.; Nakamura, T. Dual energy X-ray absorptiometry of the calcaneus: Comparison with other techniques to assess bone density and value in predicting risk of spine fracture. AJR Am. J. Roentgenol. 1994, 163, 1435–1440. [Google Scholar] [CrossRef]
- Fordham, J.N.; Chinn, D.J.; Kumar, N. Identification of women with reduced bone density at the lumbar spine and femoral neck using BMD at the os calcis. Osteoporos. Int. 2000, 11, 797–802. [Google Scholar] [CrossRef]
- Riesco Díaz, M.; Doña Naranjo, M.Á. Estudio comparativo entre la densitometría de calcáneo y la de cadera y columna lumbar, y valoración de aquélla en la práctica clínica. Reumatol. Clin. 2006, 2, 219–220. [Google Scholar] [CrossRef]
- Ivorra Cortés, J.; Román-Ivorra, J.A.; Alegre Sancho, J.J.; Beltrán Catalán, E.; Chalmeta Verdejo, I.; Fernández-Llanio Comella, N.; Muñoz Gil, S. Puntos de cribado de un densitómetro periférico de calcáneo para el diagnóstico de osteoporosis. Rev. Osteoporos. Metab. Miner. 2010, 2, 23–28. [Google Scholar]
- Caballero Uribe, C.V. Evaluation of osteoporosis by quantitative ultrasound of the calcaneus. Rev. Esp. Enf. Met. Óseas 2001, 10, 65–69. [Google Scholar]
- Vidal Neira, L.; Cabello León, E.; Rueda Fernández, C.; Laurel, W.M. Comparison of bone scan values obtained with bone densitometry by dual energy X ray absorptiometry with calcaneous ultrasound values. Rev. Soc. Peru. Med. Interna 2004, 17, 9–16. [Google Scholar]
- Chin, K.Y.; Ima-Nirwana, S. Calcaneal quantitative ultrasound as a determinant of bone health status: What properties of bone does it reflect? Int. J. Med. Sci. 2013, 10, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Trimpou, P.; Bosaeus, I.; Bengtsson, B.-Å.; Landin-Wilhelmsen, K. High correlation between quantitative ultrasound and DXA during 7 years of follow-up. Eur. J. Radiol. 2010, 73, 360–364. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, B.; Gong, J.; Xu, H.; Bai, Z. The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J. Bone Miner. Metab. 2014, 32, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Damilakis, J.; Perisinakis, K.; Gourtsoyiannis, N. Imaging ultrasonometry of the calcaneus: Optimum T-score thresholds for the identification of osteoporotic subjects. Calcif. Tissue Int. 2001, 68, 219–224. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Guirant, L.; Carlos, F.; Curiel, D.; Kanis, J.A.; Borgström, F.; Svedbom, A.; Clark, P. Health-related quality of life during the first year after a hip fracture: Results of the Mexican arm of the International Cost and Utility Related to Osteoporotic Fractures Study (MexICUROS). Osteoporos. Int. 2018, 29, 1147–1154. [Google Scholar] [CrossRef]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Sääksjärvi, K.; Heliövaara, M. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef]
- Van den Bergh, J.P.; Bours, S.P.; van Geel, T.A.; Geusens, P.P. Optimal use of vitamin D when treating osteoporosis. Curr. Osteoporos. Rep. 2011, 9, 36–42. [Google Scholar] [CrossRef][Green Version]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- D’Amico, F.; Crescenti, P.; Gaglio, G.; Granata, A.; Natoli, R.; Pipicella, T.; Russo, E.; Grippa, A. Osteoporosis and fall in elderly subjects with Parkinson disease. Bone 2010, 47, S171–S172. [Google Scholar] [CrossRef]
- Van den Brand, M.W.; Pouwels, S.; Samson, M.M.; Van Staa, T.P.; Thio, B.; Cooper, C.; Leufkens, H.G.M.; Egberts, A.C.G.; Verhaar, H.J.J.; De Vries, F. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos. Int. 2009, 20, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, M.J.; Kim, B.J.; Kim, S.R.; Chun, S.; Kim, H.-K.; Ryu, J.S.; Kim, G.S.; Lee, M.C.; Chung, S.J.; et al. Hyperhomocysteinemia due to levodopa treatment as a risk factor for osteoporosis in patients with Parkinson’s disease. Calcif. Tissue Int. 2010, 86, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Li, M.; Mok, V.; Hui, A.; Woo, J. A case control study on bone mineral density in Chinese patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.C.; Parisi, M.S.; Díaz, S.P.; Mastaglia, S.R.; Deferrari, J.M.; Seijo, M.; Bagur, A.; Micheli, F.; Oliveri, B. A pilot study on the impact of body composition on bone and mineral metabolism in Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Kamanli, A.; Ardicoglu, O.; Ozgocmen, S.; Yoldas, T.K. Bone mineral density in patients with Parkinson’s Disease. Aging Clin. Exp. Res. 2008, 20, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Gjesdal, C.G.; Vollset, S.E.; Ueland, P.M.; Refsum, H.; Drevon, C.A.; Gjessing, H.K.; Tell, G.S. Plasma total homocysteine level and bone mineral density: The Hordaland Homocysteine Study. Arch. Intern. Med. 2006, 166, 88–94. [Google Scholar] [CrossRef]
- Sleeman, I.; Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Johnston, F.; Khoo, T.K.; Burn, D.J. Urate and Homocysteine: Predicting Motor and Cognitive Changes in Newly Diagnosed Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 351–359. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; Pérez Castrillón, J.L.; de Luis Román, D.A. Influencia de la obesidad sobre el metabolismo óseo. Endocrinol. Nutr. 2016, 63, 551–559. [Google Scholar] [CrossRef]
- Cereda, E.; Cassani, E.; Barichella, M.; Caccialanza, R.; Pezzoli, G. Anthropometric indices of fat distribution and cardiometabolic risk in Parkinson’s disease. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 264–271. [Google Scholar] [CrossRef]
- Wilczyński, J.; Półrola, P. Body composition assessment by bioelectrical impedance analysis among patients treated with L-dopa for Parkinson’s disease. Med. Stud./Studia Med. 2018, 34, 120–126. [Google Scholar] [CrossRef]
- Vikdahl, M.; Carlsson, M.; Linder, J.; Forsgren, L.; Håglin, L. Weight gain and increased central obesity in the early phase of Parkinson’s disease. Clin. Nutr. 2014, 33, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, G.E.; Anachebe, T.; McCarroll, K.G.; O’Shea, F. Calcaneal quantitative ultrasound has a role in out ruling low bone mineral density in axial spondyloarthropathy. Clin. Rheumatol. 2020, 39, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.; Ahmed, M.; Chen, A.; Richwine, H.; Zia, N.; Nazar, A.; Bauer, L. Advancing Methods of Assessing Bone Quality to Expand Screening for Osteoporosis. J. Am. Osteopath. Assoc. 2019, 119, 147–154. [Google Scholar] [CrossRef]
| Parkinson’s Group N = 21 | Control Group N = 30 | p | ||||
|---|---|---|---|---|---|---|
| Age (Years) | 71.75 | 67.93 | 0.059 | |||
| Weight (kg) | 73.65 | 72.20 | 0.696 | |||
| Height (cm) | 165.05 | 166.80 | 0.509 | |||
| Sex | Frequency | % | Frequency | % | p | |
| Male | 12 | 57.14% | 13 | 43.33% | 0.332 | |
| Female | 9 | 42.86% | 17 | 56.67% | ||
| Parkinson’s Group N = 21 | Control Group N = 30 | p | |
|---|---|---|---|
| Stiffness (%) | 86.3 ± 20.4 | 92.1 ± 20.2 | 0.318 |
| Tscore | −1.05 ± 1.5 | −0.12 ± 1.4 | 0.032 * |
| BUA (dB/MHz) | 115.9 ± 16.5 | 120.6 ± 15 | 0.304 |
| SOS (m/s) | 1530.3 ± 42 | 1164 ± 646.3 | 0.013 * |
| BMD (gr/cm2) | 0.58 ± 0.13 | 0.62 ± 0.14 | 0.283 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Parkinson’s Group N = 12 | Control Group N = 13 | p | Parkinson’s Group N = 9 | Control Group N = 17 | p | |
| Stiffness (%) | 97.4 ± 4 | 97.3 ± 27.2 | 0.977 | 71.4 ± 14.7 | 87.8 ± 12 | 0.005 * |
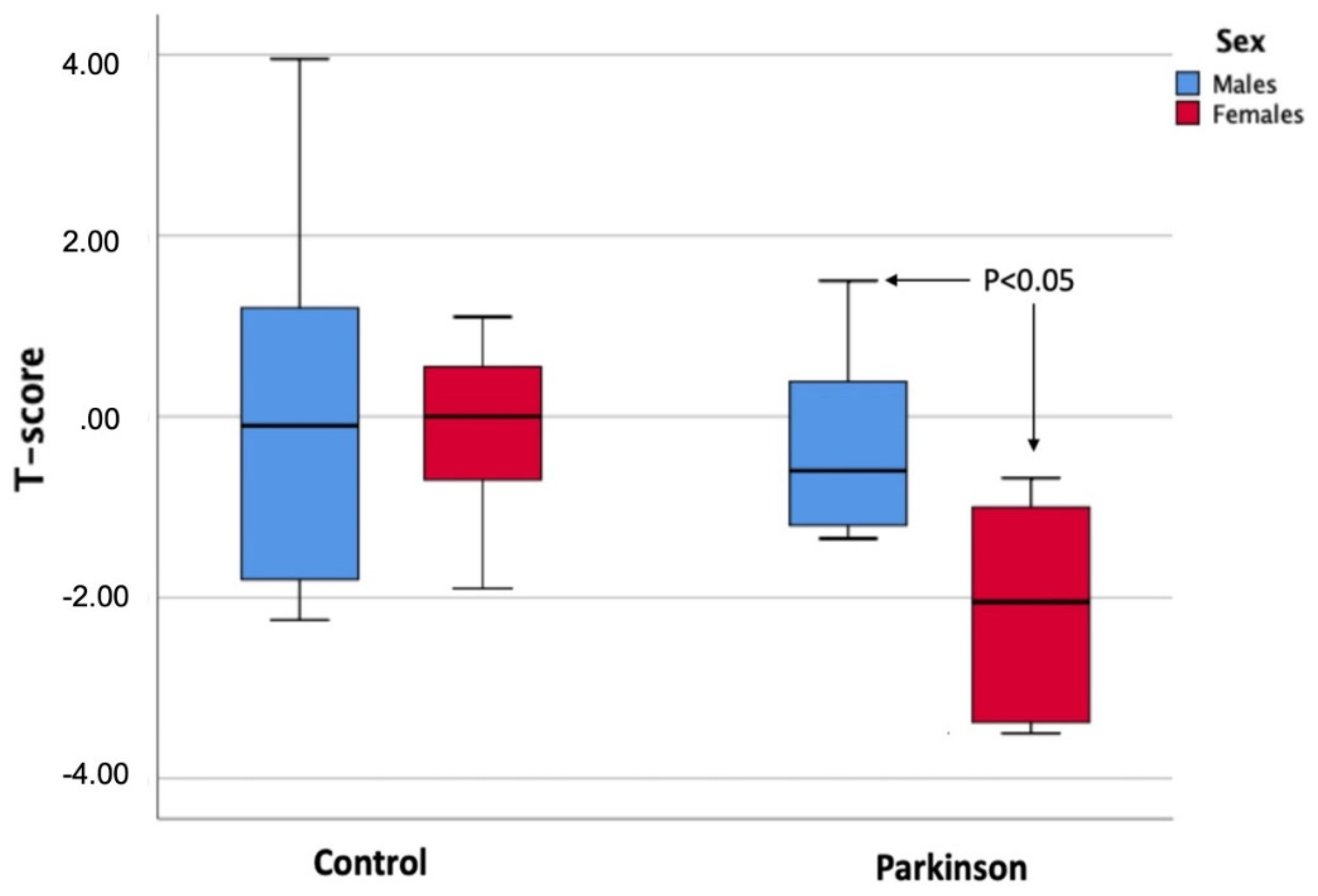
| Tscore | −0.20 ± 1.3 | −0.08 ± 1.9 | 0.863 | −2.19 ± 1.1 | −0.15 ± 0.8 | 0.001 * |
| BUA (dB/MHz) | 124.5 ± 13.8 | 126.5 ± 18 | 0.760 | 104.5 ± 13 | 116 ± 10.6 | 0.023 * |
| SOS (m/s) | 1546.9 ± 42.4 | 1333.9 ± 549.1 | 0.194 | 1508.2 ± 31.4 | 1034.2 ± 699.8 | 0.056 |
| BMD (mg/cm2) | 0.64 ± 0.1 | 0.65 ± 0.1 | 0.895 | 0.49 ± 0.09 | 0.60 ± 0.08 | 0.007 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caplliure-Llopis, J.; Escrivá, D.; Navarro-Illana, E.; Benlloch, M.; de la Rubia Ortí, J.E.; Barrios, C. Bone Quality in Patients with Parkinson’s Disease Determined by Quantitative Ultrasound (QUS) of the Calcaneus: Influence of Sex Differences. Int. J. Environ. Res. Public Health 2022, 19, 2804. https://doi.org/10.3390/ijerph19052804
Caplliure-Llopis J, Escrivá D, Navarro-Illana E, Benlloch M, de la Rubia Ortí JE, Barrios C. Bone Quality in Patients with Parkinson’s Disease Determined by Quantitative Ultrasound (QUS) of the Calcaneus: Influence of Sex Differences. International Journal of Environmental Research and Public Health. 2022; 19(5):2804. https://doi.org/10.3390/ijerph19052804
Chicago/Turabian StyleCaplliure-Llopis, Jordi, Dolores Escrivá, Esther Navarro-Illana, María Benlloch, Jose Enrique de la Rubia Ortí, and Carlos Barrios. 2022. "Bone Quality in Patients with Parkinson’s Disease Determined by Quantitative Ultrasound (QUS) of the Calcaneus: Influence of Sex Differences" International Journal of Environmental Research and Public Health 19, no. 5: 2804. https://doi.org/10.3390/ijerph19052804
APA StyleCaplliure-Llopis, J., Escrivá, D., Navarro-Illana, E., Benlloch, M., de la Rubia Ortí, J. E., & Barrios, C. (2022). Bone Quality in Patients with Parkinson’s Disease Determined by Quantitative Ultrasound (QUS) of the Calcaneus: Influence of Sex Differences. International Journal of Environmental Research and Public Health, 19(5), 2804. https://doi.org/10.3390/ijerph19052804








